Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.1 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1507-7
Phytopatological notes
Plant parasitic nematodes associated to banana roots ( Musa acuminata AA) in central Veracruz, México
1Facultad de Ciencias Agrícolas, Universidad Veracruzana; Xalapa, Veracruz, México
2Facultad de Ciencias, Universidad Autónoma del Estado de México; Toluca, Estado de México, México
3Instituto de Ecología, A.C., Xapala, Veracruz, México
Plant parasitic nematodes are among the major phytosanitary problems affecting banana crops worldwide. In México, this fruit is one of the main agricultural products and in the state of Veracruz the most important production area is located in the Atzalan and Tlapacoyan municipalities. In some farms of these municipalities banana plants with lesions and galling of the root, symptoms caused by nematodes, have been detected. To determine which nematodes were associated with these symptoms, roots were collected in six plantations of Atzalan and Tlapacoyan municipalities in April 2014. Identification of nematodes was performed according to their morphological characteristics, and in the case of Meloidogyne species, specific molecular markers were used. The number of nematodes per 100 g root was also quantified. Helicotylenchus sp. Meloidogyne incognita, Meloidogyne arenaria, Criconema sp., Helicotylenchus multicinctus and Radopholus similis were detected involved in the affecting of root system. The last four are recorded for the first time in the Veracruz banana plantations.
Key words: Meloidogyne spp.; Helicotylenchus spp.; Radopholus similis; Criconema sp.
Los nematodos fitoparásitos constituyen uno de los principales problemas fitosanitarios que afectan el cultivo de plátano a nivel mundial. En México este frutal es uno de los principales productos agrícolas y en el estado de Veracruz, la zona más importante de producción se ubica en los municipios de Atzalan y Tlapacoyan. En algunos cultivos de plátano de estos municipios se han detectado plantas con lesiones y engrosamientos en la raíz, síntomas inducidos por nematodos. Con la finalidad de determinar que nematodos estaban asociados a esta sintomatología, se colectaron raíces en seis plantaciones de los municipios Atzalan y Tlapacoyan durante el mes de abril de 2014. La identificación de los nematodos se realizó de acuerdo a sus características morfológicas y en el caso de las especies de Meloidogyne, se utilizaron marcadores moleculares específicos. Asimismo se cuantificó el número de nematodos en 100 g de raíz. Se detectaron Helicotylenchus sp. Meloidogyne incognita, Meloidogyne arenaria, Criconema sp., Helicotylenchus multicinctus y Radopholus similis involucrados en la afectación del sistema radical. Los ultimos cuatro se registran por primera vez en el cultivo de plátano en Veracruz.
Palabras clave: Meloidogyne spp.; Helicotylenchus spp.; Radopholus similis; Criconema sp.
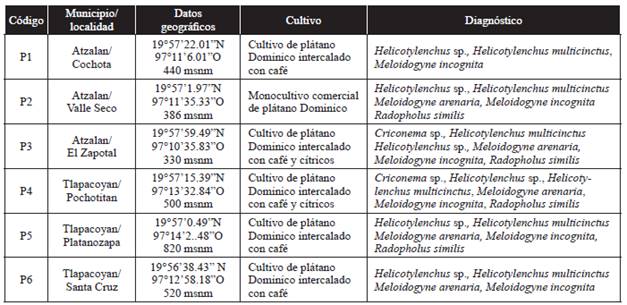
Banana is an important crop in Mexico. It accounts for over 75,000 hectares with production estimated at more than 5000 million Mexican pesos (SIAP, 2014). In the Mexican state of Veracruz, the main banana production area is located in Martínez de la Torre District (SIAP, 2014). In the last five years, characteristic nutrient deficiency symptoms have been observed in some banana plantations in this District, as well as poor development and root necrosis, which causes plants to fall. The latter symptoms are associated to those caused by plant parasitic nematodes. However, no studies have been conducted to detect their presence in this important production area. This study aimed to identify plant parasitic nematodes associated with banana root (Musa acuminata Colla AA), subgroup Pisang Mas, showing infestation symptoms in the municipalities of Atzalan and Tlapacoyan, located in the central part of the state of Veracruz, Mexico. For this study, three banana plantations were selected in each municipality where root infestation has been reported (Table 1). At each plantation, five root samples (500 g) were collected (N=30) at 15-20 depth and 20-cm from the pseudo-stalk of the selected plants (Adriano-Anaya et al., 2008).
Table 1 Geographical data of the collection sites and nematode species identified in banana plants (Musa acuminata).
Musa acuminate AA Sub grupo Pisang Mas locally called Dominico.
Nematodes were extracted from 100 g of root using the maceration, flotation and centrifugation technique (van Bezooijen, 2006); they were fixed and cleared according to Seinhorst (1962) and then taxonomically classified and quantified. At the same time, females of the genus Meloidogyne were extracted by dissecting plant material (100 g of root) to make perineal cuts. Meloidogyne spp. eggs were also extracted from 200 g of root using the maceration in sodium hypochlorite (NaOCl), flotation and centrifugation technique. The extracted nematodes were kept in an ultrafreezer (Thermo Scientific) at -80 °C for subsequent DNA extraction (Carneiro et al., 2004). Nematodes recovered from the first extraction were observed and quantified (100 g of root) under an optical microscope (Leica ICC50). To identify nematodes, the best adult specimens were selected for mounts (van Bezooijen, 2006) and according to the specialized taxonomic keys of the order of Tylenchida (Siddiqi, 1973; Orton-Williams, 1973; Orton-Williams y Siddiqi, 1973; Orton-Williams, 1975; Siddiqi, 2000). Given the similarity of morphological characteristics of some species of Meloidogyne (Carneiro and Cofcewicz 2008), were identified also using specific molecular markers. For this purpose, DNA was extracted from eggs and J2 previously extracted from roots and frozen using the DNA protocol from the DNA Tissue and Insect MiniPrep Isolation Kit (Zymo Research) to conduct a Polymerase Chain Reaction (PCR) test with SCAR markers (Sequence Characterized Amplified Region) (Sigma-Aldrich) specifics to the four Meloidogyne species most widely distributed in banana plantations in Latin America: M. arenaria (F5´-TCGGCGATAGAGGTAAATGAC-3` and R5´-TCGGCGATAGACACTACAACT-3´ 420 base pairs), M. incognita (F5´-GGGATGTGTAAATGCTCCTG-3´ y R 5´-CCCGCTACACCCTCAACTTC-3´ 399 pb), M. javanica (F5´-GGTGCGCGATTGAACTGAGC-3´ and R 5´-CAGGCCCTTCAGTGGAACTATAC-3´ 670 pb) and M. paranaensis (F 5´- GCCCGACTCCATTTGACGGA-3´ and R 5´-CCGTCCAGATCCATCGAAGTC-3´ 208 pb) (Zijlstra et al., 2000; Randig et al., 2002). PCR tests were carried out in a final volume of 30 µL with 6.3 µL of sterile distilled H2O, 6 µL of reaction buffer at 5X concentration (Promega), 3 µL of dNTP at 10 µM, 1.5 µL of MgCl2 at 25 µM (Promega) 6 µL of the corresponding SCAR primer (10 µM), 0.2 µL of Taq polimerasa at 5 u/µL (Promega) and 1 µL of DNA of each population. Additionally, a PCR was prepared using M. arenaria and M. incognita DNA provided by the Instituto de Ecología, A.C. (INECOL) (Ecology Institute, A.C.) as the positive check and a PCR without DNA as the negative check. The PCR was performed in a thermocycler (Applied Biosystems) under the following amplification conditions: 5 min at 94 °C, 30 cycles of 30 s at 94 °C, 45 s at 64 °C, 1 min at 70 °C; and a final extension of 8 min at 70 °C (Randig et al., 2002). The amplification products were isolated using the gel electrophoresis method in a 2 % agarose gel stained with ethidium bromide (0.02 %) and observed under UV light.
The population density of different nematode genera and species from the same location were analyzed using the non-parametric tests Kruskal-Wallis and multiple comparison test (p≤0.05) using Windows STATISTICA 8.0 software.
At the six sampling sites, banana plants showed symptoms characteristic of lack of nutrients such as rickets (thin stalks), yellowing, water stress and low production or racemes with small bananas. Due to damage to the root system, some plants were found uprooting (Figure 1A), or about to fall over, and showed symptoms of necrosis in most of the roots and some corm areas (Gowen et al., 2005). When the roots were analyzed in the laboratory, reddish lesions in the form of longitudinal lines, as well as necrosis, were observed in the epidermis (Figure 1B); these symptoms were consistent with infestation symptoms caused by nematodes of the genus Helicotylenchus (Orion et al., 1999). Swelling and cracking (Figure 1C), as well as female and egg masses of Meloidogyne spp. (Figure 1D) were also found.
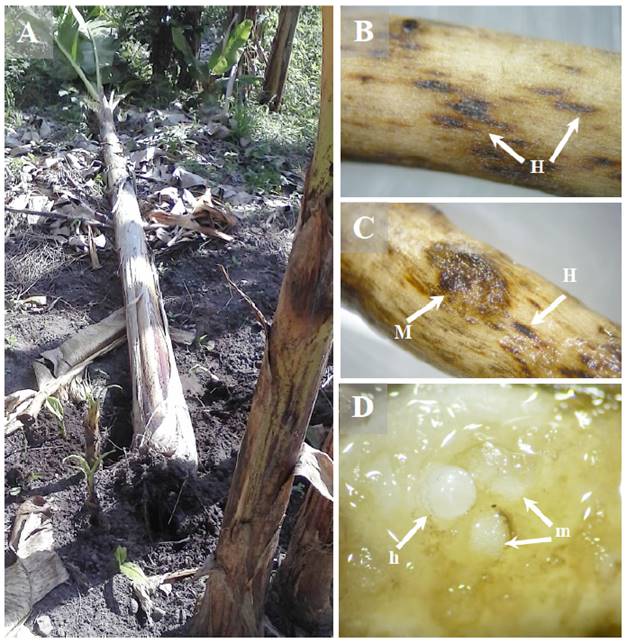
Figure 1 Infestation symptoms caused by nematodes in banana plants A) Toppling and uprooting caused by root weakness, B) Root lesions caused by Helicotylenchus spp. (H), C) Root swelling and cracking caused by Meloidogyne spp. (M) and lesions caused by Helicotylenchus spp., D) Female (h) and egg masses (m) in banana roots.
The identified nematodes were Criconema sp., Helicotylenchus sp., Helicotylenchus multicinctus, Meloidogyne arenaria, Meloidogyne incognita and Radopholus similis (Table 1). The PCR performed using SCAR markers showed that M. arenaria is present in five localities, while M. incognita is present in the six locations (Figure 2). M. javanica and M. paranaensis were not detected in the analyzed samples. A description of the morphological features of the analyzed nematodes follows:
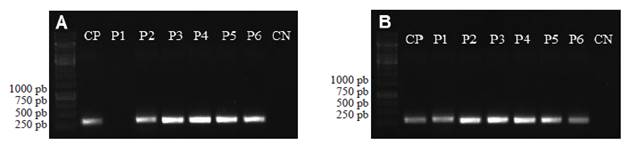
Figure 2 Amplification test using specific markers for A) 420 base pairs (pb) of Meloidogyne arenaria and B) 399 pb of M. incognita in the six populations using roots of Musa acuminata. The numbers of the populations suggest the sampling site summarized in Table 1. CP= positive check using DNA of M. arenaria and M. incognita populations previously identified. CN= negative check for PCR. The stripes on the left correspond to the molecular weight marker GeneRuler 1kb (Thermo Scientific).
Criconema sp. Female: Ornamented cuticle, 39 to 44 annulations of 4-4.8 μm long from the base, rounded, slightly directed backwards with a slight projection of 2.4-3.2 μm long at the terminal part of each annulation. From 343 to 571 μm long and 46.8 to 62.4 μm long. Two differentiated cephalic annulations of the body; the former annulation is greater than the latter. Strong stylet 70.2-78 μm long. Vulva located in the last quarter of the body, in annulation 33. The lips of the vulva were closed, cone-shaped and protruded slightly from the contour of the body. Straight vagina, conical to rounded tail (Figure 3A and B).
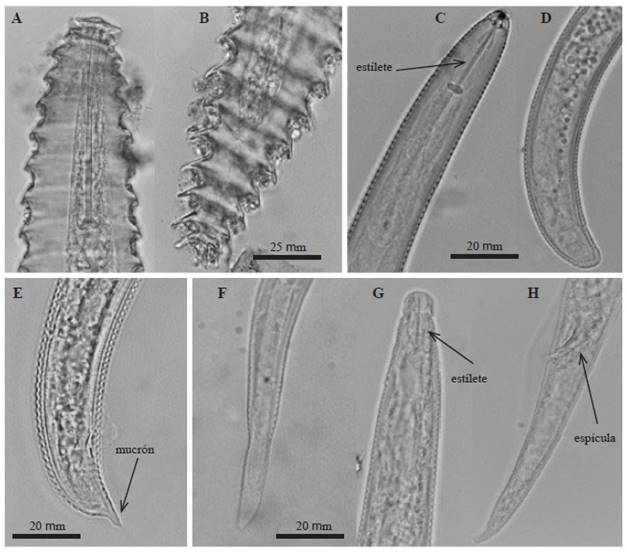
Figure 3 Photographs of some specimens observed under an optical microscope A) Cephalic region of a Criconema sp. female, B) Tail of a Criconema sp. female, C) Cephalic region of a Helicotylenchus multicintus female, D) Tail of a Helicotylenchus multicintus female, E) Tail of a Helicotylenchus sp. Female with ventral projection (mucron), F) Tail of a Radopholus similis female, G) Cephalic region of a Radopholus similis male where a degraded stylet can be seen, H) Tail of a Radopholus similis male.
Helicotylenchus sp. Female: Rolling or arching after having been fixed with formaldehyde, 398 to 671 μm long and 16 a 23 μm wide. Hemispherical labial region and slightly different from the rest of the body; 3 to 4 annulations. Style with prominent basal knobs 16 to 24 μm long . Median esophageal bulb oval-shaped (8 μm wide x 12 μm long) with a small valve apparatus in the center. Esophageal glands overlapping the intestine. Excretory pore near to the junction of the esophagus to the intestine. Well developed vulva, transversally depressed; it is located in the third quarter of the body length. Didelphic, symmetric ovaries and spermathecas (10 to 12 μm diameter). Short tail of the female, semispherical, dorsally convex, with ventral projection (mucron) (Figure 3E). Lateral fields with 4 incisures, around one quarter of the body width.
Helicotylenchus multinctus. Female: Arched after having been fixed with formaldehyde, 480 to 640 μm long and 16 to 20 μm wide. Stylet from 20 to 22 μm long with prominent basal knobs (Figure 3C). Semispherical labial region with 4 annulations. Median esophageal bulb oval-shaped (8 μm wide x 10 μm long) with a small valvular apparatus in the center. Esophageal gland overlapping intestine. Excretory pore near to the junction of the esophagus to the intestine. Didelphic, both branches of the reproduction organs well developed and functional. Symmetric ovaries, rounded spermathecas, 10 μm diameter. Vulva prominent, slightly depressed transversally; it is located in the third quarter in relation to the body length. Short tail, semispherical, dorsally convex, without ventral or terminal projection (Figure 3D). Lateral fields with 4 non-areolated incisures, around one quarter of the body width. Male: 460 to 686 μm long x 16 to 20 μm wide. Stylet 18 to 20 μm long. Spicule from 18 to 22 μm long, gubernaculum from 4 to 6 μm. Tail with conical termination and ventral- hyaline projection.
Radopholus similis. Female: Wormlike, 530 to 772 μm long, and 15 to 31 μm wide in the middle. Slightly ringed cuticle. Rounded labial region with 3 to 4 annules. Stylet from 12 to 14 μm long with well-developed basal knobs. Well developed median bulb, oval, from 10 to 12 μm diameter. Well developed valvular apparatus. Esophageal glands overlapping intestine dorsally. Vulva prominent, slightly below in the middle of the body. Didelphic, two functional reproductive organs. Spherical spermathecas. Elongated, cone-shaped tail with an oval-shaped ending (Figure 3F). Lateral fields with 4 non-aerolated incisures. Male: Wormlike slightly ventrally arched, 515 to 632 μm long and 16 to 23 μm wide. Labial region well defined from the rest of the body with 4 or 5 annulations and raised from 4 to 6 μm. Esophagus and stylet degenerated, without visible basal knobs (Figure 3G). Median bulb and valvular apparatus indistinct. Strong spicule from 18 to 20 μm long (Figure 3H). Enlarged gubernaculum from 8 to 10 μm long and pointed at the back end.
Meloidogyne arenaria. Female: White, pear-shaped body, 510-1000 μm long and 400-600 μm wide. Conical neck. A strong 14 to 16 μm long stylet. Dorsal esophageal gland opening at the base of the stylet. The perineal pattern has a depressed and rounded dorsal arch, dorsal-laterally compressed, with undulated and smooth separated marks. (Figure 4A).
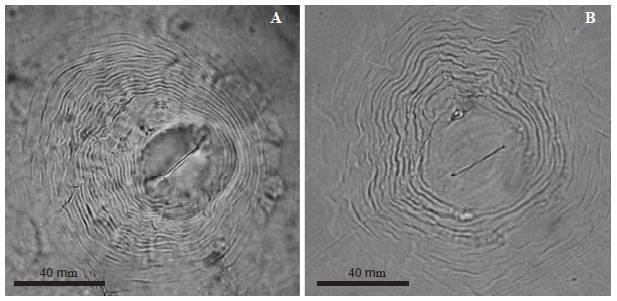
Figure 4 Photographs of perineal patterns of Meloidogyne spp., A) M. arenaria, B) M. incognita females.
Meloidogyne incognita. Female: White, spherical body with 500 to 723 μm long short neck and 331 to 520 μm wide. A 12 to 15 μm long stylet. Elongated vulva transversally oriented at the center of the body. Pear-shaped perineal pattern with a high and trapezoidal arch formed by closely-spaced marks, relatively thick, smooth, and slightly undulated or in zigzag (Figure 4B).
The nematode genera found are similar to the ones reported in Musa spp. plants in different countries, though the number of species found was low compared to those found in other studies (Kamira et al., 2013; Chitamba et al., 2013; Lima et al., 2013). Also, Helicotylenchus spp., Meloidogyne spp. and Radopholus similis found in banana correspond to genera previously reported in Mexico (Montes-Belmont, 2000; Cid del Prado-Vera et al., 2001; Adriano-Anaya et al., 2008; López-Lima et al., 2015). This is the first time Criconema sp., Helicotylenchus multicinctus, M. arenaria and Radopholus similis have been reported in banana crops in Veracruz. Helicotylenchus microcephalus, Meloidogyne incognita, M. paranaensis and Tylenchus spp. (Montes-Belmont 2000; López-Lima et al., 2015) were reported in previous studies.
Helicotylenchus sp. and Helicotylenchus multicinctus were present in all the sampling sites and were the most abundant (Table 2). These nematode genera, in particular H. multicinctus and H. dihystera species, are considered a strong constraint for banana crops worldwide (Das et al., 2014), though H. dihystera was not found in this study. The two species of root-knot (galling of the root) nematodes found in this study are the most widely distributed in banana crops (Cofcewicz et al., 2005). Recently, M. incognita was detected in banana root associated with M. paranaensis in a location near the study area in the municipality of Atzalan (López-Lima et al., 2015). However, during the PCR test no amplification of this species was detected. The presence of Helicotylenchus multicinctus populations associated with M. arenaria and M. incognita populations may represent a risk for banana production in this area, as it has happened in other sites where the two nematode genera have caused serious damages to banana crops (Brentu et al., 2004; Subbotin et al., 2011).
Table 2 Number of plant parasitic nematodes found in 100 g of root tissue ± standard deviation.
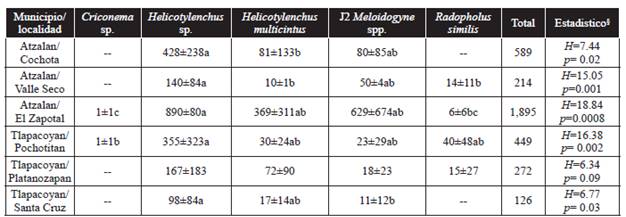
Values represent the average of five samples at each collection site. § = Kruskal-Wallis (H). Diferent letters in columns indicate significant differences between genera and species of nematodes with multiple comparisons test (p ≤ 0.05).
The most harmful and widely distributed plant parasitic nematode in banana roots is Radopholus similis (Chabrier and Queneherve, 2003; Quénéhervé et al., 2011), which was found in four out of six of the study sites, though in small populations. This may be due to the origin and variety of banana plants in this area (Musa acuminata AA subgroup Pisang Mas). This statement is based on the findings that show that many diploid accessions, such as the studied plants, are less susceptible to R. similis (Quénéhervé et al., 2009). Also, the temperature rank in the study area (14-26 °C) is low for the optimal development of R. similis (24-32 °C) (Pinochet et al., 1995; Gowen et al., 2005) and can contribute to a higher presence of Helicotylenchus and Meloidogyne. In the production area of Chiapas, Mexico, R. similis has been reported to be the dominant nematode over Musa AAA from the Cavendish subgroup (Adriano-Anaya et al., 2008).
The population density of endoparasitic nematodes found in roots was relatively low (Table 2) compared to those reported in other studies conducted in the state of Chiapas, which have found densities of more than 10,000 individuals in 100 g of roots (Adriano-Anaya et al., 2008). The reason that few Criconema sp. nematodes were found could be that they are ectoparasitic nematodes whose life cycle takes place outside of the root (Yeates et al., 1993). The Helichotylenchus species were the most abundant in all the study sites and their population was significantly higher than the Radopholus similis population it was lower in the four locations where it was found. The population density of Meloidogyne spp. J2 was low in all locations except El Zapotal, where the highest total number of nematodes was found. This could be because El Zapotal is located at the lowest altitude over sea level and weather conditions there may be more favorable for nematode reproduction. However, it is necessary to conduct studies on various aspects related to population dynamics and its influence on production.
Symptoms observed in the banana plants from the municipalities of Atzalan and Tlapacoyan are caused by plant parasitic nematodes, which are reported for the first time in the main production area in the state of Veracruz. Genera of nematodes causing the greatest damage worldwide were recorded. The information from this study is instrumental to implement control measures and prevent the spread of these plant parasitic nematodes to other areas, since banana spreading takes places through vegetative propagation. It is necessary to conduct studies to quantify the economic damage caused by these nematodes to banana crops, including studies at the field level to test control strategies.
Acknowledgements
This study is part of the outputs of the project 000174936 funded by CONACYT and the Secretariat of Economy. We would like to thank Bertha Pérez Hernández for her advice on molecular techniques. Finally, thanks to the banana producers who allowed us to take samples from their plantations.
REFERENCES
Adriano-Anaya ML, Herrera-López D, Albores-Flores V, Salvador-Figueroa M y Velasco-Zebadua ME. 2008. Nematodos endorrizosféricos del banano (Musa AAA sub grupo Cavendish) clon "grande naine" en el Soconusco, Chiapas, México. Revista Mexicana de Fitopatología 26:147-152. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61226207 [ Links ]
Brentu CF, Speijer PR, Green KR, Hemeng BMS, De Waele D and Coyne DL. 2004. Micro-plot evaluation of the yield reduction potential of Pratylenchus coffeae, Helicotylenchus multicinctus and Meloidogyne javanica on plantain cv. Apantu-pa (Musa spp., AAB-group) in Ghana. Nematology. http://dx.doi.org/10.1163/1568541042360537 [ Links ]
Carneiro RMDG, Tigano MS, Randing O, Almeida MRA and Sarah JL. 2004. Identification and genetic diversity of Meloidogyne spp. (Tylenchida Meloidogynidae) on coffee from Brazil, Central America and Hawaii. Nematology 2:287-298. http://dx.doi.org/10.1163/1568541041217942 [ Links ]
Carneiro RMDG and Cofcewicz E. 2008. Taxonomy of coffee-parasitic root-knot nematodes, Meloidogyne spp. Pp: 87-122. In: Souza R. (ed.). Plant-parasitic nematodes of coffee. Springer. Netherlands. 340p. Disponible en línea: http://link.springer.com/chapter/10.1007%2F978-1-4020-8720-2_6 [ Links ]
Cofcewicz ET, Carneiro RMDG, Randig O, Chabrier C and Quénéhervé P. 2005. Diversity of Meloidogyne spp. on Musa in Martinique, Guadalupe, and French Guiana. Journal of Nematology 3:313-322. Disponible en línea: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620976/ [ Links ]
Chabrier C and Quénéhervé P. 2003. Control of the burrowing nematode (Radopholus similis Coob) on banana: impact of the banana field destruction method on the efficiency of the following fallow. Crop Protection. http://dx.doi.org/10.1016/S0261-2194(02)00121-7 [ Links ]
Chitamba J, Manjeru P, Chinheya CC, Mudada N and Handiseni M. 2013. Plant-parasitic nematodes associated with banana (Musa spp.) in Rusitu Valley, Zimbabwe. Nematropica 43:113-118. Disponible en línea: http://journals.fcla.edu/nematropica/article/view/82440 [ Links ]
Cid del Prado-Vera I, Tovar-Soto A y Hernández JA. 2001. Distribución de especies y razas de Meloidogyne en México. Revista Mexicana de Fitopatología 1:32-39. Disponible en línea: http://www.redalyc.org/articulo.oa?id=61219105 [ Links ]
Das SC, Balamohan TN, Poornima K, Velalazan R and Seenivasan N. 2014. Breeding and evaluation of Musa hybrids to the spiral nematode, Helicotylenchus multicinctus. Indian Journal of Genetics and Plant Breeding. http://dx.doi.org/10.5958/j.0975-6906.74.1.008 [ Links ]
Gowen SR, Quénéhervé P, and Fogain R. 2005. Nematode parasites of bananas and plantains. Pp: 611-643. In: Luc M, Sikora RA, and Bridge J. (eds.). Plant parasitic nematodes in subtropical and tropical agriculture. CAB International. UK. 917p. [ Links ]
Kamira M, Hauser S, van Asten P, Coyne D and Talwana HL. 2013. Plant parasitic nematodes associated with banana and plantain in eastern and western Democratic Republic of Congo. Nematropica 2:215-225. Disponible en línea: http://journals.fcla.edu/nematropica/article/view/82710 [ Links ]
Lima RS, Muniz MFS, Castro JMC, Oliveira ERL, Oliveira PG, Siqueira KMS, Machado ACZ and Costa JG. 2013. Frequencies and population densities of the major phytonematodes associated with banana in the state of Alagoas, Brazil. Nematropica 43:186-193. Disponible en línea: http://journals.fcla.edu/nematropica/article/view/82707 [ Links ]
López-Lima D, Sánchez-Nava P, Carrion G, Espinosa de los Monteros A and Villain L. 2015. Corky-root symptoms for coffee in central Veracruz are linked to the root-knot nematode Meloidogyne paranaensis, a new report for Mexico. European Journal of Plant Pathology. http://dx.doi.org/ 10.1007/s10658-014-0564-9 [ Links ]
Montes-Belmont R. 2000. Nematología vegetal en México. Segunda edición. Sociedad Mexicana de Fitopatología, A.C. México. 158p. [ Links ]
Orion D, Levy Y, Israeli Y and Fischer E. 1999. Scanning electron microscope observations on spiral nematode (Helicotylenchus multicinctus) infested banana roots. Nematrópica 29:179-183. Disponible en línea: http://journals.fcla.edu/nematropica/article/view/64247 [ Links ]
Orton-Williams KJ. 1973. Meloidogyne incognita. C.I.H. Descriptions of Plant-parasitic nematodes, Set 2 No. 18. [ Links ]
Orton-Williams KJ. 1975. Meloidogyne arenaria. C.I.H. Descriptions of Plant-parasitic nematodes, Set 5 No. 62. [ Links ]
Orton-Williams KJ and Siddiqi MR. 1973. Radopholus similis. C.I.H. Descriptions of Plant-parasitic nematodes, Set 2 No. 27. [ Links ]
Pinochet J, Fernandez C and Sarah JL. 1995. Influence of temperature on in vitro reproduction of Pratylenchus coffeae, P. goodeyi, and Radopholus similis. Fundamental and Applied Nematology 18:391-392. [ Links ]
Quénéhervé P, Valette C, Topart P, Tezenas-du Montcel H and Salmon F. 2009. Nematode resistance in bananas: screening results on some wild and cultivated accessions of Musa spp. Euphytica. http://dx.doi.org/10.1007/s10681-008-9773-7 [ Links ]
Quénéhervé P, Barrièreb V, Salmon F, Houdin F, Achard R, Gertrude JC, Marie-Luce S, Chabrier C, Duyck PF and Tixier P. 2011. Effect of banana crop mixtures on the plant-feeding nematode community. Applied Soil Ecology. http://dx.doi.org/10.1016/j.apsoil.2011.07.003 [ Links ]
Randig O, Bongiovanni M, Carneiro RMDG and Castagnone-Sereno P. 2002. Genetic diversity of root-knot nematodes from Brazil and development of SCAR markers specific for the coffee-damaging species. Genome. http://dx.doi.org/10.1139%2Fg02-054 [ Links ]
SIAP (Servicio de Información Agroalimentaria y Pesquera) (2014) Producción Nacional de plátano. Disponible en línea: http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-estado/ [ Links ]
Siddiqi MR. 1973. Helicotylenchus multicinctus. C.I.H. Descriptions of Plant-parasitic nematodes, Set 2 No. 23. [ Links ]
Siddiqi MR. 2000. Tylenchida: Parasites of Plants and Insects. CAB International. United Kingdom 848p. [ Links ]
Seinhorst JW. 1962. On the killing, fixation and transferring to glycerin of nematodes. Nematologica. http://dx.doi.org/10.1163/187529262X00981 [ Links ]
Subbotin SA, Inserra RN, Marais M, Mullin P, Powers TO, Roberts PA, Van Den Berg E, Yeates GW and Baldwin JG. 2011. Diversity and phylogenetic relationships within the spiral nematodes of Helicotylenchus Steiner, 1945 (Tylenchida: Hoplolaimidae) as inferred from analysis of the D2-D3 expansion segments of 285 rRNA gene sequences. Nematology. http://dx.doi.org/10.1163/138855410X520936 [ Links ]
van Bezooijen J. 2006. Methods and techniques for nematology. Wageningen University, The Netherlands. 112p. Disponible en línea: https://www.wageningenur.nl/en/show/Manual-Methods-and-Techniques-for-nematology-1.htm [ Links ]
Yeates GW, Bongers T, De Goede RGM, Freckman DW, and Georgieva SS. 1993. Feeding habits in soil nematode families and genera-an outline for soil ecologists. Journal of Nematology 25:315-331. Disponible en línea: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2619405/ [ Links ]
Zijlstra C, Donkers-Venne DTHM, Fargette M. 2000. Identification of Meloidogyne incognita , M. javanica and M. arenaria using sequence characterized amplified region (SCAR) based PCR assays. Nematology . http://dx.doi.org/10.1163/156854100750112798 [ Links ]
Received: August 03, 2015; Accepted: November 16, 2015











 text in
text in 


