Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.34 n.1 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1507-5
Phytopathological notes
Etiology of wilt and basal rot of Jatropha curcas in Arriaga, Chiapas, Mexico
1Universidad Autónoma de Chiapas; Villaflores, Chiapas, México
2Instituto Nacional de Investigaciones Forestales Agrícolas y Pesqueras-Campo Experimental Centro de Chiapas; Ocozocoautla, Chiapas, México
3El Colegio de la Frontera Sur, Unidad Tapachula, Tapachula, Chiapas, México
In the municipality of Arriaga, Chiapas, the death of physic nut (Jatropha curcas L.) 1 and 2-year old plantations has been observed since 2009, with symptoms of wilting and basal stem rot. The registered incidence has been up to 85 %; the virulence of the pathogen is very high because it causes the death of the plant in a period of 20 to 30 days. The objective of this study was to determine the causal agent of the physic nut death. Laboratory isolates were conducted from field samples of diseased plant material of stem and root tissue, in Petri dishes with B King (BK) and triphenyl tetrazolium chloride (TTC) medium, incubated at 28 °C for 48 h. The isolates obtained were purified and subjected to biochemical and hypersensitivity tests on tobacco (Nicotiana tabacum) cv. Xanthi. The identification was positive for three isolates of Ralstonia solanacearum. However, it was not possible to complete Koch's postulates; therefore, R. solanacearum is only consigned at association level.
Keywords: Physic nut; Ralstonia solanacearum; diagnosis; biochemical tests
En el municipio de Arriaga, Chiapas, México, a partir del 2009 existe la muerte de plantas de piñón (Jatropha curcas L.) de 1 y 2 años de edad, con síntomas de marchitez y pudrición basal del tallo. La incidencia registrada es hasta un 85 %; con una alta virulencia del patógeno ya que ocasiona la muerte de la planta en un lapso de 20 a 30 días. El objetivo del presente trabajo fue determinar el agente causal de la muerte de plantas de piñón. A partir de muestras de campo de tejido enfermo de la base del tallo y raíz, se realizaron a nivel de laboratorio, aislamientos en cajas Petri con medio B de King (BK) y cloruro de trifenil tetrazolio (TTC); las cajas se incubaron a 28 °C durante 48 h. Los aislamientos obtenidos fueron purificados y sometidos a pruebas bioquímicas y de hipersensibilidad en tabaco (Nicotiana tabacum) cv. Xanthi. La identificación fue positiva para tres cepas de Ralstonia solanacearum. Sin embargo, no fue posible completar los postulados de Koch por lo que se consigna a R. solanacearum nivel de asociación.
Palabras clave: piñón; Ralstonia solanacearum; diagnosis; pruebas bioquímicas
The physic nut (Jatropha curcas L.) is used as a living fence, due to its fast spreading, handling, and quick growth. It is studied worldwide as a bioenergy crop to obtain biodiesel, which helps reduce the emission of greenhouse gases and mitigate global warming (Zamarripa and Solís, 2013). In Arriaga, Chiapas, since 2009, commercial plantations have been observed to contain dead 1- and 2-year old physic nut plants, which presented symptoms of wilting and basal stem rot. The aim of this study was to determine the etiology of the wilting and basal stem rot in the physic nut plant in Arriaga, Chiapas.
Samples with symptoms of wilting and basal stem rot were gathered in two physic nut plantations located in Arriaga, Chiapas: 1) a 90 ha lot with physic nuts from India, planted from seeds in 2008 in Rancho San Jacinto, located at 16° 13' 18" latitude north and 94° 01' 07" longitude west, at an altitude of 50 masl; 2) 3 ha lot with physic nut from Chiapas, planted from seeds in 2010, property of the Autonomous University of Chiapas, located at 16° 11' 05" latitude north and 93° 55' 57" longitude west, at an altitude of 38 masl. The samples were processed and analyzed under laboratory conditions. Cross sections of 5 mm of diseased plant material were obtained, disinfected superficially with 1 % sodium hypchlorite, rinsed with sterile distilled water and planted in Petri dishes with B King. The dishes were incubated at 28 °C for 48 hours; the culture formed was transferred to a new culture medium, using an inoculation loop, using the Streak Plate Method. Different tests were carried out with pure cultures, including: the hypersensitivity reaction test, with the infiltration of a bacterial solution at 1.2x109 UFC/mL in tobacco (Nicotiana tabacum L. cv. Xanthi) leaves from 60-day old plants. Concentration was calculated using the McFarland scale (1907), a test of rotting in potato tubercula of the cv. Alpha, by inoculations of bacterial growth in incision in slices under aseptic conditions; auxiliary biochemical identification tests in a triphenyl tetrazolium chloride (TTC) culture media, Gram staining, KOH test, production of the enzyme cytochrome oxidase, production of the enzyme catalase with hydrogen peroxide (H2O2), production of the enzyme urease in a Christensen culture media, oxidative and/or fermentative metabolism of glucose in a Hugh and Leifson culture media (Denny and Hayward, 2001).
The plants gathered from commercial fields displayed symptoms of wilting on leaves, bacterial rot on the base of the stem and root. This corresponds with the description of symptoms reported by Hendroko et al. (2008), Ginting and Maryono (2009), and Hidayah and Yulianti (2009). Up to 85 % less plants aged 1 to 2 years died in a time lapse of 20 to 30 days after the beginning of the epidemic. The incidence of the disease fell noticeably when the crop reached its third year of age. From the samples gathered on the field, 16 strains were obtained, 11 of which were discarded using the test of hypersensitivity on tobacco and the biochemical tests applied on it. Strains 1 and 3B showed positive results on the hypersensitivity test on tobacco after 48 h (Figure 1), and strain P3 also showed positive results or variables after 72 h; in this sense, Ji et al. (2007) mentioned that R. solanacearum shows variability in the response to hypersensitivity. Finally, negative results were obtained for this test with strains P1 and P2.

Figure 1 Positive result after 48 h for the hypersensitivity test on tobacco (Nicotiana tabacum L.) cv. Xanthi, performed on the isolated strain 1 of physic nut.
In the potato tubercule rotting tests, results were positive for all five bacterial strains, which coincides with Rodríguez (2012), who mentioned that for isolated bacterias from organisms with rotting, pathogenicity can be valued with this test.
The five isolated bacterial strains from physic nut were positive in a TTC media, with the growth of colonies colored red with white edges, distinctive features of colonies infected with R. solanacearum (Figure 2). These results coincide with reports by Perea et al. (2011), who reported that the growth of R. solanacearum colonies in a TTC growth media are characterized by a red color with white edges. The Gram staining and biochemical tests for oxidase, catalase, urease, coincide with reports by Yabuuchi et al. (1995), Denny and Hayward (2001), Dhital (2001), EPPO (2004), Rahman et al. (2010), Perea et al. (2011), Marques et al. (2012) and Rodríguez (2012) (Table 1).
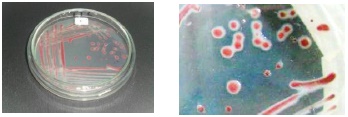
Figure 2 Growth in a TTC media of the P1 isolated strain of Jatropha curcas with symptoms of wilting and bacterial rotting; a red color is observable, surrounded by a white area, typical of R. solanacearum cultures.
Table 1 Biochemical and pathogenicity tests performed on five bacterial strains of Jatropha curcas.
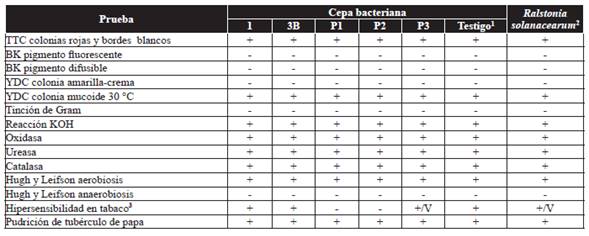
1Control = Isolated reference strain from tomato (Solanum lycopersicum L.) identified and provided by the Lab. Of Plant Disease, Dept. of Microbiology, Natinal School of Biological Science, Instituto Politécnico Nacional, Mexico City.
2Response of R. solanacearum, according to según Denny y Hayward (2001), Dhital (2001), EPPO (2004), Rahman et al. (2010), Perea et al. (2011), Marques et al. (2012), Rodríguez (2012).
3V = Vaiable.
The wilting and basal rotting of Jatropha curcas in Arriaga, Chiapas, Mexico are characterized by symptoms of flabbiness in petioles, wilting and loss of leaves, rotting in the root and base of the stem, production of adventitious roots and senescence 20 to 30 days after the first symptoms. Out of 16 isolated bacterial strains in physic nut plants that underwent the tobacco hypersensitivity test, three strains coincide with the characteristics for R. solanacearum. Although Koch's postulates were not fulfilled, this is the first report on the relation of Ralstonia solanacearum in wilting and basal rotting of the physic nut plant in Chiapas, Mexico.
Bibliografía
Denny TP and Hayward AC. 2001. Ralstonia. In: Schaad NW, Jones JB and Chun W (eds.). Laboratory guide for identification of plant pathogenic bacteria. Third Edition. American Phytopathological Society Press. St. Paul, MN, USA. Pp. 151-174. [ Links ]
Dhital SP, Thaveechai N and Shrestha SK. 2001. Characteristics of Ralstonia solanacearum strains of potato wilt disease from Nepal and Thailand. Nepal Agriculture Research Journal 5:42-47. http://www.nepjol.info/index.php/NARJ/article/download/4868/4034. [ Links ]
EPPO. 2004. EPPO Standards: Diagnostic protocols for regulated pests. European and Mediterranean Plant Protection Organization. EPPO Bulletin 34:155-157. http://archives.eppo.int/EPPOStandards/PM7_DIAGNOS/pm7-21(1).pdf [ Links ]
Ginting C and Maryono T. 2009. Physic nut (Jatropha curcas L.) diseases in Lampung province. Biotropia 16(1):45-54. http://journal.biotrop.org/index.php/biotropia/article/view/66/45 [ Links ]
Hendroko R, Karmawati E, Mahmud Z and Soekamto. 2008. The attack of disease occurred at ETF Jatropha seed garden. Cikarang, Indonesia. Presented in poster at Kuala Lumpur Jatropha Summit, Indonesia. http://www.slideshare.net/greenmile/jatropha-presentation (consulta, febrero 2014). [ Links ]
Hidayah N and Yulianti T. 2009. Development of bacterial wilt of physic nut (Jatropha curcas L.) in Muktiharjo Experimental Station. Proceedings of the Third National Workshop on Jatropha. Indonesia Tobacco and Fiber Crops Research Institute. Malang, East Java, Indonesia. Pp. 308-312. http://balittas.litbang.pertanian.go.id/eng/images/jarpag3/pen/nurul%20mp.pdf (consulta, febrero 2014). [ Links ]
Ji P, Allen C, Sánchez PA, Elphinstone JG, Jones JB and Momol MT. 2007. New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Disease 91:195-203. http://dx.doi.org/10.1094/PDIS-91-2-0195 [ Links ]
Marques E, Uesugi CH, Ferreira MASV and Rezende DV. 2012. Characterization of isolates of Ralstonia solanacearum biovar2, pathogenic to Eucalyptus "urograndis" hybrids. Tropical Plant Pathology 37(6):399-408. http://dx.doi.org/10.1590/S1982-56762012000600004 [ Links ]
McFarland J. 1907. The nephelometer: an instrument for estimating the numbers of bacteria in suspensions used for calculating the opsonic index and for vaccines. Journal of the American Medical Association 49(14):1176-1178. http://dx.doi.org10.1001/jama.1907.25320140022001f [ Links ]
Perea-S JM, García-E RS, Allende-M R, Carrillo-F JA, León-F J, Valdez-T B y López-S FSM. 2011. Identificación de razas y biovares de Ralstonia solanacearum L. aisladas de plantas de tomate. Revista Mexicana de Fitopatología 29(2):98-108. http://www.redalyc.org/articulo.oa?id=61222864002 [ Links ]
Rahman MF, Islam MR, Rahman T and Meah MB. 2010. Biochemical characterization of Ralstonia solanacerum causing bacterial wilt of brinjal in Bangladesh. Progressive Agriculture 21(1-2):9-19. http://dx.doi.org/10.3329/pa.v21i1-2.16744 [ Links ]
Rodríguez-M. ML. 2012. Manual para la identificación de bacterias fitopatógenas. 3ª edición. Universidad Autónoma Chapingo. Chapingo, México. 146 p. [ Links ]
Yabuuchi E, Kosako Y, Yano I, Hotta H and Nishiuchi Y. 1995. Transfer of two Burkholderia and an Alcaligenes species to Ralstonia gen. nov.: proposal of Ralstonia pickettii (Ralston, Palleroni and Doudoroff 1973) comb. nov., Ralstonia solanacearum (Smith 1896) comb. nov. & Ralstonia eutropha (Davis 1969) comb. nov. Microbiology and Immunology 39:897-904. http://dx.doi.org 10.1111/j.1348-0421.1995.tb03275.x [ Links ]
Zamarripa-Colmenero A y Solís-Bonilla JL. (eds.). 2013. Jatropha curcas L., alternativa bioenergética en México. Libro Científico No. 1. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Centro de Investigación Regional Pacífico Sur, Campo Experimental Rosario Izapa. Tapachula, Chiapas, México. 157 p. [ Links ]
Received: July 30, 2015; Accepted: November 16, 2015











 text in
text in 


