Meloidogyne incognita is one of the most important root-knot nematode in Mexican agriculture. In Yucatan affects several important crops such as habanero pepper (Capsicum chinense Jacq.), aloe (Aloe vera L.), and tomato (Solanum lycopersicum L.), among many others crops (Herrera-Parra et al., 2011). A number of studies have reported that the metabolic production of some fungi could be used as part of management strategy (Xalxo et al., 2013; Bhattacharjee and Dey 2014). These metabolites include caryospomycins (Dong et al., 2007), chitinases, glucanases, peroxidases, viridine, gliotoxin, Trichodermine (Szabo et al., 2013) among others.
However, there is highly necessary development more investigations on fungal potential in this topic (Hernández-Carlos and Gamboa-Angulo, 2011). In this sense, our natural products research group has focused on screening potential regional sources of eco-friendly compounds to be developed as natural nematicides to control this important nematode. As results, the Selenosporella sp. MR26 strain has shown to have good effectivity against M. incognita (Reyes-Estebanez et al., 2011). However, in this study the nematotoxic effect detected was low (2 %), when strains were grown in fermented rice.
Also, is well documented that microbial biosynthesis of metabolites are in dependence on the substrate and conditions of culture (Shinya et al., 2008; Regaieg et al., 2010). Then, to continue the screening of our fungal collections, nine active strains were choosen (Acremonium kiliense TA31, Aspergillus sp. 2XA5, Gliocladium sp. MR41, Selenosporella sp. MR26, Stagonospora sp. TA34, and four unidentified strains: TA13, 2TA6, 2TA7, and 2XA7). These strains were grown in Czapeck Dox, a defined liquid medium (Gamboa-Angulo et al., 2012; Reyes-Estebanez et al., 2011).
Therefore, the aim of this study was to test in vitro culture filtrates, and mycelia organic extracts (methanol and ethyl acetate) against second juvenile stage (J2) of M. incognita, and to measure the median effective concentration of the most active samples.
Materials and methods
Fungal material
Fungal strains (Table 1) from the culture collection of the biotechnology unit of the Scientific Research Center of Yucatán, C.A., were originally isolated from leaves and water samples collected in Tabasco, Veracruz, and Yucatan, Mexico (Reyes-Estébanez et al., 2011; Gamboa-Angulo et al., 2012).
Table 1 Effect of fungal culture filtrate (1 mL) and mycelium methanol extracts (0.3 mg mL-1) from micromycetes strains against J2 of Meloidogyne incognita.
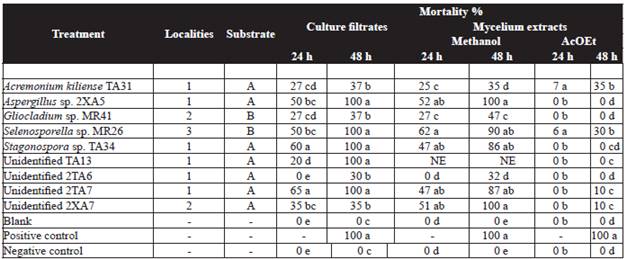
Means with the same letter(s) in the column are not significantly different at P = 0.05 according to Tukey's studentized range test. Positive control: Vydate (Oxamyl 1 mL L-1). Blank: Czapeck-Dox medium/ organic extract.
Negative control: water. Nt= no tested.
1: Yucatán 2:Veracruz 3:Tabasco. A:water B: leaves.
Culture conditions and metabolites fungal extraction
All strains were cultured in potato dextrose agar (PDA) media at room temperature (25 ± 2 °C, 16/8 hours light/darkness) for one week, to obtain a suspension of hyphal fragments-spore suspension (Reyes-Estebanez et al., 2011). One mL of the suspension was transferred to Czapek-Dox liquid medium (200 mL) contained in Roux bottles, with three replicates per isolate. These cultures were incubated for 2 weeks at room temperature, in stationary conditions. Then the culture y s were filtered through filter paper (Whatman(r) No. 42), to separate the mycelial mat and the culture filtrate. Mycelial mat was lyophilized (Labconco), ground to powder using a glass mortar, and the respective extract was obtained using ethyl acetate (3×, 100 mL for 24 h, each time), followed by methanol (3×, 100 mL for 24 h, each time) to obtain non-polar and polar compounds, respectively. Solvent was eliminated under reduced pressure until dryness to get organic crude extracts which were stored at 4 °C in the dark. Culture filtrates, ethyl acetate extracts (from mycelia and filtrate), as well as methanol extracts were tested for nematotoxic effects (Reyes-Estébanez et al., 2008).
Nematotoxic assay
The nematode inoculum was prepared as previously described (Cristóbal-Alejo et al., 2006). All fungal extracts (300 mg mL-1) were dissolved in 0.25 % tween 20, and culture filtrates (1 mL) were sterilized by filtration through 0.45 µm filter (Millipore) before use in bioassays (Cristóbal-Alejo et al., 2006; Candelero-de la Cruz et al., 2015). Initial assessments were conducted with freshly hatched J2 (10 each replicate) which were placed in suspension, and incubated at room temperature in special glass dishes for J2 mortality studies. Vydate (Oxamyl, 1 mL L-1 of water) was used as positive control and negative control included distilled water and a blank (Czapeck-Dox medium extracts). Each extract was replicated four times and maintained under laboratory conditions using a completely randomized design. After 24 and 48 h, under a stereoscopic microscopic, the J2 were touched with a needle and those that did not respond were classified as immobile. All J2 were counted and classified as mobile and immobile. After 72 h, death of J2 was confirmed by the transfer of immobile J2 to distilled water and further examination (24 h).
Dose-inhibitory response curves using a dilution series (0, 50, 100, 200, 300, 400 and 500 mg mL1) were prepared for three of the most active extract in culture filtrates and extracts (Aspergillus sp. 2XA5, Selenosporella sp. MR26, and unidentified 2XA7). In the case of culture filtrates, these were diluted to 50 and 25 % (v:v).
Results were reported as percentage of J2 M. incognita mortality. For the analyses of variance data were arcosin transformed [y = arcosin (sqrt (y/100))] and significant differences among treatments were detected by Tukey´s test (P=0.05) (Steel and Torrie, 1988). Effective concentrations (EC50 and EC95) were obtained by transforming to "Probit" and ten-base logarithms the calculated percent mortality of data from the second assay (Throne et al., 1995).
Results and discussion
Nematotoxic activity of culture filtrates and mycelium methanol extracts on M. incognita is summarized in Table 1. Ethyl acetate extracts did not show nematotoxic effect (<50 %). The results showed that the extracts from six strains (67 %) immobilize J2 M. incognita in at least one of their filtrate or mycelial extract tested. Five culture filtrates at undiluted concentration of Aspergillus sp. 2XA5, Selenosporella sp. MR26, Stagonospora sp. TA34, and two unidentified strains (TA13 and 2TA7), together two methanol extracts (Aspergillus sp. 2XA5 and unidentified 2XA7) displayed good effect (85-100 % mortality) on J2. Moreover, there was observed that Aspergillus sp. 2XA5, followed by Selenosporella sp. MR26 displayed the most significant nematotoxic effect, in both, their culture filtrate and methanol mycelia extracts.
The toxicity of the filtrates and methanol extract obtained from liquid culture medium from Selenosporella sp. MR26 was higher as compare to the one shown by extracts derived from solid medium (Reyes-Estebanez et al., 2011).
When the juveniles were exposed to three different concentrations of the Aspergillus sp. 2XA5, Selenosporella sp. MR26 and unidentified 2XA7 active filtrates (Figure 1), there was a concentration-dependent effect. The number of immobile J2 was higher at 50 % concentration and almost all nematodes were paralyzed with a mean value of 75.8 % mortality at 48 h. The highest toxicity was observed with all undiluted filtrates from the active isolates. Similar results were reported for culture filtrate from Nigrospora sp., cultivated on Czapeck medium, when tested against juveniles of M. incognita (Amin, 2013) The nematicidal action of culture filtrates can be attributed to the production of toxic metabolites or enzymes, as in F. solani, where the action was a result of fungal toxins and unused sugar and salt residues present in the culture filtrate (Jain et al., 2008; Regaieg et al., 2010). On the other hand, enzymes could play a key role in fungal infection processes (Segers et al., 1994). Extracellular hydrolytic enzymes such as lipases, chitinases and proteases are considered to be virulence determinants of entomopathogenic fungi and they are involved in complex processes leading to host cuticle penetration and cell digestion (Regaieg et al., 2010).
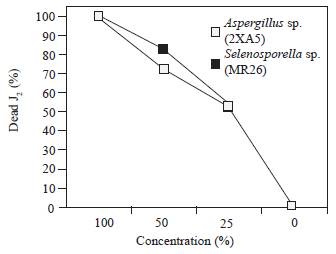
Figure 1 Nematotoxic effect of fungal culture filtrates at different dilutions (0, 25, 50, 100 % v/v) against J2 of Meloidogyne incognita at 48 h.
The results of nematotoxic assays from culture filtrates and mycelia methanol extracts against M. incognita showed to be effective with only five polar extracts (Table 1). The best effect was produced by Aspergillus sp. 2XA5 and the unidentified strain 2XA7 (100 % mortality), followed by Selenosporella sp. MR26 (90 % mortality) at 48 h of exposure. Other strains such as Stagonospora sp. TA34 and the unidentified strain 2TA7 were less active than those mentioned above with 47 % nematode mortality at 24 h and up to 80 % mortality at 48 h. This nematotoxic effect was particularly interesting since the fungal strains could be causing nematode immobility as a result of intracellular (endotoxins) and extracellular metabolites (exotoxins). The nematotoxic effect of the unidentified fungus 2XA7 only occurred in the methanol extract and not in filtrate, indicating that the toxic substance is not secreted by fungal hyphae (endotoxin).
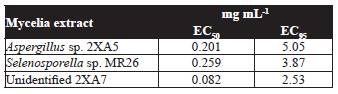
According to Table 1, mycelia methanol extracts with the highest nematotoxic activity obtained from Aspergillus sp. 2XA5, Selenosporella sp. MR26, and the unidentified strain 2XA7 were chosen for estimation of EC50 and EC95 (Table 2). The most potent fungal extracts was the unidentified strain 2XA7 with a median effective dose of 0.08 mg mL-1, followed by Aspergillus sp. 2XA5 and Selenosporella sp. MR26 (EC50 values of 0.20 and 0.26 mg mL-1, respectively). These values are good in comparison with other fungal compounds isolated from Paecilomyces lilacinus 6029 (LC values of 3.03 mg mL1) and Verticilllium chlamydosporum (500 mg L-1) (Khambay et al., 2000; Sharma et al., 2014). Furthermore, it was interesting to detect that Selenosporella sp. MR26 produces non polar nematotoxic metabolites when grown on solid fermented rice media (EC50 values of 0.91 mg mL-1) and polar in Czapeck-Dox liquid medium (Reyes-Estebanez et al., 2011). Actually, the isolation and identification of the metabolites of Selenosporella sp. MR26 are in progress.
Table 2 Effective concentrations (EC50 and EC95) of methanol extracts of those strains with the highest nematotoxic activity against J2 of Meloidogyne incognita at 48 h.
On other hand, Aspergillus is an extensively known genus with a highly prolific production of active metabolites, several species have shown nematotoxic properties such as A. awamori and A. niger against M. incognita, and A. quadrilineatus against M. javanica (Bath and Wani, 2012). From A. niger were brevianamide A, itaconitin, canadensic and mycophenolic acids, and A. quadrilineatus produces flavoskyrin, dehydrocanadensolide and α-Collatolic acid (Siddiqui and Futai, 2009; Akhtar and Panwar, 2013).
Conclusions
The experimental data obtained indicate that Aspergillus sp. 2XA5 and Selenosporella sp. MR26 are the most promissory fungi herein detected to control J2 of M. incognita. Both fungi produce great nematotoxic effect in their culture filtrates and methanol mycelia extracts which indicate polar nature of the metabolites responsible of the activity. Furthermore investigations are required to identify and characterize the molecules responsible for the activity of the potential candidates detected in this study. It will also be necessary to carry out greenhouse trials to tests these nematotoxic metabolites, and finally verify the environmental safety of their use.











 texto en
texto en 


