Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.34 no.1 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1506-7
Revision articles
Role of lignin in the plant-sedentary endoparasitic nematodes interaction
1Laboratorio de Fisiología y Fitopatología Molecular, Especialidad de Fitopatología, Instituto de Fitosanidad, Colegio de Posgraduados.
Sedentary endoparasitic nematodes introduce into their host, through its stylet, effector molecules that are previously synthesized in their esophageal glands, which induce reprogramming of gene expression in the host cells to promote changes in their metabolism, physiology and structure, necessary for the formation of the specialized feeding sites. The plant responds by expressing defense mechanisms such as the increase in the activity of key enzymes in the phenylpropanoid pathway, involved in the synthesis of secondary metabolites with antimicrobial properties and monomers that make up the lignin, which has a structural and defense roles in plants. The plant-nematode interaction established (compatible or incompatible), will depend on whether the changes induced in the lignin synthesis are favorable or not for the differentiation of the specialized feeding site. The aim of this review is to present information on the biosynthesis, composition and deposition of lignin, as well as the updated knowledge on its role as a potential physical barrier against the establishment of phytonematodes in incompatible interactions and their possible participation in the formation of specialized feeding sites in compatible interactions.
Key words: lignin, monolignols; cell wall; interactions; endoparasitic nematodes
Los nematodos endoparásitos sedentarios introducen en el hospedante, a través de su estilete, moléculas efectoras que son previamente sintetizadas en sus glándulas esofágicas, las cuales inducen una reprogramación de la expresión génica en las células del hospedante para provocar modificaciones en el metabolismo, fisiología y estructura celular del hospedante, necesarios para la formación de sitios especializados de alimentación. La planta responde expresando mecanismos de defensa como el incremento de la actividad de enzimas clave de la ruta de los fenilpropanoides, mediante la cual se sintetizan metabolitos secundarios con propiedades antimicrobianas y monómeros que conforman la lignina, que tienen un papel estructural y de defensa en las plantas. Dependiendo de los cambios que se induzcan en su síntesis será el tipo de interacción planta-nematodo que se establezca (compatible o incompatible), en función de si los cambios son o no favorables para la diferenciación del sitio especializado de alimentación. Esta revisión tiene como objetivo presentar información sobre la biosíntesis, composición y deposición de lignina, así como el conocimiento que a la fecha se tiene, en su función como barrera física potencial contra el establecimiento de nematodos fitopatógenos en interacciones incompatibles, y su posible participación durante el proceso de formación de los sitios especializados de alimentación en las compatibles.
Palabras clave: lignina; monolignoles; pared celular; interacciones; nematodos endoparásitos
Lignin is a complex three-dimensional polymer, whose aromatic subunits are synthesized through the phenylpropanoid pathway (Dixon et al., 2002). It is also a basic component that reinforces and provides rigidity to plant tissues (Dixon et al., 2002; Weng and Chapple, 2010) and is deposited abundantly in specific plant cells such as sclereids, tracheids, elements of the vessels, and xylem and phloem fibers (Vance et al., 1980; Dixon et al., 2002). Lignin is also considered an important defense mechanism; its biosynthesis and deposition in cell walls increases when plants undergo biotic or abiotic stress (Weng and Chapple, 2010). On its own, lignin is an initial physical barrier aginst the entry of pathgens in the host and, in some cases, it limits their growth or confines them (Wuyts et al., 2006). Deposited on cell walls, it increases its resistance to degradation by enzymes (Wuyts et al., 2006; Menden et al., 2007), it limits the diffusion of toxins secreted by the pathogen and of nutrients from the host to the pathogen, and is also a source for the production of toxic precursors and free radicals (Nicholson and Hammerschmit, 1992).
In plant-nematode interactions, the stylets of nematodes participate in the penetration of the cell walls of its host, they help ingest the cell content and inject the effectors produced in their esophageal glands, to induce the modification of the host cells to form feeding cells. In the case of root-knot nematodes, they are known as giant cells, and are basically a product of repeated nuclear divisions without cytokinesis, whereas those induced by cyst nematodes are known as syncytia, and are a result of the fusion between protoplasts and the gradual breakup of the walls of cells that form them; both especilize feeding sites (SFS) make up a highly metabolic and multinucleated structure which will be the nematode's food source, and it will provide the nutrients required for its growth and development (Hussey, 1989; Ithal et al., 2007; Gheysen and Mitchum, 2011; Mitchum et al., 2013). An important modification that takes place in the host cells is the alteration in the lignification of cell walls; whether the nematode will stay and complete its life cycle will depend on the successful formation of the SFS. This review describes the biosynthesis, structure, and composition of lignin and the knowledge there is to this day on its function in the plant-nematode interaction, both compatible and incompatible.
Biosynthesis and Composition of Lignin
Lignin is a basic component of the cell wall (CW) in plants; the CW is a dynamic, extracellular complex that is formed by lignin, but also by cellulose, hemicellulose, pectin, proteins, cutin, suberin, mineral salts, and others (Bonawitz and Chapple, 2010).
Lignin biosynthesis implies the participation of two metabolic pathways, starting with the shikimate pathway for the production of L-phenylalanine and L-tyrosine, and the phenylpropanoid pathway from L-phenylalanine to the final biosynthesis of the cinnamyl alcohols (Figure 1.) (Boerjan and Baucher, 2003); in the latter pathway, not only lignin is synthesized; also, other phenolic compounds, such as (e)-stilbenes, coumarins, flavonoids and some phytoalexins (Dixon et al., 2002).
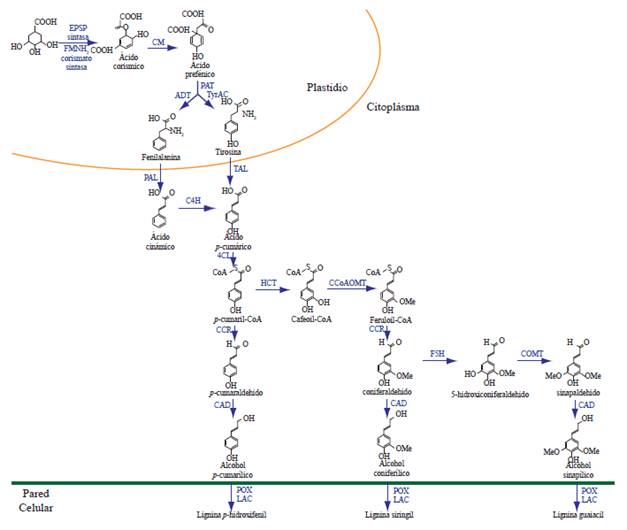
Figure 1 Pathways of shikimate (plastid) and phenylpropanoid (cytoplasm) for the biosynthesis of lignin. EPSP synthase: acid 5-enolpyruvylshikimate-3-P synthase; FMNH2 chorismate synthase: flavin mononucleotice chorismate mutase; CM: chorismate mutase; PAT: prephenate aminotransferase; ADT: Arogenate dehydratase; TyrAC: arogenate dehydrogenase; PAL: phenylalanine ammonia lyasa; TAL: tyrosine ammonia lyase; C4H: cinnamate-4-hydroxylase; 4CL: 4-coumarate:CoA ligase; HCT: hydroxycinnamoyl transferase; CCoAOMT: caffeoyl CoA-O-methyltransferase; CCR: cinnamoyl-CoA reductase; F5H: ferulate 5-hydroxylase; COMT: caffeic acid O-methyltransferase; CAD: cinnamyl alcohol-dehydrogenase; POX: peroxidases; LAC: lacasses. Modified from Liu (2012).
The synthesis of lignin monomers (monolignols) begins with the de-amination of the phenylalanine to form cinnamic acid, and later a series of hydroxylations and subsequent O-methylation modify the aromatic rings of the cinnamic acid, reducing its lateral chain from an acid to an alcohol. This results in the production of monolignols: paracoumaryl alcohol (4-hidroxycinnamyl), conferyl alcohol (3-methoxy 4-hidroxycinnamyl), and sinapyl alcohol (3,5-dimethoxy 4-hidroxycinnamyl); these differ in their degrees of methoxylation, coniferyl alcohol displays a methoxyl group (-OCH3) in the position 3 of the aromatic ring, sinapyl has two methoxyls in its positions 3 and 5, and paracoumaryl lacks substitutes in its aromatic ring. After their incorporation into the lignin polymer, these monomers are referred as p-hidroxyphenyl (H), guaiacol (G), and syringyl (S) units respectively, and their dispositions and abundance determine the physical properties of the CW (Rastogi and Dwivedi, 2007; Vanholme et al., 2008; Bonawitz and Chapple, 2010; Vanholme et al., 2010). In general, monomers G and S are the main components of lignin in dicotyledon angiosperms, whereas in gymnosperms, lignin consists mostly of G monomers with low levels of H units, and grasses contain more H units than dicotyledons (Bonawitz and Chapple, 2010). Although lignin is generally compose of H, G, and S units, in transgenic plants with alterations of the genes that codify for both O-methyltransferases: CCoAOMT and COMT; atypical lignin units have also been identified, such as catechol (C) and 5-hydroxy-guaiacyl (5H) (Marita et al., 2003; Vanholme et al., 2010; Weng and Chapple, 2010).
The biosynthesis of monolignols requires the participation of 10 enzymes: phenylalanine amonnia lyase (PAL), three cytochrome P450-monoxygenases (cinnamate-4-hydroxilase, p-coumarate-3-hydroxylase and ferulate-5-hydroxylase, C4H, C3H, and F5H, respectively), two methyltransferases (caffeoyl-CoA-O-methyltransferase and caffeic acid caffeate-O-methyltransferase, CCoAOMT and COMT, respectively), and two oxidoreductases (cinnamoyl-CoA-reductase and cinnamyl-alcohol dehydrogenase, CCR and CAD, respectively), as well as the enzymes 4-coumarate CoA ligase (4CL) and hydroxicinnamoyl-CoA shikimate (HCT), which are required for the synthesis of the different intermediaries that act as substrates in later reactions (Bonawitz and Chapple, 2010).
Lingin monomers are bonded covalently to henicelluloses and provide the plant's CW with strength and rigidity and gives the vascular system with the hydrofobicity it needs to carry water and solutes (Vanholme et al., 2008); lignin can be classified as condensed and non-condensed, based on the type of bond established between the monolignols and between the former and latter components of the CW (Table 1). Also involved in the composition of the CW are p-hydroxycinnamic acids (mainly p-coumaric and ferulic acids) which contribute to the linkage of lignin with the hemicelulose by ester-ester bonds; ferulic acid is responsible for the bridges between lignin and polysaccharides, whereas bonds between monomers give way to the formation of a new three-dimensional structure (Sun et al., 2002).
Table 1 Characteristics of condensed and non-condensed lignin.

C-C carbon-carbon bonds; H: p-hydroxyphenyl; G: guaiacol; S: syrinhgyl.
Both the lignification of the CW and the incorporation of each monomer are regulated in space and time and vary with the plant species, age, and tissue, and also depending on whether the cell walls are primary or secondary. In early stages of lignification, the coniferyl alcohol is coplymerized with small amounts of p-coumaryl in the primary wall to form lignins G and H and during the growth of the secondary CW the amount of sinapyl alcohol is copolymerized with the coniferyl alcohol to form lignin S and G (Grabber, 2005).
The monolignols precursors to lignina are synthesized in the endoplasmic reticulum of the cytosol and are later transported to the cell wall, where they are finally deposited (Santiago et al., 2013). Given its relative toxicity for the cell, it has been suggested that they are exported in the form of monolignols-glucosides and once in the CW, they are converted by the action of peroxidases and/or laccases, to the units H, G, or S. The deposition of each unit in the plant tissue is controlled spacially and temporarily; first, the H units are deposited, followed by G, and finally, S (Dixon et al., 2002; Bonawitz and Chapple, 2010; Santiago et al., 2013). The composition and characteristics of lignin are determined by the abundance of each one of the monolignols and vary considerably bewteen taxons, cell types, and even between layers of the cell walls; environmental factors and the growth of the plant itself also contribute (Boerjan and Baucher, 2003; Bonawitz and Chapple, 2010; Vanholme et al., 2010; Weng and Chapple, 2010).
Lignification is a highly coordinated process, and is regulated by a set of harmonized metabolic events; so far the action of 10 enzymes have been identified in the monolignols synthesis process (Sattler and Funnell-Harris, 2013). In the different species of plants, the synthesis of most of these enzymes is directed by the action of different genes and proteins present in multiple isoforms that vary in their kinetic properties and their distribution in the plant; however, the large amount of substrates, as well as the extense family of genes has made it difficult to identify and characterize the isoforms that are specifically involved in the lignification process (Bonawitz and Chapple, 2010).
PAL participates in the syntheses of different compounds of the primary and secondary metabolism, it catalizes the non-oxidative de-amination of L-phenylalanine to trans-cinnamic acid and is involved in the biosynthesis of salicylic acid (SA) and other phenolic compounds such as chlorogenic acid and the phytoalexins, flavonoids with antimicrobial properties, all involved in the defense of plants (Huang et al., 2010). Consequently, alterations in the expression of the gene PAL has a crucial impact during plant-pathogen interactions (Sattler and Funnell-Harris, 2013), since it participates in the formation of enzymatic complexes, known as metabolons or metabolic compartments, responsible for the metabolic channeling for the synthesis of the diverse phenolic compounds (Rasmussen and Dixon, 1999). The enzyme synthesis is induced by different types of biotic and abiotic stress (Dixon and Paiva, 1995; MacDonald and D'Cunha, 2007).
The enzyme C4H catalyzes the hydroxylation of the cinnamate to produce 4-Coumarate, also known as paracoumarate (Fraser and Chapple, 2011), which along with enzymes F5H and C3H are mono-oxygenases dependant on cytochrome P450 that are involved in the biosynthesis of monolignols (Ehlting et al., 2006; Boudet, 2007).
Enzyme 4CL catalyzes the formation of ATP dependant on the CoA thioester 4-coumaroyl CoA (p-coumaroyl CoA). This enzyme not only is the point of entry for the biosynthesis of phenylpropanoids compounds, but also for other types of secondary metabolites such as proantocianidins, tannins, flavonoids, isoflavonoids, and phytoalexins (Allina et al., 1998; Ehlting et al., 1999; Ehlting et al., 2001; Fraser and Chapple, 2011). Like PAL and C4H, 4CL exists as a family of multigenes, and although its resulting isoforms have not been fully characterized, they seem to have different functions in the secondary metabolism of plants; since its acivity in different substrates and space and time expression patterns suggest different physiological functions (Ehlting et al., 1999; Ehlting et al., 2001; Raes et al., 2003; Costa et al., 2005). For example, in Arabidopsis isoforms At4CL1 and At4CL2 seem to be involved in lignification, whereas At4CL3 acts in the biosynthesis of flavonoids (Ehlting et al., 1999) and At4CL4 probably acts in the activation of sinapate (Hamberger and Hahlbrock, 2004). During the last stage of the biosynthesis of monolignols in gymnosperms, aldehydes paracoumaryl, coniferyl, and sinapyl aldehydes are transformed into their corresponding alcohol by the enzyme CAD (Fraser and Chapple, 2011), while in angiosperms SAD (sinapyl alcohol dehydrogenase) is required for the biosynthesis of S units (Li et al., 2001).
Modifications of the CW during the formation of the SFS induced by Sedentary Endoparasitic Nematodes
Although in early stages the cells that give way to the formation of giant cells and the syncytia undergo similar changes, throughout the period of formation of SEAs the progressive molecular dialog between the nematode and its host presents distinctive features, depending on whether it is a nematode that induces the formation of giant cells or syncytia; this in turn produces structural differences between both types of SFS. Changes in the CW include modifications in the thickness and extension of the CW, as well as the formation of invaginations in areas adjacent to the xylem vessesl, both in giant cells and in syncytia.
In the formation of giant cells, the initial expansion zones where the thickening of the CW begins, are the first to form; these changes then extend to cover large areas of it (Rodiuc et al., 2014). The mechanisms used to regulate the deposition of new material to the CW and its thickening vary, and as the giant cells form, highly reticulated regions are created from these expansion zones that resemble CW labyrinths called "invaginations", through which the transportation of solutes into and out of the giant cell intensifies (Vieira et al., 2012; Rodiuc et al., 2014). Such invaginations develop throughout the process of maturation of the nematode and degenerate once it reaches its maturity and completes its life cycle (Rodiuc et al., 2014).
There are reports that reveal the importance of the plasmodesmata during the formation of the SFS. Plasmodesmata are membrane canals located in the cell walls, which provide cytoplasmic continuity between cells, creating a network of intercellular exchange. In a natural way, these plasmodesmata are found dispersed in the CW, while in the case of giant cells, a large amount of them is formed between the walls of giant cells and on the walls that surround them (Hofmann et al., 2010). This suggests the existence of a massive solute transportation system via symplast (Hofmann et al., 2010; Vieira et al., 2012). In addition, the flow of nutrients to and from the giant cells can also be mediated by specialized transport proteins located in the membrane (Rodiuc et al., 2014).
From the beginning of the formation of the syncytium, the dissolution of cell walls, alterations in their configuration, and increases in their syntheses are evident. These changes are necessary for both the formation of invaginations in areas near the xylem and for the thickening of the CW that surrounds the syncytium (Golinowski et al., 1996; Rodiuc et al., 2014); since although the cell walls that make up the syncytium break, the cell walls that confine it extend and thicken to resist the increase in pressure created inside the SFS (Golinowski et al., 1996). In addition, the deposition of new material obstructs the existing plasmodesmata and some neighboring cells divide and fuse and others differentiate in the new xylem (vessels) and phloem (sieve tube elements) tissues (Hoth et al., 2008; Rodiuc et al., 2014). As in giant cells, during the conformation of the syncytium, CW invaginations takes place near the xylem and phloem, which are elongated, branched, and form sophisticated reticulations that expand apically, making the basal parts of the invaginations fuse and wide CW thicknesses form. However, these invaginations are only evident 5 to 7 days after the infection occurs, once the process of SEA formation has advanced (Golinowski et al., 1996). It seems that in the syncytia the formation of invaginations in the CW is a secondary reaction, unrelated to the differenciation of the syncytium and could be caused by an increase in the flow of solutes to the SFS (Golinowski et al., 1996; Rodiuc et al., 2014).
The CW of immature syncytia thickens in a uniform manner, except in sections of the wall that are in direct contact with the sieve tubes, the walls of which remain thin until the neighboring walls thicken (Grundler et al., 1998). Plasmodesmata are important points of initial degradation of cell walls, and are therefore essential for the formation and expansion of the syncytium (Hofmann et al., 2010); in early stages of the syncytium formation, due to the temporary deposition on callose, nutrients are transported from the phloem apoplastically through transmembrane transporters (Hofmann and Grundler, 2006; Rodiuc et al., 2014); between 4 and 7 days after innoculation (dai),the amount of plasmodesmata increases and the deposition of callose decreases (Hofmann and Grundler, 2006), and in later stages, after 10 dai, the syncytia connect through the plasmodesmata, making the transfer of nutrients possible (Hofmann and Grundler, 2006; Hofmann et al., 2010).
The modifications that take place during the formation of the SFS could be the result of coordinated alterations induced by the nematode through the manipulation of the expression of host genes that codify for proteins such as extensins (EXT), expansins (α- y β expansinas), pectin acetylesterases (PAE), pectate lyases (PEL), and endoglucanases (endo-β-1,4-glucanasas), which participate in the modification of cell walls in giant cells, whereas in syncytia it is attributed to the participation of genes that codify for expansins (α- and β expansins), endoglucanases (endo-β-1,4-glucanasas), EXT, polygalacturonases (PG) y pectin acetilasas (PE) (Rodiuc et al., 2014).
Modifications in the synthesis and accumulation of lignin in the SFS
SFS are the only food source for sedentary endoparasitic nematodes and are essential for their growth and reproduction, which is why nematodes, through the induction of extensive changes in the gene expression, must include complex changes in the morphology, metabolism, and physiology of host cells, for their formation. SFS are found in the vascular cylinder, ensuring the necessary contact with the xylem and phloem to provide the SFS with nutrients, therefore alterations in the morphology, thickness, and composition of the CW are a necessary requirement for the formation of the SFS, and therefore, for the successful establishment of sedentary endoparasitic nematodes.
The effectors, synthesized in the oesophageal glands and secreted through the stylet of nematodes, induce the de-differentiation and re- differentiation of the root cells in SFS , although the identification of these secretions is limited (Mitchum et al., 2013). It is known that of the three oesophageal glands, the two subventrals have a greater activity during the invasion of the root and the migration of the nematodes in early stages, whereas the dorsal gland increases its activity during the formation and manintenance of the SFS, that is, in the nematode´s sedentary stage (Davis et al., 2008; Mitchum et al., 2013).
Nematodes such as Rotylenchulus spp., Tylenchulus spp., Nacobbus spp., and Xiphinema spp. Also induce the formation of SFS in the roots of hosts, although they have been studied little in comparison to those induced by the root-knot nematodes Meloidogyne spp. (giant cells) and those that form cysts (Globodera spp. and Heterodera spp., syncytiums). Both types of SFS share some structural characteristics, yet their ontogeny is different (Jones and Northcote, 1972; Rodiuc et al., 2014). Root-knot nematodes induce the formation of giant cells when the J2 are hosted in the area of differentiation of the vascular cylinder and each individual induces the differentiation of five to seven parenchymal cells in giant multinuclear cells (Abad et al., 2009). The syncytium, in turn, is formed when the J2 are hosted near the vascular cylinder and from only one cell begins the progressive thickening or narrowing of the plasmodesmata, until the cell from which the SFS formation began fuses with neighboring cells by the CW dissolution to form a large multinuclear syncytium (Turner and Rowe, 2006). In both feeding sites there is an increase in metabolic activity and cytoplasmic density, numerous small vacuoles, proliferation of organelles, particularly of the Golgi apparatus, mitochondria, plastids, ribosomes, and endoplasmic reticula (Rodiuc et al., 2014).
Nematode infection promots the synthesis of phenylpropanoids compounds (Edens et al., 1995; Balbridge et al., 1998). The induction of lignin biosynthesis occurs as a response to biotic and abiotic stress and is considered an important defense mechanism during the plant-pathogen interaction. Although it is true that this induction will occur in every plant-nematode interaction, it is also true that the regulation of its synthesis will vary, depending on whether the interaction is compatible or incompatible. In order for the restructuring of host cells to take place during the formation of the SFS, changes in the expression in a large number of genes are crucial. In this regard, Jammes et al. (2005) carried out a global analysis of the A. thaliana transcriptome during its compatible interaction with M. incognita and found that out of 22089 genes monitored, 15% displayed a differential expression during the development of giant cells; the expression of genes involved in the regulation of the cell cycle, DNA processing, protein synthesis and energy presented high levels of expression, as well as the expression of genes related to the metabolism of the cell wall that codify for pectate lyases, expansins, glycoside hyrdolases, xyloglucan endotransglycosylases and one glucose oxidase; in contrast, two genes related to defense, patatin and a protein similar to germin, turned out to be the most strongly repressed; however, they do not report the monitoring of genes specifically involved in the biosynthesis of lignin.
Ithal et al. (2007) reported that in soya plants innoculated with the nematode Heterodera glycines the activity of genes implied in the pathway of the phenylpropanoids, favoring the synthesis of a wide variety of secondary metabolites in plants, such as flavonoids and anthocyanins, and lignin and suberin, which make up the cell walls. The accumulation of phytoalexins, the deposition of lignin and the accumulation of phenolic compounds are characteristic of the defense response in plants; however, the functions of these secondary metabolites in the plant-nematode interactions are not entirely clear, since although its overexpression could be a part of the response of the plant to the infection by the nematodes in an incompatible interaction, these components could play another part in a compatible interaction (Ithal et al., 2007).
During the development of syncytia, the extensive thickening of its cell walls takes place, and although the composition of the new material deposited has not been characterized, histological studies show that the center of the external cortical walls, as well as the center of some fragments of cell wall inside the syncytia, become dyed with tolonium chloride, indicating the presence of lignin and some polyphenoles (Jones and Northcote, 1972). In this way, the increase in the synthesis and accumulation of lignin, not only becomes part of the plant's defense against infection by nematodes, but can also contribute to the formation of the cell wall de novo, to help protect and strengthen the syncytium (Ithal et al., 2007). The transcription of POX genes that codify for peroxidases has been reported to increase during the formation of syncytia. Peroxidases not only eliminate the oxygen-reactive species produced typically as a plant's early defense response against a wide variety of pathogens (Marrs, 1996; Blokhina et al., 2003), but they also seem to be involved in strengthening the cell wall of the syncytium by the crossing of cell wall polymers (Schopfer, 1996; Darley et al., 2001), or by its contribution to polymerization of extensins and to the crossing of polysaccharides by the dimerization of phenols (Darley et al., 2001).
The fact that most of the genes that codify for enzymes involved in the biosynthesis of lignin (PAL, C4H, C3H, F5H, CCoAOMT, COMT, CCR, and CAD) have been overexpressed in soybean during the formation of syncytium at 2, 5, and 10 days after infection by H. glycines, indicates that these genes also have an important function in the formation of SFS (Ithal et al., 2007). In Arabidopsis thaliana plants that overexpressed the C4H (early enzyme in the pathway of the phenylpropanoids) and F5H (enzyme that catalyzes the irreversible hydroxylation of G precursors toward the biosynthesis of S units) enzymes, in which the production of G units was inhibited and the amount of S units increased by 50%, as compared to the wild plants, the nematode reproduction was significantly reduced (Wuyts et al., 2006). The increase of S lignin in the vascular bundles might had inhibited the flow of nutrients towards the giant cells or impeded nematode feeding (Wuyts et al., 2006). Similarly when the content of S units was reduced by 10% in tobacco plants in which the expression of the enzyme isoflavona O-metiltransferasa (OMT) was blocked, M. incognita completed its life cycle in a shorter time and there was a higher number of juveniles as compared to the plants where OMT was not bocked (Wuyts et al., 2006). In contrast to the above, Quentin et al. (2009) found that in A. thaliana plants in which the levels of S lignin fell due to the blockage of activity in enzyme COMT, the growth of the nematode and its life cycle were similar to that observed in control plants in which the enzyme was not blocked. However, A. thaliana is, by nature, susceptible to M. incognita, and therefore in this case the differences in the content of lignin monomers (G or S) in the plant tissues did not influence the infection and life cycle of the nematode in A. thaliana, although one cannot exclude the possibility that in other plants, changes in the activity of enzyme COMT can determine the type of interaction (compatible or incompatible) (Pegard et al., 2005).
Based on investigations cited, it is possible to infer that it is very likely that the type and predominance of the monolignols that form the lignin, specifically the type of monomeric unit (S, G, or H) deposited, can influence the infection and reproduction of sedentary endoparasitic nematodes during its interaction with its host, since these nematodes establish their SFS inside the vascular cylinder, where there is an abundant deposition of polymers for the formation of secondary walls and a high lignification of roots.
On the other hand Wuyts et al. (2007) point out that it is possible for the pathway of phenylpropanoids to be redirected towards the synthesis of compounds related to resistance. In this way, the hydroxycinnamic acids covalently bonded to the polysaccharides of the CW constitute a possible second physical (and chemical) barrier to the nutrition and migration of the nematodes in the cortex of the roots. Given the physiological importance of the products in the pathway of the phenylpropanoids in the plant-sedentary endoparasitic nematode, the pathway seems to be directed, in the new CW synthesis, towards the formation of the monomers that form a type of lignin that favors the formation of functional SFS. As opposed to what occurs in incompatible ones, the pathway is directed towards the biosynthesis of lignin, which is a barrier for physical defense and secondary metabolites with antimicrobial properties.
Conclusions
The changes that take place in plant cells as a result of the reprogramming of the genetic expression induced by the nematode in the root of its host are perfectly coordinated and synchronized. Metabolic, physiological, and structural alterations also include the modification of the components of the plant's CW. Lignin is more frequently conceived as a potential physical barrier against pathogens. In the plant-sedentary endoparasitic nematode interaction, its biosynthesis, deposition, and composition are a part of the modifications involved in the restructuring of the cell walls of the host's roots. This process is crucial for the formation of the specialized functional nutrition site, which will allow the nematode to complete its life cycle. In the particular case of lignin, the nematode modifies the abundance and disposition of the monolignols that compose it, and depending on the dialog that takes place between plant and nematode (compatible or incompatible interaction) the lignin will contribute to the creation of a favorable environment for the successful establishment of the nematode or an environment that hinders its growth and life cycle completion. The potential of nematodes is well-known for modifying important metabolical pathways during its interaction with its host, such as the pathway of phenylpropanoids. However, given the complexity of the pathway, due, on one hand, to the existence of multiple isoforms of the genes responsible for codifying the enzymes involved, and on the other hand, to the abundant variation of metabolites produced through it, it has become difficult to elucidate the behavior and function of the genes implied in the pathway during the plant-nematode interaction. The functional characterization of these genes and the explanation of the part they play in the biosynthesis and deposition of monolignols is a challenge that has begun to be tackled using techniques such as infrared spectroscopy, separation by gas chromatography (GC) and identification by mass spectrometry (MS). Likewise, molecular techniques such as hybridation with microarrangements, which helps compare the genic expression of a set of genes in different conditions, and the availability of new sequencing equipment can contribute to the identification and characterizatio of the isoforms implied in the biosynthesis of monolignols. In this way, it will be possible to know more about the role played by lignin in the plant-nematode interactions, which could provide elements for the design of new ways to control these plant pathogens.
Acknowledgements
We would like to thank the Consejo Nacional de Ciencia y Tecnología (CONACYT) for the scholarship granted to Erika Lagunes Fortiz, as well as the Fideicomiso Revocable de Administración e Inversión No. 167304 para el Establecimiento y Operación de los Fondos para la Investigación Científica y Desarrollo Tecnológico del Centro Público Colegio de Postgraduados.
REFERENCES
Abad P, Castagnone-Sereno P, Rosso MN, de Almeida EJ and Favery B. 2009. Invasion, feeding and development. In: Perry RN, Moens M and Starr JL. 2009. Root-knot nematodes. CAB International, Wallingford, UK. pp:163-181. [ Links ]
Allina SM, Pri-Hadash A, Theilmann DA, Ellis BE and Douglas CJ. 1998. 4-Coumarate: Coenzyme A ligase in hybrid poplar. Properties of native enzymes, cDNA cloning, and analysis of recombinant enzymes. Plant Physiology. http://dx.doi.org/10.1104/pp.116.2.743 [ Links ]
Balbridge GD, O'Neill NR and Samac DA. 1998. Alfalfa (Medicago sativa L.) resistance to root-lesion nematode, Pratylenchus penetrans: defense-response gene mRNA and isoflavonoid phytoalexin levels in roots. Plant Molecular Biology. http://dx.doi.org/10.1023/A:1006182908528 [ Links ]
Blokhina O, Virolainen E and Fagerstedt KV. 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany. http://dx.doi.org/10.1093/aob/mcf118 [ Links ]
Boerjan W and Baucher MR. 2003. Lignin Biosynthesis. The Annual Review of Plant Biology. http://dx.doi.org/10.3114/sim.2007.58.01 [ Links ]
Bonawitz ND and Chapple C. 2010. The genetics of lignin biosynthesis: connecting genotype to phenotype. Annual Review of Genetic. http://dx.doi.org/10.1146/annurev-genet-102209-163508 [ Links ]
Boudet AM. 2007. Evolution and current status of research in phenolic compounds. Phytochemistry. http://dx.doi.org/10.1016/j.phytochem.2007.06.012 [ Links ]
Costa MA, Bedgar DL, Moinuddin SG, Kim KW, Cardenas CL, Cochrane FC, Shockey JM, Helms GL, Amakura Y, Takahashi H, Milhollan JK, Davin LB, Browse J and Lewis NG. 2005. Characterization in vitro and in vivo of the putative multigene 4-coumarate:CoA ligase network in Arabidopsis: Syringyl lignin and sinapate/sinapyl alcohol derivative formation. Phytochemistry. http://dx.doi.org/10.1016/j.phytochem.2005.06.022 [ Links ]
Darley CP, Forrester AM and McQueen-Mason SJ. 2001. The molecular basis of plant cell wall extension. Plant Molecular Biology. http://dx.doi.org/10.1023/A:1010687600670 [ Links ]
Davis EL, Hussey RS, Mitchum MG and Baum TJ. 2008. Parasitism proteins in nematode plant interactions. Current Opinion in Plant Biology. http://dx.doi.org/0.1016/j.pbi.2008.04.003 [ Links ]
Dixon RA and Paiva N. 1995. Stress-induced phenylpropanoid metabolism. Plant Cell. http://dx.doi.org/10.1105/tpc.7.7.1085 [ Links ]
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MSS, and Wang L. 2002. The phenylpropanoid pathway and plant defence-a genomics perspective. Molecular Plant Pathology. http://dx.doi.org/10.1046/j.1364-3703.2002.00131.x. [ Links ]
Edens RM, Anand SC and Bolla RI. 1995. Enzymes of the phenylpropanoid pathway in soybean infected with Meloidogyne incognita or Heterodera glycines. Journal of Nematology. Disponible en línea: http://www.ncbi.nlm.nih.gov/pubmed/19277292 [ Links ]
Ehlting J, Büttner D, Wang Q, Douglas CJ, Somssich IE and Kombrink E. 1999. Three 4-coumarate: Coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. The Plant Journal. http://dx.doi.org/10.1046/j.1365-313X.1999.00491.x [ Links ]
Ehlting J, Hamberger B, Million-Rousseau R and Werck-Reichhart D. 2006. Cytochromes P450 in phenolic metabolism. Phytochemistry Reviews. http://dx.doi.org/10.1007/s11101-006-9025-1 [ Links ]
Ehlting J, Shin, JK andDouglas CJ. 2001. Identification of 4-coumarate:Coenzyme A ligase (4CL) substrate recognition domains. The Plant Journal. http://dx.doi.org/10.1046/j.1365-313X.2001.01122.x [ Links ]
Fraser CM and Chapple C. 2011. The Phenylpropanoid Pathway in Arabidopsis. The Arabidopsis Book. e0152. http://dx.doi.org/10.1199/tab.0152 [ Links ]
Gheysen G and Mitchum MG. 2011. How nematodes manipulate plant development pathways for infection. 2011. Current Opinion in Plant Biology. http://dx.doi.org/10.1016/j.pbi.2011.03.012 [ Links ]
Golinowski W, Grundler FMW and Sobczak M. 1996. Changes in the structure of Arabidopsis thaliana during female development of the plant parasitic nematode Heterodera schachtii. Protoplasma. http://dx.doi.org/10.1007/BF01273172 [ Links ]
Grabber JH. 2005. How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Crop Science. http://dx.doi.org/10.2135/cropsci2004.0191 [ Links ]
Grundler FMW, Sobczak M and Golinowski W. 1998. Formation of cell wall openings in root cells of Arabidopsis thaliana following infection by the plant parasitic nematode Heterodera schachtii. The European Journal of Plant Pathology. http://dx.doi.org/10.1023/A:1008692022279 [ Links ]
Hamberger B and Hahlbrock K. 2004. The 4-coumarate:CoA ligase gene family in Arabidopsis thaliana comprises one rare, sinapate-activating and three commonly occurring isoenzymes. Proceedings of the National Academy Sciences of the United States of America. http://dx.doi.org/10.1073/pnas.0307307101 [ Links ]
Hofmann J and Grundler FMW. 2006. Females and males of root parasitic cyst nematodes induce different symplasmic connections between their syncytial feeding cells and the phloem in Arabidopsis thaliana. Plant Physiology and Biochemistry. http://dx.doi.org/10.1016/j.plaphy.2006.06.006 [ Links ]
Hofmann J, Banora MY, de Almeida-Engler J and Grundler FMW. 2010. The role of callose deposition along plasmodesmata in nematode feeding sites. Molecular Plant-Microbe Interactions Journal. http://dx.doi.org/10.1094/MPMI-23-5-0549 [ Links ]
Hoth S, Stadler R, Sauer N and Hammes UZ. 2008. Differential vascularization of nematode induced feeding sites. Proceedings of the National Academy Sciences of the United States of America. http://dx.doi.org/10.1073/pnas.0803835105 [ Links ]
Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, Yu JQ and Chen Z. 2010. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiology . http://dx.doi.org/10.1104/pp.110.157370 [ Links ]
Hussey RS. 1989. Disease-inducing secretions of plant-parasitic nematodes. Annual Review of Phytopathology. http://dx.doi.org/10.1146/annurev.py.27.090189.001011 [ Links ]
Ithal N, Recknor J, Nettleton D, Maier T, Baum TJ and Mitchum MG. 2007. Developmental transcript profiling of cyst nematode feeding cells in soybean roots. Mol. Plant-Microbe Interactions. http://dx.doi.org/10.1094/MPMI-20-5-0510 [ Links ]
Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette ML, Renou JP, Abad P and Favery B. 2005. Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. The Plant Journal. http://dx.doi.org/10.1111/j.1365-313X.2005.02532.x [ Links ]
Jones MGK and Northcote DH. 1972. Nematode induced syncytium a multinucleate transfer cell. Journal of Cell Science 10:789-809. http://www.ncbi.nlm.nih.gov/pubmed/5038416 [ Links ]
Li L, Cheng, XF, Leshkevich J, Umezawa T, Harding SA and Chiang VL. 2001. The Last Step of Syringyl Monolignol Biosynthesis in Angiosperms Is Regulated by a Novel Gene Encoding Sinapyl Alcohol Dehydrogenase. The Plant Cell. http://dx.doi.org/10.1105/TPC.010111 [ Links ]
Liu CJ. 2012. Deciphering the enigma of lignification: precursor transport, oxidation, and the topochemistry of lignin assembly. Molecular Plant. http://dx.doi.org/10.1093/mp/ssr121 [ Links ]
MacDonald MJ and D'Cunha GB. 2007. A modern view of phenylalanine ammonia lyase. Biochemistry and Cell Biology. http://dx.doi.org/10.1139/O07-018 [ Links ]
Marita JM, Ralph J, Hatfield RD, Guo D, Chen F andDixon RA. 2003. Structural and compositional modifications in lignin of transgenic alfalfa down-regulated in caffeic acid 3-O-methyltransferase and caffeoyl coenzyme A 3-O-methyltransferase. Phytochemistry. http://dx.doi.org/10.1016/S0031-9422(02)00434-X [ Links ]
Marrs KA. 1996. The functions and regulation of glutathione S-transferases in plants. Annual Review of Plant Physiology and Plant Molecular Biology. http://dx.doi.org/10.1146/annurev.arplant.47.1.127 [ Links ]
Menden B, Kohlhoff M and Moerschbacher B. 2007. Wheat cells accumulate a syringyl-rich lignin during the hypersensitive resistance response. Phytochemistry. http://dx.doi.org/10.1016/j.phytochem.2006.11.011 [ Links ]
Mitchum MG, Hussey RS, Baum TJ, Wang X, Elling AA, Wubben M and Davis EL. 2013. Nematode effector proteins: an emerging paradigm of parasitism. New Phytologist. http://dx.doi.org/10.1111/nph.12323 [ Links ]
Nicholson RL and Hammerschmidt R. 1992. Phenolic compounds and their role in disease resistance. Annual Review of Phytopathology. http://dx.doi.org/10.1146/annurev.py.30.090192.002101 [ Links ]
Pegard A, Brizzard G, Fazari A, Soucaze O, Abad P and Djian-Caporalino C. 2005. Histological characterization of resistance to different root-knot nematode species related to phenolics accumulation in Capsicum annuum. Phytopathology. http://dx.doi.org/10.1094/PHYTO-95-0158 [ Links ]
Quentin M, Allasia V, Pegard A, Allais F, Ducrot PH, Favery B, Levis C, Martinet S, Masur C, Ponchet M, Roby D, Schlaich NL, Jouanin L and Keller H. 2009. Imbalanced Lignin Biosynthesis Promotes the Sexual Reproduction of Homothallic Oomycete Pathogens. Plos Pathogens. http://dx.doi.org/10.1371/journal.ppat.1000264 [ Links ]
Raes J, Rohde A, Christensen JH, Peer YV and Boerjan W. 2003. Genome-wide characterization of the lignification toolbox in Arabidopsis. Plant Physiology. http://dx.doi.org/10.1104/pp.103.026484 [ Links ]
Rasmussen S and Dixon RA. 1999. Transgene-mediated and elicitor-induced perturbation of metabolic channeling a the entry point into the phenylpropanoid pathway. The Plant Cell. http://dx.doi.org/10.1105/tpc.11.8.1537 [ Links ]
Rastogi S y Dwivedi UN. 2007. Manipulation of lignin in plants with special reference to O-methyltransferase. Plant Science. http://dx.doi.org/10.1016/j.plantsci.2007.11.014 [ Links ]
Rodiuc N, Vieira P, Banora MY y de Almeida-Engler J. 2014. On the track of transfer cell formation by specialized plant-parasitic nematodes. Frontiers in Plant Science. http://dx.doi.org/10.3389/fpls.2014.00160 [ Links ]
Santiago R, Barros-Rios J and Malvar RA. 2013. Impact of Cell Wall Composition on Maize Resistance to Pests and Diseases. International Journal of Molecular Sciences. http://dx.doi.org/10.3390/ijms14046960 [ Links ]
Sattler SE and Funnell-Harris DL. 2013. Modifying lignin to improve bioenergy feedstocks: strengthening the barrier against pathogens?. Frontiers in Plant Science. http://dx.doi.org/10.3389/fpls.2013.00070 [ Links ]
Schopfer P. 1996. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta. http://dx.doi.org/10.1007/BF00196879 [ Links ]
Sun RC, Sun XF, Wang SQ, Zhu W y Wang XY. 2002. Ester and ether linkages between hydroxycinnamic acids and lignins from wheat, rice, rye, and barley straws, maize stems, and fast-growing poplar wood. Industrial Crops and Products. http://dx.doi.org/10.1016/S0926-6690(01)00112-1 [ Links ]
Turner SJ and Rowe JA. 2006. Cyst nematodes. In: Perry RN yMoens M. Plant nematology. CAB International. Wallingford, UK. Pp:91-122. [ Links ]
Vance CP, Kirk TK and Sherwood RT. 1980. Lignification as a mechanism of disease resistance. Annual Review of Phytopathology. http://dx.doi.org/10.1146/annurev.py.18.090180.001355 [ Links ]
Vanholme R, Demedts B, Morreel K, Ralph J and Boerjan W. 2010. Lignin biosynthesis and structure. Plant Physiology. http://dx.doi.org/10.1104/pp.110.155119 [ Links ]
Vanholme R, Morreel K, Ralph J and Boerjan W. 2008. Lignin engineering. Current Opinion in Plant Biology. http://dx.doi.org/10.1016/j.pbi.2008.03.005 [ Links ]
Vieira P, Engler G and de Almeida Engler J. 2012. Whole mount confocal imaging of nuclei in giant feeding-cells induced by root knot nematodes in Arabidopsis. New Phytologist. http://dx.doi.org/10.1111/j.1469-8137.2012.04175.x. [ Links ]
Weng JK and Chapple C. 2010. The origin and evolution of lignin biosynthesis. New Phytologist. http://dx.doi.org/10.1111/j.1469-8137.2010.03327.x [ Links ]
Wuyts N, Lognay G, Swennen R and Waele D. 2006. Nematode infection and reproduction in transgenic and mutant Arabidopsis and tobacco with an altered phenylpropanoid metabolism. Journal of Experimental Botany. http://dx.doi.org/10.1093/jxb/erl044 [ Links ]
Wuyts N, Lognay G, Verscheure M, Marlier M, De Waele D and Swennen R. 2007. Potential physical and chemical barriers to infection by the burrowing nematode Radopholus similis in roots of susceptible and resistant banana (Musa spp.). Plant Pathology. http://dx.doi.org/10.1111/j.1365-3059.2007.01607.x [ Links ]
Received: July 01, 2015; Accepted: November 16, 2015











 texto en
texto en 


