Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.34 no.1 Texcoco 2016
https://doi.org/10.18781/R.MEX.FIT.1509-1
Scientific articles
Identification and management alternatives of powdery mildew in rosebush
1Centro Universitario Tenancingo, Universidad Autónoma del Estado de México, México
2Facultad de Ciencias Agrícolas, Universidad Autónoma del Estado de México, México
Rose powdery mildew caused by Podosphaera pannosa affects all aerial parts of the plant, which affects their quality as a major component of economic loss. In this work, the causal agent of powdery mildew of rose was identity and confirmed, the effect of potassium phosphite (K3PO3), silicon, chitosan and dodemorph acetate on the incidence and severity of the disease was evaluated, as well as their response in the quality of flower stalks. Two trials (February to April and from May to July) were performed. The morphometric and molecular characteristics of the causal agent corresponded to Podosphaera pannosa. The K3PO3, silicon and chitosan they reduced incidence and severity compared to the control for both assays, however, only the K3PO3 and silicon, exhibited a control similar to provided by the fungicide dodemorph acetate. The treatment with chitosan increased the length and diameter of the stem and flower bud in contrast to K3PO3 and silicon, but was not significant to the control in both tests. Based on the results, the K3PO3, silicon and chitosan are alternatives for the rosebush powdery mildew management under greenhouse conditions.
Keywords: Podosphaera pannosa; Rosa spp.; severity; incidence; inductors
La cenicilla del rosal causada por Podosphaera pannosa afecta todas las partes aéreas de la planta, lo que repercute en su calidad como principal componente de la pérdida económica. En el presente trabajo, se confirmó la identidad del agente causal de la cenicilla en rosa y se evaluó el efecto del fosfito de potasio (K3PO3), silicio, quitosano y acetato de dodemorf sobre la incidencia y severidad de la enfermedad, así como, su respuesta en la calidad de tallos florales. Se realizaron dos ensayos (febrero-abril y mayo-julio). Las características morfométricas y moleculares del agente causal de la cenicilla correspondieron a Podosphaera pannosa. El K3PO3, silicio y quitosano redujeron la incidencia y severidad con respecto al testigo para ambos ensayos; sin embargo, solo el K3PO3 y silicio manifestaron un control similar al proporcionado por el fungicida acetato de dodemorf. El tratamiento con quitosano incrementó la longitud y diámetro del tallo y del botón floral en relación con K3PO3 y silicio, pero no fue diferente del testigo en ambos ensayos. Con base a los resultados obtenidos, el K3PO3, silicio y quitosano pueden ser alternativas en el manejo de la cenicilla del rosal bajo condiciones de invernadero.
Palabras clave: Podosphaera pannosa; Rosa spp.; severidad; incidencia; inductores
Introduction
The Rosa spp. is one of the ornamental species of the highest economic importance worldwide (Debener and Linde, 2009) with a yearly production estimated in 18 trillion stems cut, 60-80 million roses in pots, and 220 million garden roses (Pemberton et al., 2003). However, it is vulnerable to a large number of diseases (Horst and Cloyd, 2007) such as powdery mildew, caused by Podosphaera pannosa (Wallr.: Fr) de Bary, one of the most destructive diseases for roses grown in the open air and greenhouses (Leus et al., 2006; Suthaparan et al., 2012). The fungus can infect all the aerial parts of the plant, and under favorable conditions, causes the distortion of leaves and premature defoliation (Shetty et al., 2012), which causes significant economic losses in the productivity, quality, and therefore, in marketing (Suthaparan et al., 2010).
The control of P. pannosa is bases mainly on the spraying of fungicides at intervals of 7-14 days (Debener and Byrne, 2014). Continuously applying these chemicals increase production costs and may bring about selections of resistant P. pannosa populations (Daughtrey and Benson, 2005); also, the need to minimize the use of fungicides leads to search for control alternatives such as the use of defense inducers. Such is the case of silicon, that has proven to have potential to improve the structural and biochemical potential for resistance to diseases such as powdery mildew in different crops such as cucumbers (Liang et al., 2005), strawberries and grapes (Botta et al., 2011), as well as in pot roses (Shetty et al., 2012). Likewise, phosphites are capable of controlling diseases in diverse crops, acting directly upon the pathogen, and indirectly, by the stimulation of defense responses from the host (Deliopoulos et al., 2010); in this regard, potassium phosphite was reported to induce resistance in potato to Phytophthora infestans (Mont) from Bary (Machinandiarena et al., 2012) and significantly reduces the incidence and severity of Oidium sp. In cucumber (Cucumis sativus L.) (Yañez et al., 2012), in cocoa plantlets (Theobroma cacao L.), it had an antifungal effect, inhibiting the germination of Verticillium dahliae Kleb. (Ribeiro et al., 2006) conidia. Another inducing molecule is chitosan, a derivative of the deacetylation of chitin, considered an efficient biopolymer in the prevention of fungal diseases, since it interferes directly on fungal growth or by activating defenses in plants (Iriti et al., 2011). Borkowski and Szwonek (2004) report that chitosan displays high levels of effectiveness in the control of powdery mildew in tomato plants, (Oidium lycopersicum Cooke & Massee), and when applied on the leaves of barley (Hordeum vulgare L.), it induces resistance against Blumeria graminis (DC.) Speer. f. sp. hordei (Faoro et al., 2008). On the other hand, Moret et al. (2009) report that applying chitosan at concentrations of 1 and 2.5 % reduces the severity of Sphaerotheca fuliginea Schlecht ex Fr. Poll. and Erysiphe cichoracearum DC. ex Merat in cucumber (Cucumis sativus L.).
Despite these compounds showing potential in disease control, the information of these products in treating powdery mildew in ornamental plants, and particularly in rose crops, is limited. Therefore, the aims of this study were to verify the morphometric and molecular identity of the rosebush powdery mildew, evaluate the effect of silicon, potassium phosphite, chitosan and dodemorph acetate on the incidence and severity of the pathogen under study, and quantify its effect on the length and diameter of the stem and floral bud.
Materials and methods
Morphometric Characterization
In April 2014 young Samourai(r) variety rose leaves with symptoms and signs of powdery mildew were gathered in a greenhouse in the municipality of Tenancingo, Mexico. Structures of asexual reproduction such as hyphae, conidiophores, and conidia were detached from the surfaces of young leaves, and they were used for permanent and temporary preparations with Scotch tape in distilled water and potassium hydroxide at 3 % (Braun and Cook, 2012). In these, observations and measurements were taken in the compound microscope (Carl Zeiss(r)), using a 40X lens, of the morphometric characteristics of the 30 repetitions for each structure: diameter of the mycelium, size and shape of conidia, presence of fibrin bodies in conidia, characteristics of the conidiophore (size and shape of the cell base, position of the basal septum), shape of the appresoria on the hyphae and the position of the conidial germ tubes, following the taxonomical key by Braun and Cook (2012). Pfor the observation of the conidial germ tubes, onion cataphylls were innoculated with fungal conidia (To-anun et al., 2005).
Scanning Electron Microscopy (SEM)
Sections of young leaves (0.5 cm2) from a rosebush with signs of powdery mildew were fixed in glutaraldehyde at 3 % for 24 h, and were later washed with Sorensen᾽s phosphate buffer (0.2 M). The samples were dehydrated by sinking them in ethanol at gradual concentrations (30, 40, 50, 60, 70, 80, 90 %) for 40 min each, and at 100 % three times for 20 min. Next, they were dried with CO2 in a Samdri-780A(r) critical point dryer for 40 min, placed on a copper specimen rack and coated in gold in a JFC-1100(r) ionizer for 1 min. Finally, the preparations were observed and photographed in a JEOL(r) JSM-6390 scanning electron microscope.
Molecular Characterization
DNA was extracted from leaves with signs of having the fungus, using the Plant DNAzol Reagent(r) (Invitrogen(tm)) extraction kit, according to the protocol described by the manufacturer, with modifications to avoid the efect of phenols; for this reason, five washings were carried out with 300 µL of ethanol at 75 %. DNA integrity was observed in an agarose gel (Ultrapure(tm)) at 1 %, the DNA strands were seen in a Syngene(r) GVM20 transiluminador, quality and concentration were determined using an Eppendorff(r) D-5000-3000 biophotometer. The DNA obtained was resuspended in 50 µL of molecular biology degree water and stored at -20 °C to be used later.
Polymerase Chain Reaction (PCR)
For the PCR test, primers ITS1F (5'-CTTGGTCATTTAGAGGAAGTAA-3') (Gardes and Bruns, 1993) and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') were used (White et al., 1990). The PCR reactions were carried out in a final volume of 20 μL de la mezcla: 6.60 μL de agua estéril desionizada (Gibco(r)), 10 μL of 2X Phire Plant PCR Buffer (includes 200 μM of every dNTP and 1.5 mM of MgCl2), 1 μL of each ITS1F and ITS4 primer at 10 ρmol, 1 μL of ADN, 0.4 μL of DNA Polymerase (Phire(r) Hot Start II). Amplification was carrried out in an MJ ResearchThermal(r) PTC-100 thermocycler, according to the procedure described by Leus et al. (2006). The product of the amplification was verified by electrophoresis at 90 V for 30 min in agarose gel at 1 % and stained with 1 µL of ethidium bromide, visualization was carried out in a Syngene(r) GVM20 transilluminator. The DNA was purified using the DNA Clean & Concentrator TM-5 (Zymo Research(r)) commercial kit. Later, the fragments amplified using the PCR were sequenced in both directions in an ABI Prism 3130XL genetic analyzer. The sequence obtained was aligned in the National Center for Biotecnology Information database. The sequence was deposited in the base of the GenBank. A cladogram was taken using the Neighbor-Joining method with the program Mega 6.0.
Experiments in Greenhouses
In Samourai(r) variety rose bushes planted in greenhouse conditions, two trials were carried out: the first, in the months of February-April, and the second, from May-July 2014; both were carried out under a randomized block design with five treatments and four repetitions, and 20 experimental units were used. Each experimental unit consisted of a plot of land 2.70 m long by 1 m wide, and contained 27 rose bushes distributed in rows. By trimming the bushes, a homogenous production of sprouts was stimulated, on which the treatments were evaluated.
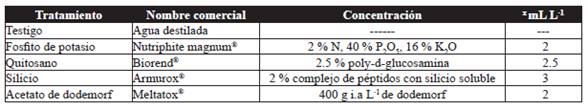
Treatments
These were potassium phosphite, chitosan, silicon as resistance inductors; the fungicide dodemorph acetate and a control (distilled water) (Table 1). Treatments were assigned at random to each experimental unit; its application began eight days after trimming, and afterwards, at weekly intervals until reaching cutting point. Application was carried out using a motorized spray pump (Maruyama, MS072H) with a fan-shaped nozzle, during the early hours of the morning, covering the entire foliage (usage of 0.5 L per plot).
Variables
Ten stems were chosen at random in each experimental unit for the evaluation of the incidence and severity of the disease, length and diameter of the sten and floral bud.
Evaluation of Incidence and Severity of P . pannosa
To enhance the natural growth of the mildew during the experiment, the temperature was raised (25-33 °C) during the day, and relative humidity by night (70-90 %) using the vents in the greenhouse. Incidence and severity were evaluated immediately after the apprearance of signs and symptoms. Later evaluations were performed on a weekly basis. The percentage of incidence was calculated by counting the number of stems with symptoms and signs in relation to the 10 stems evaluated for each experimental unit.
The severity of the disease was determined using the Horsfall and Barratt scale (1945), with the classes: 0= No symptoms, 1=1-2.5 %, 2=2.6-5 %, 3=6-10 %, 4=11-25 %, 5=26-50 %, 6=51-75 %, and 7=76-100 % of the surface of the leaf damaged. The values were converted to percentages of severity using the Townsend and Heuberger equation:
Where: P= percentage of damage; n= total number of leaves for each class on the scale; v= respective degree of the scale; N= total number of leaves evaluated; and i= highest degree of the scale.
Incidence and severity data were converted into Area under the disease progress curve (AUDPC), applying trapezoidal integration method (Campbell and Madden, 1990), starting directly from the percentages of the diseased stems and leaves in each evaluation dates.
Evaluation of Length and Diameter of Stem and Floral Bud
At the end of the experiment, the length of the stem was measured (cm), from the base to the apex. The diameter was determined using a CALDI-6MP digital caliper, taking the reading one centimeter bove the base of the stem. The length and diameter of the floral bud were measured in the cutting point using a digital caliper.
Results
Characterization of Symptoms
The symptoms were characterized by the sporadic development of reddish to purple stains on the underside of the leaves. In the second trial, the leaves were completely colonized by the signs of the pathogen, which caused the leaflets to become deformed into a curved and/or twisted shape (Figure 1A). Some stems and peduncles showed sings of the disease (Figure 1B).
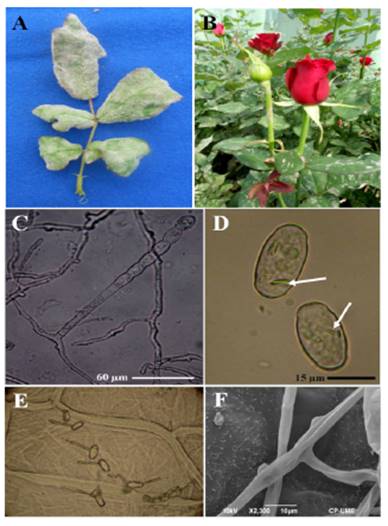
Figure 1 A). Deformation of leaflets caused by P. pannosa Samourai(r) var. rose bushes. B). Leaves and peduncle with signs of the fungus. C). Conidiophore emerging from the mother cell of the hyphae with a catenulation of conidia. D). Elipsoidal to doliformed Conidia with fibrin bodies. E). Conidial germination patterns on onion catáphylls. F). Almost indistinguishable appressoria as protuberances on the hyphae.
Morphometric Characterization
The causative agent of the disease characteristically produced mycelia, superficial and hyaline, 3-8 µm in diameter; almost indistinguishable appresoria as protuberances; erect conidiophores up to 115 µm long emerging from the surface of the hyphal mother cells, centrally or not; straight basal cells, subcylindrical, 31-88 x 7-11 µm, followed by 1-2 short basal cells, forming catenescent conidia, elipsoidal to doliform, 20-35 x 11-18.7 µm with fibrin bodies; germ tubes ± terminal to lateral, 3.75-4 µm wide, of the type Fibroidium, subtype Orthotubus (Figura 1C -1F). According to this, characteristics correspond to P. pannosa.
Scanning Electron Microscopy (SEM)
The surface of the wall of the P. pannosa conidia was smooth (Figure 2A), with occasional fine waviness; in the terminal section of these, which represents the location of the septum that separates the conidia when they form chains, slight concentric rings were observed (Figure 2B and 2C). In partially collapsed conidia, the external wall presented wrinkiling that produced sinuous longitudinal and transversal lines (Figure 2D).
Molecular Characterization
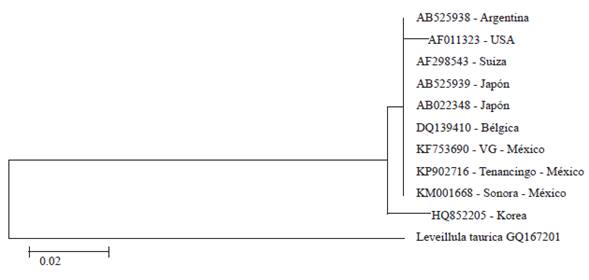
By amplification by PCR with the primers ITS1F and ITS4, a sequence of nucleotides was obtained of 574 pb. When comparing the sequence of nucleotides in this study (Deposit number KP902716), with those deposited in the GenBank, the BLAST analysis showed a 100 % of identification with accessions AB525939 (P. pannosa in Rosa maltiflora), AB022348 (P. pannosa n Rosa sp.), DQ139410 (P. pannosa in Rosa sp.), and KF753690 (P. pannosa in Rosa sp.), and 99 % with accesions AF011323 (P. pannosa in Rosa sp.), AF298543 (P. pannosa in Rosa sp.), AB525938 (P. pannosa in Rosa rubiginosa), HQ852205 (P. pannosa in Rosa rugosa), and KM001668 (P. pannosa in Rosa sp.) (Figure 3).
Evaluation of Incidence and Severity of P. pannosa
The AUDPC of incidence varied significantly (P˃0.05) between different treatments. In the first trial, the plants treated with chitosan, silicon, and potassium phosphite presented low incidence rates, significantly different to the control. For the second trial, only plants treated with silicon and potassium phosphite were significantly different (P˃0.05) to the control. The best treatment to reduce the incidence of the disease was dodemorph acetate, which presented a AUDPC of 70.0 and 1400.0 for the first and second trials, respectively (Table 2).
Table 2 Area under the disease progress curve (AUDPC) for the incidence and severity of powdery mildew (P. pannosa) in rose plantations.
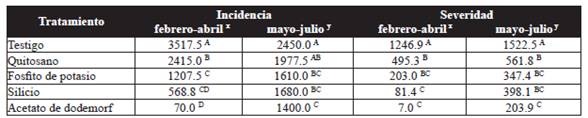
Averages with a letter in common are not significantly differen, Tukey ( P > 0.05), xFirst trial, ysecond trail.
In terms of severity, plants treated with chitosan, potassium phosphite, and silicon displayed a reduction of 60.3, 83.7, and 93.5 % respectively in the first trial, and 63.1, 77.2, and 73.9 % in the second, in comparison to the control (Table 2). In both trials, the plants treated with silicon and potassium phosphite were statistically equal (P˃0.05) with the fungicide dodemorph acetate, which displayed the least severity.
Evaluation of the Length and Diameter of the Stem and Floral Bud
The treatment with chitosan showed an increase in stem length of 9.4 % and 20.1 % in the first and second trials, respectively, constrasting with the potassium phosphite, which induced the shortest length, with 66.7 and 64.7 cm for the first and second trials, respectively. However, the treatment with chitosan was not significantly different to the control (Table 3). In regard to the stem diameter, the control showed the greatest diameter, with 8.0 mm, and it wa statistically different (P˃0.05) to the diameter in plants treated with potassium phosphite (7.3 mm) in the first trial. For the second trial, plants treated with potassium phosphite and silicon displayed lower stem diameters with 6.4 mm, whereas the treatment with chitosan showed the greatest stem diameter with 7.8 mm and no significant differences (P˃0.05) with the contol (Table 3).
Table 3 Effect of the treatments on the length and diameter of the stem of the Samurai(r) variety rose.
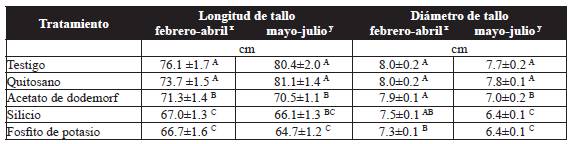
Results are the average of 10 plants per treatment. ± Standard Error. Values with a letter in common are not significantly different, Tukey (P > 0.05). xFirst trial, y second trial.
Regarding the length of the floral bud, potassium phosphite and silicon were significantly different (P˃0.05) to the rest of the treatments and displayed the shortest bud length with 35.6 and 37.1 mm respectively in the first trial. However, in the second trial, chitosan displayed an increase on bud length of up to 29.5 % in relation to the potassium phosphite, which induced the shortest bud lengt, with 34.6 mm (Table 4). The diameter of the floral bud, with the potassium phosphite treatment, was 21.6 mm in the first treatment and 21.2 mm in the secon, and was statistically different (P˃0.05) to the chitosan that showed diameters of 27.8 and 26.5 mm for the first and second trials, respectively. However, chitosan dis not show any significant difference with the control in trials (Table 4).
Discussion
Based on the results of this investigation Podosphaera pannosa (Wallr. Fr.) de Bary was identified as the causing agent of the powdery mildew in roses; the morphometric characteristics were similar to those reported by Braun and Cook (2012). However, the number of basal cells observed in this study were 1-2 short cells, which is different to reports by Havrylenko (1995) and Félix-Gastélum et al. (2014). These differences may be due to diverse factors such as temperature and humidity, the host (variety), age of the leaves, and the sampling period (Salmon, 1900). Primers ITS1F and ITS4 amplified a product of 574 pb, corresponding to P. pannosa; similarly, Félix-Gastélum et al. (2014) and Romberg et al. (2014) used the same primers to identify and detect P. pannosa in the Rosa spp. crop and Catharanthus roseus (L) G. Don crops, respectively. The comparison of the nucleotide sequence obtained (KP902716), with thse deposited in the GenBank showed an identity of 99 to 100 % with P. pannosa accessions presents in Rosa sp. in Mexico, Belgium, the United States, Japan and Switzerland (Saenz and Taylor, 1999; Mori et al., 2000; Cunnington et al., 2003; Leus et al., 2006; Félix-Gastélum et al., 2014), in Rosa maltiflora Thunberg ex Murray from Japan, Rosa rubiginosa L. from Argentina (Takamatsu et al., 2010), and in Rosa rugosa Thunb. from Korea (Lee et al., 2011).
With the application of the inducers sillicon (Si), potassium phosphate (K3PO3) and chitosan, there was a reduction in the incidence and severity of powdery mildew under greenhouse conditions. However, these treatments were not as effective as the dodemorph acetate fungicide, which displayed the lowest values for incidence and severity of the disease in both trials. Its effect is due to it being a systemic fungicide with acropetal absorption that acts inhibiting the ergosterol and protein syntheses, affecting the permeability of the membrane (Brent and Hollomon, 2007). Likewise, Benyagoub and Bélanger (1995), report that applying dodemorph acetate affects the integrity of hyphae, resulting in the collapse of conidiophores and conidia. On the other hand, there are reports claiming that applying dodemorph acetate can generate toxicity in rose plants (Bélanger et al., 1994), although in our results,the fungicide displayed an excellent control of the disease and caused no loss of vigor in plants.
Within the inductors, the treatment with Si at doses of 3 mL L-1 reduced significantly the incidence and severity of P. pannosa and was statistically equal to the dodemorph acetate fungicide. Regarding this, Shetty et al. (2012) reported that applying 3.6 mM of Si (100 ppm) in a nutrient solution reduces the severity of de P. pannosa in up to 48.9 %, depending on the genotype of the host, as Datnoff et al. (2006), demonstrated that applying Si reduces the severity of powdery mildew significantly, in up to 57 % in roses in pots. These reports suggest that the application of Si plays an important part in suppressing powdery mildew in rose bushes, which could be explained by an increase in the concentration of antimicrobial phenolic compounds and flavonoids in response to the infection with P. pannosa (Shetty et al., 2011).
It has also been shown that applying Si has benefitial effects on the growth and quality of the roses (Hwang et al., 2005; Reezi et al., 2009). However, in this study, applying Si induced a reduction of the length and diameter of stems and floral bud in relation to the control, as reported by Reezi et al. (2009), who observed that with high doses of Si (150 ppm), there was a noticeable reduction in the length and diameter of rose stems. Likewise, it has been documented that applying potassium silicate (200 mg L-1) in sunflower (Helianthus annuus L.) causes deformity in flowers and hinders growth (Kamenidou et al., 2008), whereas applying sodium silicate (150 mg L-1) reduces the length of stems and causes deformity in gerbera flowers (Kamenidou et al., 2010). Another possible explanation is that the addition of Si in plants could improve biotic or abiotic stress or alter their morphology (Ma and Yamaji, 2006), as observed in thie present study.
Phosphites are commonly used to control oomycetes in different crops. Their efficiency has been proven against species such as Oidium sp. (Yáñez et al., 2012), Erysiphe polygoni D.C. (Salamanca-Carvajal and Alvarado-Gaona, 2012), Penicillium expansum Link. (Amiri and Bompeix, 2011), Phytophthora cactorum (Lebert and Cohn) Scröt (Rebollar-Alviter et al., 2010), and Peronospora sparsa Berkeley (Rebollar-Alviter et al., 2012). In this study, the treatment with K3PO3 at doses of 2 mL L-1 significantly reduced the incidence and severity of P. pannosa. Similar results have been reported by Chavarro-Carrero et al. (2012), who demonstrated that applying potassium phosphite regularly on Bingo White(r) variety roses reduce incidence by up to 35 % and severity by 6.3 % de P. sparsa, with a biological effectiveness of 93.4 % against 14.8 % of a fungicide based on cymoxanil + copper hydroxide + mancozeb. It is important to highlight that the success of phosphites in the control of some diseases is due to its systemic action, which is why they act upon all areas of the plant. Several authors report that phosphite displays a complex form of action, acting directly on the development of the pathogen, inhibiting the growth of the mycelia and the cell wall synthesis (King et al., 2010), or indirectly by the stimulation of the plants' defense responses, such as the production of phytoalexins (Lovatt and Mikkelsen, 2006; Lobato et al., 2011), deposition of callose, reactive species of oxygen and the induction of proteins related to pathogenesis (Eshraghi et al., 2011; Machinandiarena et al., 2012).
There have been reports on the fungistatic effect of phosphites, but also on their ability to increase flowering, yield, fruit size, total of soluble solids, and concentration of anthocyanins in some crops (Lovatt and Mikkelsen, 2006). However, in this study the potassium phosphite reduced the length and diameter of stems and floral buds in comparison to the control in both trials, which could be due to the doses applied, since there is evidence that shows that high concentrations of phosphites lead to plant toxicity, which affects yield (Lovatt and Mikkelsen, 2006). Similar results were reported by Yáñez et al. (2012), who documented that applying mineral salts, including potassium phosphite, showed no significant effects with reprecussions on the length and number of leaves in cucumber plants.
On the other hand, chitosan, a heavy cationic polysaccharide taken from the deacetylation of the exoskeletons of crabs, is a biodegradable and non/toxic biopolymer, efficient in preventing diseases caused by fungi, since they interfere directly in their growth (Bautista-Baños et al., 2006) or in the activation of biological processes in host tissues (Bautista-Baños et al., 2006; Iriti et al., 2011). In this investigation, applying at 0.013 % reduced incidence in up to 31.2 and 19.3 % in the first and second trials, respectively, and severity was also reduced by 60.3 % in the first and 63.1 % in the second trial in comparison to the control. Wojdyla (2001) reported that applying chitosan at a concentration of 0.025 to 0.2 % reduced the development of powdery mildew in roses between 43.5 and 85.4 %, similar to the chemical treatment with triforine (0.03 %). When used on P. sparsa, its efficiency varied between 50 and 73 %, and on Botrytis cinerea Pers: Fr. at concentrations of 0.1 and 0.2 %, severity decreased by 5 and 35 %, respectively. Related results indicate that weekly applications of chitosan (Biochikol 020 PC), increase tolerances to Diplocarpon rosae Wolf from 18 to 60 %, yet in chrysanthemums, at a concentration of 0.01 to 0.05 % the control of Oidium chrysanthemi DC. was from 69 to 79 %, whereas with Puccinia horiana Henning, it was from 54 to 97 % (Wojdyla, 2004).
In the present study, it has been shown that applying chitosan increases the length and diameter of the stem and floral bud in relation to silicon and potassium phosphite. Some reports have shown that the efficiency of chitosan to protect plantlets against pests and diseases, improve seed germination, enhance plant growth, and therefore, increase crop yield. In ornamental plants, chitosan had a positive influence on gladioli (Gladiolus spp.), since it increases the sprouting of corms, as well as the number of flowers per spike and prolongued its vase life (Ramos-García et al., 2009); in freesia (Freesia spp.) corms it showed a fast emergence and reduced its vegetative cycle (Startek et al., 2005), whereas in orchids (Dendobrium phalaenopsis Fitzg.) it influenced the growth of meristematic sprouts in tissue culture (Nge et al., 2006), and in Lilium spp. It displayed an increase in vase life when the stems were submerged in, or sprayed with, a solution of chitosan + pure AG colloidal nanoparticle + ion (Kim et al., 2005). There is other evidence, such as that described by Ohta et al. (2001), who reported that seeds from lisianthus (Eustoma grandiflorum (Raf.) Shinners) submerged in chitosan at 1 % for one hour, and applying it onto the soil, significantly increased the number of flowers and the length and diameter of the stem.
Conclusions
Morphometric and molecular characterizations confirmed that Podosphaera pannosa is the agent related to the powdery mildew in rose bushes in the municipality of Tenancingo, Mexico. Applying silicia and potassium phosphite reduced the incidence and severity of Podosphaera pannosa, and are therefore considered feasible alternatives that can be incorporated in the integrated management of this disease. Chitosan can be an alternative in handling rose plantations, since it has positive effects on the length and diameter of the stem and floral bud. Dodemorf acetate presented an excellent potential for the control of P. pannosa and good selectivity in the crop.
Literatura citada
Amiri, A and Bompeix G. 2011. Control of Penicillium expansum with potassium phosphite and heat treatment. Crop Protection 30:222-227. http://dx.doi.org/10.1016/j.cropro.2010.10.010 [ Links ]
Bautista-Baños S, Hernández-Lauzardo AN, Velázquez-del Valle MG, Hernández-López M, Ait Barka E, Bosquez-Molina E and Wilson CL. 2006. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Protection 25:108-118. http://dx.doi.org/10.1016/j.cropro.2005.03.010 [ Links ]
Bélanger RR, Labbé C and Jarvis WR. 1994. Commercial-scale control of rose powdery mildew with a fungal antagonist. Plant Disease 78:420-424. http://dx.doi.org/10.1094/PD-78-0420 [ Links ]
Benyagoub M and Bélanger RR. 1995. Development of a mutant strain of Sporothrix flocculosa with resistance to dodemorph-acetate. Phytopathology 85:766-770. http://dx.doi.org/10.1094/Phyto-85-766 [ Links ]
Borkowski J and Szwonek E. 2004. Powdery mildew control on greenhouse tomatoes by chitosan and other selected substances. Acta Horticulturae 633:435-438. http://dx.doi.org/10.17660/ActaHortic.2004.633.53 [ Links ]
Botta A, Sierras N, Marín C and Piñol R. 2011. Powdery mildew protection with Armurox: An improved strategy for silicon application. Journal of Agricultural Science and Technology A 1:1032-1039. http://dx.doi.org/10.17265/2161-6256/2011.11A.012 [ Links ]
Braun U and Cook RTA. 2012. Taxonomic manual of the Erysiphales (powdery mildews). CBS-KNAW Fungal Biodiversity Centre. Utrecht, The Netherlands. 707p. [ Links ]
Brent KJ and Hollomon DW. 2007. Fungicide resistance in crop pathogens. Second Edition Fungicide Resistance Action Committee. Brussels, Belgium. 56p. Disponible en línea: http://www.frac.info/docs/default-source/publications/monographs/monograph-1.pdf?sfvrsn=8 [ Links ]
Campbell CL and Madden LV. 1990. Introduction to plant disease epidemiology. John Whiley and Sons Inc. New York, USA. 532p. [ Links ]
Chavarro-Carrero EA, García-Velasco R, González-Díaz JG, González-Cepeda LE y Jiménez-Ávila LJ. 2012. Uso del fosfito de potasio para el manejo de Peronospora sparsa Berkeley en Rosa spp. Fitopatología Colombiana 36:53-56. [ Links ]
Cunnington JH, Takamatsu S, Lawrie AC and Pascoe IG. 2003. Molecular identification of anamorphic powdery mildews (Erysiphales). Australasian Plant Pathology 32:421-428. http://dx.doi.org/10.1071/AP03045 [ Links ]
Datnoff LE, Nell TA, Leonard RT and Rutherford BA. 2006. Effect of silicon on powdery mildew development on miniature potted rose. Phytopathology 96:S28. Disponible en línea: http://apsjournals.apsnet.org/toc/phyto/96/6s [ Links ]
Daughtrey ML and Benson DM. 2005. Principles of plant health management for ornamental plants. Annual Review of Phytopathology 43:141-169. http://dx.doi.org/10.1146/annurev.phyto.43.040204.140007 [ Links ]
Debener T and Byrne DH. 2014. Disease resistance breeding in rose: Current status and potential of biotechnological tools. Plant Science 228:107-117. http://dx.doi.org/10.1016/j.plantsci.2014.04.005 [ Links ]
Debener T and Linde M. 2009. Exploring complex ornamental genomes: The rose as a model plant. Critical Reviews in Plant Science 28:267-280. http://dx.doi.org/10.1080/07352680903035481 [ Links ]
Deliopoulos T, Kettlewell PS and Hare MC. 2010. Fungal disease suppression by inorganic salts: A review. Crop Protection 29:1059-1075. http://dx.doi.org/10.1016/j.cropro.2010.05.011 [ Links ]
Eshraghi L, Anderson J, Aryamanesh N, Shearer B, McComb J, Hardy GEStj and O'Brien PA. 2011. Phosphite primed defence responses and enhanced expression of defence genes in Arabidopsis thaliana infected with Phytophthora cinnamomi. Plant Pathology 60:1086-1095. http://dx.doi.org/10.1111/j.1365-3059.2011.02471.x [ Links ]
Faoro F, Maffi D, Cantu D and Iriti M. 2008. Chemical-induced resistance against powdery mildew in barley: The effects of chitosan and benzothiadiazole. Biocontrol 53:387-401. http://dx.doi.org/10.1007/s10526-007-9091-3 [ Links ]
Félix-Gastélum R, Herrera-Rodríguez G, Martínez-Valenzuela C, Maldonado-Mendoza IE, Quiroz-Figueroa FR, Brito-Vega H and Espinoza-Matías S. 2014. First report of powdery mildew (Podosphaera pannosa) of roses in Sinaloa, México. Plant Disease 98:1442. http://dx.doi.org/10.1094/PDIS-06-14-0605-PDN [ Links ]
Gardes M and Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes: application to the identification of mycorrhizae and rusts. Molecular Ecology 2:113-118. http://dx.doi.org/10.1111/j.1365-294X.1993.tb00005.x [ Links ]
Havrylenko M. 1995. Erysiphaceous species from Nahuel Huapi National Park, Argentina. Part I. New Zeal. New Zealand Journal of Botany 33:389-400. http://dx.doi.org/10.1080/0028825X.1995.10412965 [ Links ]
Horsfall JG and Barratt RW. 1945. An improved grading system for measuring plant diseases. Phytophatology 35:655. Disponible en línea: http://www.garfield.library.upenn.edu/classics1986/A1986A666500001.pdf [ Links ]
Horst RK and Cloyd RA. 2007. Compendium of rose diseases and pests. Second Edition. The American Phytopathological Society. St. Paul, Minnesota, USA. 96p. [ Links ]
Hwang SJ, Han-Min P and Jeong BR. 2005. Effects of potassium silicate on the growth of miniature rose 'Pinocchio' grown on rockwool and its cut flower quality. Journal of the Japanese Society for Horticultural Science 74:242-247. http://dx.doi.org/10.2503/jjshs.74.242 [ Links ]
Iriti M, Vitalini S, Di Tommaso G, D'Amico S, Borgo M andFaoro F. 2011. New chitosan formulation prevents grapevine powdery mildew infection and improves polyphenol content and free radical scavenging activity of grape and wine. Australian Journal of Grape and Wine Research 17:263-269. http://dx.doi.org/10.1111/j.1755-0238.2011.00149.x [ Links ]
Kamenidou S, Cavins TJ and Marek S. 2008. Silicon supplements affect horticultural traits of greenhouse-produced ornamental sunflowers. HortScience 43:236-239. Disponible en línea: http://hortsci.ashspublications.org/content/43/1/236.full [ Links ]
Kamenidou S, Cavins TJ andMarek S. 2010. Silicon supplements affect floricultural quality traits and elemental nutrient concentrations of greenhouse produced gerbera. Scientia Horticulturae 123:390-394. http://dx.doi.org/10.1016/j.scienta.2009.09.008 [ Links ]
Kim JH, Lee AK and Suh JK. 2005. Effect of certain pre-treatment substances on vase life and physiological character in Lilium spp. Acta Horticulturae 673:307-314. http://dx.doi.org/10.17660/ActaHortic.2005.673.39 [ Links ]
King M, Reeve W, Van der Hoek MB, Williams N, McComb J, O'Brien PA and Hardy GE. 2010. Defining the phosphite-regulated transcriptome of the plant pathogen Phytophthora cinnamomi. Molecular Genetics and Genomics 284:425-435. http://dx.doi.org/10.1007/s00438-010-0579-7 [ Links ]
Lee SH, Han KS, Park JH and Shin HD. 2011. Occurrence of Podosphaera pannosa Teleomorph on Rosa rugosa from Korea. Plant Pathology Journal 27:398. http://dx.doi.org/10.5423/PPJ.2011.27.4.398 [ Links ]
Leus L, Dewitte A, Van Huylenbroeck J, Vanhoutte N, Van Bockstaele E and Höfte M. 2006. Podosphaera pannosa (syn. Sphaerotheca pannosa) on Rosa and Prunus spp.: Characterization of pathotypes by differential plant reactions and ITS sequences. Journal of Phytopathology 154:23-28. http://dx.doi.org/10.1111/j.1439-0434.2005.01053.x [ Links ]
Liang YC, Sun WC, Si J and Römheld V. 2005. Effects of foliarand root-applied silicon on the enhancement of induced resistance to powdery mildew in Cucumis sativus. Plant Pathology 54:678-685. http://dx.doi.org/10.1111/j.1365-3059.2005.01246.x [ Links ]
Lobato MC, Machinandiarena MF, Tambascio C, Dosio GAA, Caldiz DO, Daleo GR, Andreu AB and Olivieri FP. 2011. Effect of foliar applications of phosphite on post-harvest potato tubers. European Journal of Plant Pathology 130:155-163. http://dx.doi.org/10.1007/s10658-011-9741-2 [ Links ]
Lovatt CJ and Mikkelsen RL. 2006. Phosphite fertilizers: What are they? Can you use them? What can they do?. Better crops With Plant Food 90:11-13. Disponible en línea: http://www.ipni.net/publication/bettercrops.nsf/issue/BC-2006-4 [ Links ]
Ma JF and Yamaji N. 2006. Silicon uptake and accumulation in higher plants. Trends in Plant Science 11:392-397. http://dx.doi.org/10.1016/j.tplants.2006.06.007 [ Links ]
Machinandiarena MF, Lobato MC, Feldman ML, Daleo GR and Andreu AB. 2012. Potassium phosphite primes defense responses in potato against Phytophthora infestans. Journal of Plant Physiology 169:1417-1424. http://dx.doi.org/10.1016/j.jplph.2012.05.005 [ Links ]
Moret A, Muñoz Z and Garcés S. 2009. Control of powdery mildew on cucumber cotyledons by chitosan. Journal of Plant Pathology 91:375-380. http://dx.doi.org/10.4454/jpp.v91i2.967 [ Links ]
Mori Y, Sato Y and Takamatsu S. 2000. Evolutionary analysis of the powdery mildew fungi using nucleotide sequences of the nuclear ribosomal DNA. Mycologia 92:74-93. http://dx.doi.org/10.2307/3761452 [ Links ]
Nge KL, Nwe N, Chandrkrachang S and Stevens WF. 2006. Chitosan as a growth stimulator in orchird tissue culture. Plant Science 170:1185-1190. http://dx.doi.org/10.1016/j.plantsci.2006.02.006 [ Links ]
Ohta K, Asao T and Hosoki T. 2001. Effects of chitosan treatments on seedling growth, chitinase activity and flower quality in Eustoma grandiflorum (Raf.) Shinn. 'Kairyou Wakamurasaki'. Journal of Horticultural Science and Biotechnology 76:612-614. Disponible en línea: http://www.jhortscib.org/Vol76/76_5/18.htm [ Links ]
Pemberton HB, Kelly JW and Ferare J. 2003. Pot rose production. Pp:587-593. : Roberts AV, Debener T and Gudin S (eds.). Encyclopedia of rose science. Academic press. Oxford, USA. 1450p. http://dx.doi.org/10.1016/B0-12-227620-5/00074-4 [ Links ]
Ramos-García M, Ortega-Centeno S, Hernández-Lauzardo AN, Alia-Tejacal I, Bosquez-Molina E and Bautista-Baños S. 2009. Response of gladiolus (Gladiolus spp) plants after exposure corms to chitosan and hot water treatments. Scientia Horticulturae 121:480-484. http://dx.doi.org/10.1016/j.scienta.2009.03.002 [ Links ]
Rebollar-Alviter A, Silva-Rojas HV, López-Cruz I, Boyzo-Marín J and Ellis MA. 2012. Fungicide sprays programs to manage downy mildew (dryberry) of blackberry caused by Peronospora sparsa. Crop Protection 42:49-55. http://dx.doi.org/10.1016/j.cropro.2012.06.007 [ Links ]
Rebollar-Alviter A, Wilson LL, Madden LV and Ellis MA. 2010. A comparative evaluation of post-infection efficacy of mefenoxam and potassium phosphite with protectant efficacy of azoxystrobin and potassium phosphite for controlling leather rot of strawberry caused by Phytophthora cactorum. Crop Protection 29:349-353. http://dx.doi.org/10.1016/j.cropro.2009.12.009 [ Links ]
Reezi S, Babalar M and Kalantari S. 2009. Silicon alleviates salt stress, decreases malondialdehyde content and affects petal color of salts-tressed cut rose (Rosa x hybrida L.) 'Hot Lady'. African Journal of Biotechnology 8:1502-1508. http://dx.doi.org/10.5897/AJB09.180 [ Links ]
Ribeiro JPM, Vilela RML, Borges PR, Rossi CF, Rufino AD and De Padua MA. 2006. Effect of potassium phosphite on the induction of resistance in cocoa seedlings (Theobroma cacao L.) against Verticillium dahliae Kleb. Ciência e Agrotecnologia 30:629-636. http://dx.doi.org/10.1590/S1413-70542006000400006 [ Links ]
Romberg MK, Kennedy AH and Ko M. 2014. First report of the powdery mildews Leveillula taurica and Podosphaera pannosa on rose periwinkle in the United States. Plant Disease 98:848. http://dx.doi.org/10.1094/PDIS-09-13-0981-PDN [ Links ]
Saenz GS and Taylor JW. 1999. Phylogeny of the Erysiphales (powdery mildews) inferred from internal transcribed spacer ribosomal DNA sequences. Canadian Journal of Botany 77:150-168. http://dx.doi.org/10.1139/b98-235 [ Links ]
Salamanca-Carvajal M y Alvarado-Gaona A. 2012. Efecto de la proteína harpin y el fosfito de potasio en el control del mildeo polvoso (Erysiphe polygoni D.C.) en tomate, en Sutamarchán (Boyacá). Ciencia y Agricultura 9:65-75. Disponible en línea: http://dialnet.unirioja.es/servlet/articulo?codigo=4986456 [ Links ]
Salmon ES. 1900. A monograph of the Erysiphaceae. Torrey Botanical Club. New York, USA. 292p. http://dx.doi.org/10.5962/bhl.title.97215 [ Links ]
Shetty R, Fretté X, Jensen B, Shetty NP, Jensen JD, Jørgensen HJL, Newman MA and Christensen LP. 2011. Silicon-induced changes in antifungal phenolic acids, flavonoids, and key phenylpropanoid pathway genes during the interaction between miniature roses and the biotrophic pathogen Podosphaera pannosa. Plant Physiology 157:2194-2205. http://dx.doi.org/10.1104/pp.111.185215 [ Links ]
Shetty R, Jensen B, Shetty NP, Hansen M, Hansen CW, Starkey KR and Jørgensen HJL. 2012. Silicon induced resistance against powdery mildew of roses caused by Podosphaera pannosa. Plant Pathology 61:120-131. http://dx.doi.org/10.1111/j.1365-3059.2011.02493.x [ Links ]
Startek L, Bartkowiak A, Salachna P, Kaminska M and Mazurklewicz-Zapalowicz K. 2005. The influence of new methods of corm coating on freesia growth, development and health. Acta Horticulturae 673:611-616. http://dx.doi.org/10.17660/ActaHortic.2005.673.84 [ Links ]
Suthaparan A, Stensvand A, Solhaug KA, Torre S, Mortensen LM, Gadoury DM, Seem RC and Gislerød HR. 2012. Suppression of powdery mildew (Podosphaera pannosa) in greenhouse roses by brief exposure to supplemental UV-B radiation. Plant Disease 96:1653-1660. http://dx.doi.org/10.1094/PDIS-01-12-0094-RE [ Links ]
Suthaparan A, Stensvand A, Torre S, Herrero ML, Pettersen RI, Gadoury DM andGislerød HR. 2010. Continuous lighting reduces conidial production and germinability in the rose powdery mildew pathosystem. Plant Disease 94:339-344. http://dx.doi.org/10.1094/PDIS-94-3-0339 [ Links ]
Takamatsu S, Niinomi S, Harada M and Havrylenko M. 2010. Molecular phylogenetic analyses reveal a close evolutionary relationship between Podosphaera (Erysiphales: Erysiphaceae) and its rosaceous hosts. Persoonia 24:38-48. http://dx.doi.org/10.3767/003158510X494596 [ Links ]
To-anun C, Kom-un S, Sunawan A, Fangfuk W, Sato Y andTakamatsu S. 2005. A new subgenus, Microidium, of Oidium (Erysiphaceae) on Phyllanthus spp. Mycoscience 46:1-8. http://dx.doi.org/10.1007/S10267-004-0202-Z [ Links ]
White TJ, Bruns TS, Lee S and Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Pp:315-322. : Innis MA, Gelfand DH, Sninsky JJ and White TJ (eds.). PCR protocols: A guide to methods and applications. Academic Press. San Diego, USA. 1990p. http://dx.doi.org/10.1016/B978-0-12-372180-8.50042-1 [ Links ]
Wojdyla AT. 2001. Chitosan in the control of rose diseases: 6-year-trials. Bulletin of the Polish Academy of Sciences, Series Biological Sciences 49:243-252. Disponible en línea: http://agris.fao.org/agris-search/search.do?recordID=PL2003000183 [ Links ]
Wojdyla AT. 2004. Chitosan (biochikol 020 PC) in the control of some ornamental foliage diseases. Communications in Agricultural Applied Biology Science 69:705-715. Disponible en línea: http://www.ncbi.nlm.nih.gov/pubmed/15756862 [ Links ]
Yáñez JMG, León RJF, Godoy ATP, Gastélum LR, López MM, Cruz OJE y Cervantes DL. 2012. Alternativas para el control de la cenicilla (Oidium sp.) en pepino (Cucumis sativus L.). Revista Mexicana de Ciencias Agrícolas 3:259-270. Disponible en línea: http://www.redalyc.org/articulo.oa?id=263123201004. [ Links ]
Received: September 07, 2015; Accepted: November 16, 2015











 texto em
texto em