Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista mexicana de fitopatología
versión On-line ISSN 2007-8080versión impresa ISSN 0185-3309
Rev. mex. fitopatol vol.33 no.2 Texcoco 2015
Phytopatological notes
In vitro sensitivity of two species of Sclerotinia spp. and Sclerotium cepivorum to agents of biological control and fungicides
1Cuerpo Académico de Protección Vegetal (UGTO-CA-81), Departamento de Agronomía, División de Ciencias de la Vida, Campus Irapuato-Salamanca, Universidad de Guanajuato, km 9 Carretera Irapuato-Silao, Irapuato, Gto., C. P. 36821. México.
The in vitro response of two Sclerotinia minor-, five S. sclerotiorum- and two Sclerotium cepivorum-isolates to 16 biological control agents and eight fungicides was evaluated. A split plot experimental design was used, with a factorial arrangement correction for each pathogen. Factor A corresponded to fungi isolates and Factor B to control agents. The comparison of means was carried out using a Tukey test (P<0.05), having 11 evaluations every 24 h of the Mycelial radial growth rates (Mrgr). In any case no effect was found, alone or in interactions with control agents. Dicloran, Benomyl, Tebuconazole and Cyprodinil-Fludioxonil inhibited the mycelial growth of all fungi with a final average of 1.0 Mrgr, and in greater proportion than biological agents (BA). The BA that propitiated the lesser mycelial growth towards S. minor were: Microorganisms (BPG-Plus), Trichoderma sp. (Trichoderma), T. viride (Esporalis) and Bacillus subtillis (Serenade max); with isolates of S. sclerotiorum, Trichoderma sp. (Trichoderma), Trichoderma harzianum (Natucontrol), Microorganisms (BPG-Plus) and T. harzianum (Biotricho-H), with a Mrgr of 1.48, 1.56, 2.35 and 2.53, respectively. For S. cepivorum, the best management was obtained with Trichoderma sp. (Trichoderma), T. viride (Esporalis), T. harzianum (Natucontrol) and Microorganisms (BPG-plus), with a Mrgr of 1.03, 1.73, 2.55 and 2.70, respectively. In summary, Thichoderma sp. and Microorganisms were the most consistents in their ability to inhibit the three pathogens.
Key words: Vegetables; soil borne fungi; biofungicides; chemical control
Se evaluó la respuesta in vitro de dos aislados de Sclerotinia minor, cinco de S. sclerotiorum, y dos de Sclerotium cepivorum a 16 agentes biológicos y ocho fungicidas en un diseño experimental de parcelas divididas con arreglo factorial por cada patógeno. El factor A correspondió a los aislados del hongo y el factor B a los productos de control. La comparación de medias se realizó con la prueba de Tukey (P < 0.05). Se hicieron 11 evaluaciones cada 24 horas del crecimiento promedio radial micelial (Cprm). En ningún caso se encontró efecto de aislado, solo o en interacción con los productos de control. Dicloran, Benomilo, Ciprodinilo-Fludioxonilo y Tebuconazole inhibieron el desarrollo micelial de todos los hongos en mayor proporción que los agentes biológicos (AB) con un promedio final de 1.0 Cprm. Los AB que propiciaron el menor crecimiento promedio radial micelial (Cprm, cm), en S. minor fueron Microrganismos (BPG-Plus), Trichoderma sp. (Trichoderma), T. viride (Esporalis) y Bacillus subtillis (Serenade max) con 1.2, 1.3, 1.5 y 1.9 Cprm, respectivamente. En S. sclerotiorum, Trichoderma sp. (Trichoderma), Trichoderma harzianum (Natucontrol), Microorganismos (BPG-Plus) y T. harzianum (Biotricho-H) fueron los mas inhibidores con 1.5, 1.6, 2.4 y 2.5 Cprm, respectivamente. S. cepivorum, Trichoderma sp., (Trichoderma), T. viride (Esporalis), T. harzianum (Natucontrol) y Microorganismos (BPG-plus) fueron las más sobresalientes con 1.0, 1.7, 2.6, y 2.7, respectivamente. En todos estos casos se superó estadísticamente al testigo (6.6-7.9 Cprm, p=0.05). En general, Trichoderma sp. y Microorganismos fueron los más consitentes en su capacidad de inhibición de los tres fitopatógenos.
Palabras clave: Hortalizas; hongos fitopatógenos del suelo; biofungicidas; control químico
The state of Guanajuato has an exceptional potential for horticulture; it is among the first places in the production of 70 species of agricultural importance, some of the most outstanding of which include garlic (Allium sativum L.), lettuce (Lactuca sativa L.), and potato (Solanum tuberosum L.) (SIAP, 2013).
Diseases are one of the most risk factors for the production of vegetables. In recent years, fungal diseases have caused major economic losses in the production of different vegetable species in Mexico and the rest of the workd (Fisher et al., 2012). These have increased in the productive areas of the country, with Bajio as one of the zones with the highest incidence of fungal diseases (Montes et al., 2003).
The resistance structures of some phythopathogenic soil fungi, such as sclerotia, can remain viable in the soil for over 20 years, such as in the case of S. cepivorum (Pérez et al., 2009), originating different space and time distribution patterns as a product of reincorporation of the sclerotia and remains of diseased plants due to inadequate cultural practices carried out by farmers, as well as by natural processes. In this way, the inocula of S. cepivorum Berk., S. sclerotiorum, and S. minor can be scattered in the fields and remain latent in them, making disease control more difficult (Adams and Papavizas, 1986; Ibarra et al., 2010).
Most farmers use fungicides as a disease control method, although its excessive use may lead to resistance in the pathogens, while generating toxic residues in foods and the environment, thus becoming a risk to human health (Fisher et al., 2012); this has lead to a search for new fungicides, which, in some cases, are found in other organisms (biofungicides) (Angulo et al., 2009). Biological control (BC) of phythopathogens presents itself as an efficient and affordable alternative, as well as risk-free in the face of numerous and increasing problems deriving from the excessive use agrochemicals (Agrios, 2005). Most antagonic agents used in biological control are asprophytes, due to their adaptability to the surroundings, and their high capability of competition for nutrients with other organisms (Nelson, 1991; Michel et al., 2009).
Based on the problem described above, the aim of this work was to determine the in vitro sensibility to biological control agents and isolated fungicides of S. minor, S. sclerotiorum, and S. cepivorum in plantations of garlic, bean, lettuce, potato, and radicchio.
The investigation was carried out in commercial vegetable fields in the state of Guanajuato and in the Plant Pathology Lab of the Life Sciences Division of the Irapuato-Salamanca Campus of the University of Guanajuato.
Obtaining isolates. S. minor was isolated from symptomatic lettuce (L. sativa) plants in San Miguel de Allende and Salamanca counties, Guanajuato; S. sclerotiorum was isolated from symptomatic lettuce (L. sativa) plants in Salamanca and Irapuato counties, Guanajuato; radicchio (Cichorium intybus) in Celaya municipality, Guanajuato; bean (Phaseolus vulgaris) in Guasave county, Sinaloa; and potato (S. tuberosum) in Santiago Tangamandapio municipality, Michoacán. Finally, S. cepivorum was isolated from symptomatic garlic plants (A. sativum) in Salamanca county, Guanajuato, and in Cordoba, Spain.
From each isolate, sclerotia were taken, along with part of the tissue that displayed symptoms of the disease; they were disinfected with sodium hypochlorite at 1%, and then planted in Potato Dextrose Agar (PDA) for eight days at 20±2 °C for S. minor; and at room temperature for S. sclerotiorum and S. cepivorum.
Biological control agents and fungicides. Biological control agents and fungicides evaluated (Tables 1, 2 and 3 ) were applied based on commercial recommendations.
Table 1 In vitro Mycelial radial average growth (Mrag) of two S. minor isolates obtained from lettuce (L. sativa) with regard to 24 treatments with biological control products (16) and fungicides (8).
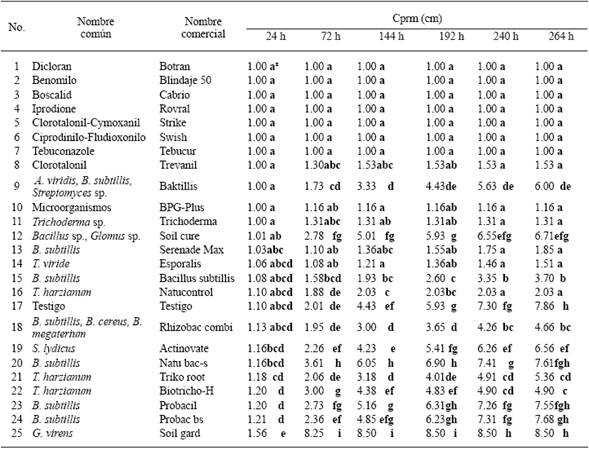
xEach value represents the average of both isolates. Values in each column with different letters are statistically different. Multiple comparison of averages DSH Tukey P≤0.05.
Table 2 In vitro Mycelial radial average growth (Mrag) of five S. sclerotiorum isolates, obtained from lettuce (L. sativa), bean (P. vulgaris), potato (S. tuberosum), and radicchio (Cichorium sp.) plants with regard to 24 treatments with biological control products (16) and fungicides (8).

xEach value represents the average of two isolates. Values in each column with different letters are statistically different. Multiple. Comparison of averages DSH Tukey P≤0.05.
Table 3 In vitro Mycelial radial average growth (Cprm) vitro of two S. cepivorum isolates obtained from plantations of garlic (A. sativum) with 24 treatments of biological products (16) and fungicides (8).
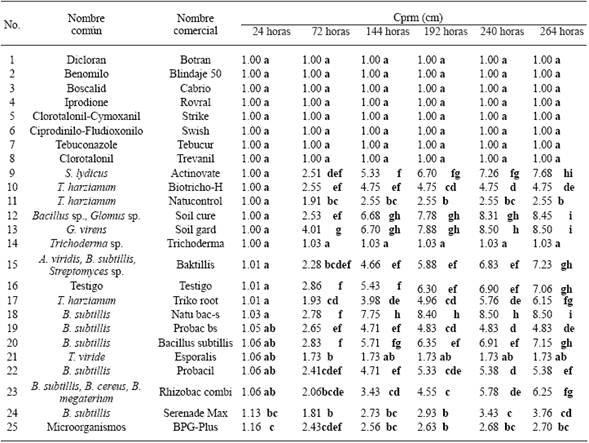
xEach value represents the average of two isolates. Values in each column with different letters are statistically different. Multiple Comparison of averages DSH Tukey P≤0.05.
In vitro sensibility and evaluation of biological products and fungicides. We weighed the commercially recommended doses of the biological products and fungicides to be evaluated and added to the agar, then poured into Petri dishes. When the agar solidified, disks, 1 cm in diameter were placed in the center of the dish from the periphery of the colonies obtained from each isolation and incubated at 20±2°C.
Evaluation of mycelium growth. The colony diameter was measured in two directions - the longest and shorteste length - and mycelial growth was obtained as the average of both values in the directions at 24, 48, 72, 96, 120, 144, 168, 192, 216, 240, and 268 hours after placing the disk with the fungi on the edge of each Petri dish in each of the three treatment repetitions (Pérez et al., 1997; Pérez et al., 2009).
Statistical analysis. In the three experiments, a split plot experimental design was used, with a factorial arrangement correction with three repetitions. In each experiment there was a factorial arrangement: factor A was assigned to fungal isolations, which had two, five, and two levels; factor B was assigned to the biological control agents and fungicides, which had 25 levels. This gave a total of 50, 125, and 50 treatments, respectively. In all three experiments, the multiple comparison of averages was carried out using Tukey's test (P≤0.05).
Sclerotinia minor. The two isolates of S. minor were sensitive to seven of the eight fungicides (Dicloran, Benomyl, Boscalid, Iprodione, Tebuconazole, Cyprodinil-Fludioxonil, and Chlorothalonil), considering this as those which did not develop a mycelial radial average growth (Mrag), 264 hours after the confrontation (Table 1). The biological control agents with the greatest fungistatic effects towards S. minor isolates, that is, those which caused the least Mrag 264 hours after confrontation, were: Microrganisms (BPG-Plus), Trichoderma sp. (Trichoderma), T. viride (Esporalis) and Bacillus subtillis (Serenade max), with 1.16a, 1.31a, 1.51a, and1.85a, respectively; in comparison to Gliocadium virens (Soil gard), B. subtillis (Probac bs), Bacillus sp.-Glomus sp. (Soil cure) and B. subtillis (Natubacs), with 8.50h, 7.68gh, 6.71efg, and 7.61fgh, respectively, which had the lowest or null fungistatic effects towards the isolates (Table 1).
Sclerotinia sclerotiorum. The five isolates sensitive to four of the eight fungicides (Dicloran, Benomyl, Tebuconazole, and Cyprodinil-Fludioxonil) i.e., those which showed no Mrag 264 hours after confrontation (Table 2). The biological control agents that had the greatest fungistatic effects towards the isolates, understood as those which caused the lowest Mrag 264 hours after confrontation were Trichoderma sp. (Trichoderma), Trichoderma harzianum (Natucontrol), Microorganismos (BPG-Plus), and T. harzianum (Biotricho-H), with 1.48abc, 1.56abc, 2.35bc, and 2.53cd, respectively, in comparison with T. harzianum (Triko root), B. subtillis (Probac bs), G. virens (Soil gard), and Streptomyces lydicus (Actinovate), with 7.12h, 6.79gh, 6.80gh, and 6.32fgh, respectively, which had the lowest or null fungistatic effects towards the S. sclerotiorum isolates (Table 2).
Sclerotium cepivorum. Both isolates were sensitive to the eight fungicides (Dicloran, Benomyl, Boscalid, Iprodione, Chlorothalonil-Cymoxanil, Cyprodinil-Fludioxonil, Tebuconazole, and Clorotalonil), therefore they did not present Mrag 264 hours after confrontation (Table 3). The biological control agents that had a greater fungistatic effect on the S. cepivorum, which means that they caused the least Mrag 264 hours after confrontation, were Trichoderma sp., (Trichoderma), T. viride (Esporalis), T. harzianum (Natucontrol), and Microorganisms (BPG-plus), with 1.03a, 1.73ab, 2.55b, and 2.70bc, respectively; in comparison with G. virens (Soil gard), Bacillus sp.-Glomus sp. (Soil cure), and B. subtillis (Natubacs), with 8.50i, 8.45i, and 8.50i, respectively, which led to a lower or null fungistatic effect on the isolates (Table 3).
A uniform behavior was found in S. cepivorum towards fungicides Tebuconazole (Tebucur), Iprodione (Rovral), Dicloran (Botran), Benomyl (Blindaje 50), Boscalid (Cabrio), Clorotalonil (Trevanil), Clorotalonil+Cymoxanil (Strike), and Ciprodinilo+Fludioxonilo (Swish). Contrasting results were found in the behavior of S. cepivorum towards the fungicides reported by Pérez et al. (1997) and Pérez et al. (2009), who showed the presence of variability of the pathogen towards the fungicides used. In relation to the response of S. cepivorum towards the biological control agents evaluated, a wide variability was found, observing that the biological products to which there was a higher sensitivity to in vitro were those formed with Trichoderma spp.; the cause of this greater benefitial effect may be the biocontrolling action of Trichoderma spp., in which there have been several forms of action described that regulate the development of phythopathogenic fungi. Among these, the main ones are the competition for space and nutrients, microparasitism and antibiosis, which have a direct action on the phythopathogenic fungi (Infante et al., 2009); it is also known that Trichoderma spp. displays other mechanisms, the bioregulatory action of which is indirect, such as the biochemical defense mechanisms, that consist in the deactivation of enzymes of the phythopathogenic fungi during the infection process (Infante et al., 2009); this could explain the high inhibitory percentage of mycelial growth of S. cepivorum. This suggests an alternative for the control of white rotting, as pointed out by Ulacio et al. (2011). The use of T. harzianum as an alternative of biological control in soils that display high densities of S. cepivorum inocula, requires high doses of the antagonist combined with other alternatives to achieve an efficient control of the disease (Delgadillo et al., 2002; Ulacio et al., 2011). A uniform response was found in S. minor and S. sclerotiorum towards most fungicides and isolates evaluated. The isolates of radicchio from Celaya and lettuce from Irapuato grew in the presence of some fungicides, in comparison to the other isolates of both fungi that did not, probably due to the treatments undergone by the fields the isolates were taken from, since these lands are treated with biological controllers, and this may have caused the isolates to have a more vigorous mycelial growth with some of the fungicides evaluated. Something that was unexpected in this study was that the isolates of S. sclerotiorum provenientes from Celaya and from Irapuato grew in the presence of Boscalid (Cabrio), which is a fungicide that belongs to the group of Carboxanilides, and Iprodione (Rovral), which belongs to the Dicarboximide, in which there was Mrag between 24 and 264 hours after confrontation; this is probably related, among other things, to the history of the fields the S. sclerotiorum isolates were obtained from, regarding the continuous use of fungicides for the control of root diseases in different crops (Pérez et al., 2009). The insensitivity to Boscalid and Iprodione obtained in this study contrasts with the sensitivity reported by Tarazona (2009), who found that the Boscalid and Iprodione inhibited fungal growth by 100 %. The sensitivity presented by isolates S. minor and S. sclerotiorum towards Tebuconazole, which brought about an inhibitory effect of 100 %, coincides with reports by Pérez et al. (1997), who claim these were similar with S. cepivorum. The biological control agents which caused the most inhibition of mycelial growth in S. minor and S. sclerotiorum were those that contained the fungus Trichoderma spp. The resuls obtained in this study coincide with those by Villalta et al. (2012), who report a strain of T. hamatum with a high percentage of inhibition of the growth of S. minor. In the case of B. subtillis (Serenade max), there were no favorable effects on S. minor and S. sclerotiorum, in comparison with the favorable effect obtained with Trichoderma spp., since B. subtillis (Serenade max) was one of the biological control agents with the lowest fungistatic effect on the S. minor and S. sclerotiorum isolates. These results contrast with those reported by Tarazona (2009), who points out that B. subtillis had a greater controlling effect towards the phythopathogenic fungi analyzed.
REFERENCES
Adams PB and Papavizas GC. 1986. Effect of inoculum density of Sclerotium cepivorum and some soil environmental factors on disease severity. Phytopathology 61:1253-1256. [ Links ]
Agrios GN. 2005. Plant Pathology. Fifth Edition. Elsevier Academic. University of Florida. Florida United States of America. 948p. [ Links ]
Angulo EMA, Armenta RE, García ERS, Carrillo FJA, Salazar VE y Valdez TJB. 2009. Extractos de Semilla de Swietenia humilis Zucc., con Actividad Antifúngica en Rhizopus stolonifer (Ehrenb.:Fr) Vuill. Revista Mexicana de Fitopatología 27:84-92. [ Links ]
Delgadillo SF, Zavaleta ME, Osada KS, Arévalo VA, González HVA, Nieto AD y Torres PI. 2002. Densidad de inóculo de Sclerotium cepivorum Berk. y su control mediante tebuconazole en ajo (Allium sativum L.). Revista Fitotecnia Mexicana 25:349-354. [ Links ]
Fisher MC, Henk, DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL and Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186-194. doi:10.1038/nature10947 [ Links ]
Infante D, Martínez B, González N, y Reyes Y. 2009. Mecanismos de acción de Trichoderma frente a hongos fitopatógenos. Revista Protección Vegetal 24 (1):14-21. [ Links ]
Ibarra MVA, Ferrera CR, Alarcón A, Lara HME, Valdez CJM. 2010. Isolation and screening of Trichoderma strains antagonistic to Sclerotinia sclerotiorum and Sclerotinia minor. Revista Mexicana de Micología 31:53-63. [ Links ]
Michel A AC, Otero S MA, Solano P LY, Ariza F R, Barrios AA y Rebolledo M A. 2009. Biocontrol in vitro con Trichoderma spp; de Fusarium subglutinans (Wollenweb. y Reinking) Nelson, Toussoun y Marasas y F. oxysporum Schlecht., agentes causales de la "escoba de bruja" del mango (Mangifera indica L.). Revista Mexicana de Fitopatología 27:18-26. [ Links ]
Montes BBR, Nava JRA, Flores MHE y Mundo OM. 2003. Hongos y nematodos en raíces y bulbos de cebolla (Allium cepa L.) en el estado de Morelos, México. Revista Mexicana de Fitopatología 21:300-303. [ Links ]
Nelson EB. 1991. Handbook of Applied Mycology. pp. 327-355. : Arora DK, Rai B, Mukerji KG, and Knudsen GR (eds.). Current limits to biological control of fungal phytopathogens. Volume 1: Soil and Plants. Marcel Dekker, Inc. New York, USA. 800p. [ Links ]
Pérez ML, Olalde PV, Sánchez PJR, Castañeda CC y Entwistle AR. 1997. Sensibilidad in vitro de aislados del hongo Sclerotium cepivorum Berk., a los fungicidas comúnmente usados para su combate. Revista Mexicana de Fitopatología 15:9-14. [ Links ]
Pérez ML, Villalpando MJJ, Castañeda CCy Ramírez MR. 2009. Sensibilidad in vitro de Sclerotium rolfsii Saccardo, a los fungicidas comúnmente usados para su combate. Revista Mexicana de Fitopatología 27:11-17. [ Links ]
SIAP, Servicio de Información Agroalimentaria y Pesquera. 2013. Avance de siembras y cosechas. Resumen Nacional por producto, Otoño -Invierno 2012 de riego y temporal. http://www.siap.gob.mx/index (consulta, julio 2014). [ Links ]
Tarazona MLS. 2009. Control biológico y químico de Sclerotinia sclerotiorum (Lib.) de Bary en alcachofa (Cynara scolymus L.). Tesis de Maestría, Especialidad de Fitopatología. Universidad Nacional Agraria La Molina. Perú. 98p. [ Links ]
Ulacio D, Jiménez MA y Perdomo W. 2011. Estrategias de manejo integrado de Sclerotium cepivorum Berk., y la pudrición blanca del ajo en Carache, estado Trujillo, Venezuela. Bioagro 23:105-114. [ Links ]
Villalta ON, Wite D, Hunt J, Stewart A and Porter IJ. 2012. Biological control of sclerotinia minor on lettuce using trichoderma and coniothyrium species. Acta Horticulturae 944:51-58. [ Links ]
Received: June 22, 2015; Accepted: July 09, 2015











 texto en
texto en 


