Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.33 n.2 Texcoco 2015
Scientific articles
Actinoycetes isolated from compost and antagonistic activity against potato phytopathogens ( Solanum tuberosum spp. andigena Hawkes)
1Laboratorio de Ecología Microbiana, Facultad de Ciencias Biológicas - Universidad Nacional Mayor de San Marcos, Av. Venezuela s/n, Ciudad Universitaria, Lima - Perú.
One way of controlling phytopathogens is through the use antagonistic microorganisms. Compost is a source of microorganisms capable of producing secondary metabolites of agricultural interest. We isolated 85 actinomycetes in vitro cultures and evaluated their antagonist capacity against phytopathogens affecting Solanum tuberosum. Of the isolates, 23.5 % showed antagonistic activity against Ralstonia solanacearum, 16.4 % against Pectobacterium carotovorum, 43.5 % against Phytophthora infestans, 25.8 % against Fusarium sp., and 61.1% against Rhizoctonia solani. Organic extracts obtained from the selected strains (AACI-5, ACZI-22, ACZII-35, ACZIII-84 y ACZIII-88) with ethyl acetate and dichloromethane showed antimicrobial activity. The most important was the ethyl acetate extract from the AACI-5 strain, which had a broader spectrum of activity and showed a minimum inhibitory concentration (MIC) against Phytophthora infestans of 0.0625 mg.mL-1. The most active strains were identified as members of the genus Streptomyces. Due to the antagonist capacity showed by actinomycetes in this work, they could be considered as potential candidates for controlling potato pathogens under the framework of sustainable agriculture.
Key words: Streptomyces ; bioactive compounds; soil microorganisms; potato pathogens; antagonism
Una de las formas de control de fitopatógenos es a través del uso de microorganismos antagonistas. El compost, un producto orgánico es fuente de microorganismos capaces de producir metabolitos secundarios de interés agrícola. Se aislaron y evaluaron mediante cultivos in vitro la capacidad antagonista de 85 actinomicetos frente a fitopatógenos que afectan a Solanum tuberosum. De los aislados, 23.5% tuvieron actividad antagonista a Ralstonia solanacearum, 16.4% a Pectobacterium carotovorum, 43.5% a Phytophthora infestans, 25.8% a Fusarium sp y 61.1% a Rhizoctonia solani. Extractos orgánicos obtenidos con acetato de etilo y diclorometano de las cepas seleccionadas (AACI-5, ACZI-22, ACZII-35, ACZIII-84 y ACZIII-88) mostraron actividad antimicrobiana, siendo el de mayor importancia el extracto de acetato de etilo de la cepa AACI-5 por tener mayor espectro de actividad y presentar una Concentración Mínima Inhibitoria (CMI) de 0.0625 mg.mL-1 frente a Phytophthora infestans. Las cepas con mayor actividad se identificaron como miembros del género Streptomyces. Los actinomicetos por su capacidad antagonista mostrada en el presente trabajo podrían ser considerados potenciales candidatos en el control de patógenos de este tubérculo en el marco de una agricultura sostenible.
Palabras clave: Streptomyces ; compuestos bioactivos; microorganismos de suelos; patógenos de la papa; antagonismo
Biological control (or biocontrol) through the use of microorganisms has become an alternative for the treatment of plant pathogens; it is considered one of the most efficient and ecologically sound practices for a sustainable agriculture (Franco-Correa, 2009). Phytopathogens cause great economic losses in agriculture, reducing both the quantity and the quality of harvested products, and thus their commercial value. The control of these pathogens has usually been done with pesticides and other chemicals, which has had negative effects on the environment and the quality of life of human populations (Serrano and Galindo, 2007). The systematic application of chemicals in agriculture currently faces difficulties such as the resurgence of pests, the development of genetic resistance in microorganisms, environmental pollution and damage to human health (Pérez, 2004).
The interest in biocontrol of plant pathogens has increased recently, particularly as a response to consumer concerns over the use of agrochemicals. Currently, biocontrol is already considered as one of the standard management practices of plant diseases caused mainly by pathogens of the genera Rhizoctonia, Sclerotium, Pythium, Phytophthora and Fusarium (Serrano and Galindo, 2007). In the control of these pathogens, it is important to select potential antagonists that can be used as biological controls, for which it is necessary, initially, to perform in vitro assays that provide information about their antagonist capacity in order to use them later in the field (Gohel et al., 2006).
Among the microorganisms useful for biocontrol of phytopathogenic bacteria are Streptomyces, Pseudomonas, Agrobacterium and Bacillus (Whipps, 2001). These microorganisms are found in fertile soils; the most prolific group are the actinomycetes, aerobic Gram positive bacteria that are the main degraders of organic matter in the soil, with a capacity to produce antimicrobial compounds, terpenoids, pigments and extracellular enzymes (Ezziyyani et al., 2004). They represent about 20-60 % of the microbial population of fertile soil; their main activities are to fix nitrogen, solubilize phosphates, promote plant growth and produce a series of secondary metabolites such as vitamins, enzymes and other beneficial compounds for plants (Bobadilla and Rincon, 2008). In addition, actinomycetes help improve soil fertility and produce bioactive compounds that allow them to serve directly or indirectly as biocontrol organisms of potential pathogens (Franco-Correa, 2008).
Actinomycetes are characterized by producing enzymes with antimicrobial activity, including the biocontrol of phytopathogens (Crawford et al., 1993; Park et al., 2002). Strains of Streptomyces sp. have shown an ability to suppress in vitro growth of pathogens, and, therefore, many of their metabolites have been proposed to inhibit the growth of these pathogens in vivo (Franco-Correa, 2009). Extracellular enzymes such as chitinases and β-1,3- glucanases play a significant role in antifungal and biocontrol activity, and are responsible for the mycoparasitism of certain strains of Streptomyces and the suppression of plant diseases (Goodfellow and Williams, 1983; Gonzales et al., 2003).
The potato (Solanum tuberosum L.) is the fourth most important crop worldwide, with an annual output of 328,865,936 MT (FAO, 2008). The potato crop of Peru is the most important in the Andean region, with a cultivated surface of 249,000 ha. Potato production in Peru increased from 3.8 million MT in 2004 to 4.5 million MT in 2013, an increase of 45 % and an average annual growth rate of 3.8 % (INEI, 2014). However, the cultivation of potatoes is affected by various diseases; among the most important are those caused by fungi and bacteria (Farfan and Gutierrez, 2009). The "late blight", caused by Chromista Phytophthora infestans, has been regarded as the major pathogen worldwide (Arios, 2005). Rhizoctonia solani Kühn, which causes rhizoctoniasis or black scurf (due to the formation of sclerotia) and stem blight (due to the necrotic lesions on the stems), is a fungal pathogen present in all potato producing areas of the world (Torres, 2002). Several species of Fusarium are cited as responsible for the "dry rot". The bacterium Ralstonia solanacearum, which causes "bacterial wilt" (Yabuuchi et al., 1995), has a great impact on potato crops in cold climates, although its optimum growth temperature is 28 °C (He et al., 1983). The bacteria Pectobacterium carotovorum subsp. atrosepticum, P. carotovorum subsp. caratovorum (previously Erwinia carotovora) and P. chrysanthemi, cause the "soft rot" of potato tubers in both the field and in the store, as well as the "stem rot" or "blackleg" in growing plants (Elphinstone, 1987).
Compost is a highly humified organic fertilizer, rich in nutrients and a source of a variety of aerobic microorganisms, including actinomycetes, which develop in response to different levels of temperature, humidity, oxygen and pH (Arslan et al., 2008). This research seeks to select strains of actinomycetes isolated from compost with potential inhibitory activity against pathogens affecting the potato crop (Solanum tuberosum L) and that could be subsequently used as biocontrol agents in the field.
Materials and Methods
Compost samples. We collected 10 samples of compost (an average of 100 g of sample) from the facilities of the land property known as "Casa Blanca" located at 47 km from Lima, Peru. Sampling was conducted under sterile conditions; the samples were placed in single-use containers with a tight lid. The samples were then transported to the laboratory of Microbial Ecology at the Universidad Nacional Mayor de San Marcos for immediate processing.
Pathogens of Solanum tuberosum. The pathogenic strains were provided by the International Potato Center (CIP) in Lima, Peru. These included bacterial cultures of Ralstonia solanacearum (strain 204 Biovar 2A, isolated from Solanum tuberosum; Huambos, Cajamarca, Peru) and Pectobacterium carotovorum subsp. atrosepticum (isolated from Solanum tuberosum; Chiras, Comas, Peru), as well as the fungi Rhizoctonia solani (isolated from Solanum tuberosum; Huancayo, Peru), Fusarium sp., (isolated from Solanum tuberosum; Huancayo, Peru) and Chromista Phytophthora infestans (isolated from Solanum tuberosum L. var canchan; Serrana, Oxapampa, Peru).
Isolation of Actinomycetes from compost. To isolate the actinomycetes, the compost samples were diluted one-tenth in saline 0.85 % (w/v) to 10-6, and 0.1 mL of each dilution was seeded by spreading it on Agar Czapek Dox, pH 7.2, and Agar starch Casein, pH 7.2 (Gurung et al., 2009), adding in both cases nystatin (Merck) (50μg.mL-1) and cycloheximide (Merck) (50 μg.mL-1). The cultures were incubated at 28 °C for 15 d. The actinomycetes colonies were collected and stored refrigerated in vials containing agar Czapek Dox plus glycerol (20 % v/v), according to Leon et al.(2007).
Selection of actinomycetes with antagonistic activity against plant pathogenic bacteria. This was performed by the modified "second layer method" (Dopazo et al., 1988). The actinomycetes were seeded as macro-colonies on Agar Czapek Dox (pH 7.2) and incubated at 28 °C for 7 d. The phytopathogenic bacteria were reactivated in nutrient broth plus 1% glucose (w/v), pH 7.0, and then inoculated (10µL) (0.5 McFarland scale) into 3 mL of liquefied semisolid agar, tempered at 50 °C and then added quickly on the actinomycetes cultures. The cultures were incubated at their respective growth temperatures (R. solanacearum at 30 °C for 48 h and P. carotovorum at 26 °C for 48 h); afterwards, we evaluated the size (in mm) of the inhibition zones (mm diameter). A diameter ≥ 5 mm was considered positive.
Selection of actinomycetes with antagonistic activity against chromista and fungi. This test was performed by the coculture method indicated by Taechowisan et al. (2005). Rhizoctonia solani and Fusarium sp. were reactivated and seeded on potato dextrose agar (PDA; Merck), while the oomycete Phytophthora infestans was seeded on rye agar; all cases were incubated at 28 °C for 7 d. The actinomycetes were seeded on PDA at one end of the plates. The pathogen cultures were cut into small pieces (0.5 cm per side) and placed at the opposite end from each actinomycete culture. This was incubated at 28 °C for 4-5 d; we then proceeded to assess the extent of the growth distance (mm) between the actinomycete and the phytopathogen. A distance ≥ 5 mm was considered positive.
Microscopic and macroscopic analysis of selected actinomycetes. This was done using microcultures of actinomycetes previously selected for their higher antagonistic activity. We used the "method of agar blocks" according to Holt et al. (1994), with incubation in a moist chamber at 28 °C for 5-7 d. The morphological characteristics of the mycelial and sporulation structure were observed using a compound microscope (Nikon) with immersion objective (100X) on slides prepared with Lactophenol blue (Merck). For the macroscopic evaluation, we considered the phenotypic characteristics of the colonies, such as color, shape, size, elevation, texture and diffusible pigment.
Obtaining the crude extract of antimicrobial compound. This was done according to the method of Pandey et al. (2004) modified for soil actinomycetes. Selected actinomycetes were inoculated in 500 mL Erlenmeyer flasks with 150 mL of the yeast extract-glucose medium (Sultan et al., 2002). The cultures were incubated at 28 °C for 10 d with constant stirring of 250 rpm, and then centrifuged at 4,000 rpm for 25 min. The recovery of antimicrobial metabolites from the supernatant was carried out by extraction with ethyl acetate and dichloromethane (Merck) (1:1, v/v), and vigorous stirring for 1 h (Liu et al., 1986). The crude extract was concentrated and separated using a rotary evaporator (Buchi R-3000) at 40 °C; the residue was then weighed and reconstituted with dimethyl sulfoxide (DMSO; 10%) for storage and later analysis.
Determining the antimicrobial activity of the crude extract. Volumes of 50 µL of crude extract recovered and reconstituted (4 mg/mL) were placed in wells (5mm) bored in Nutrient Agar medium plus 1 % glucose, where the control phytopathogenic bacteria had been previously seeded. The cultures were incubated at 30 °C for 48 h (R. solanacearum) and at 26 °C for 48 h (P. carotovorum). The medium used in the tests with phytopathogenic fungi was PDA, with incubation at 28° C for 5 d. In all cases, the antimicrobial activity was determined by measuring the size of the inhibition zone (mm in diameter) according to Pandey et al. (2004).
Minimum Inhibitory Concentration (MIC) of crude extract. This was performed according to Caviedes et al. (2002) in 96-well microplates (Pure Grade-Merck). The tests were performed by spreading the dilutions of the crude extracts of the selected actinomycetes on the nutrient broth plus 1 % glucose, with double concentration of the bacteria and the yeast extract broth plus glucose, and double concentration also of the fungi and the oomycete P. infestans. The volume of the phytopathogenic strain added to each well was 20 µL. This was incubated for 48 h in the case of bacteria and for 5 d in the case of fungi and chromista. After this time, 50 µL of tetrazolium (Sigma) were added to all wells, which were then incubated for 24 h. A color change from yellow to purple was interpreted as bacterial growth.
Results and Discussion
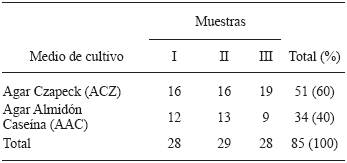
Isolation of Actinomycetes from compost. A total of 85 strains of actinomycetes were isolated from compost piles from the land property "Casa Blanca". Of these isolates, 51 were recovered from Czapek Dox agar (CDA) and 34 from starch casein agar (SCA). The isolates stored for further study were labelled according to their growth in the primary isolation medium and the sample number. In this work, the primary isolate of actinomycetes from compost was slightly higher in CDA compared to SCA (Table 1).These results are similar to those obtained by Horna (1996), although it should be noted that in that case the isolation was made from marine sediment, a completely different substrate to the one used in the present work.
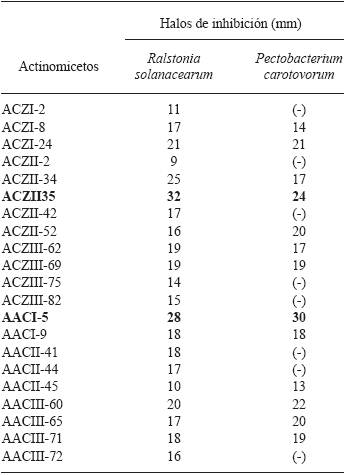
Table 1 Isolated actinomycetes from piles of compost in the final process, in the property of "Casa Blanca", located in Lurin , Lima, Peru.
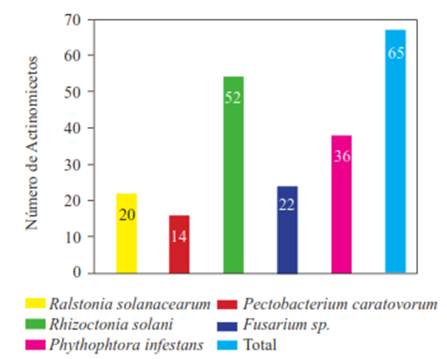
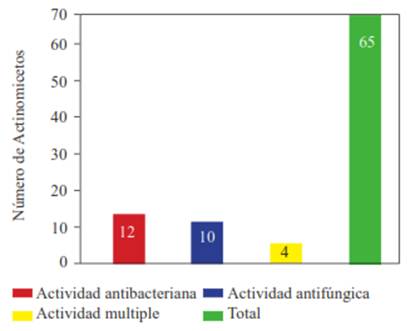
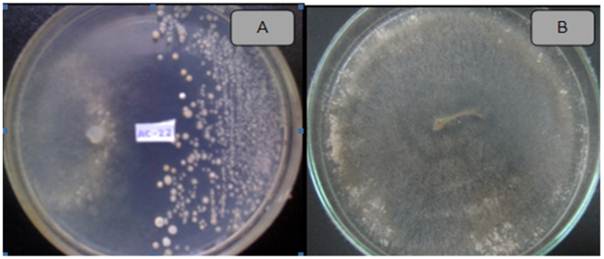
Actinomycetes antagonistic to potato pathogens. Of all the isolated actinomycetes (n=85), 65 (76.4 %) showed antagonistic activity against at least one plant pathogen in the tests, while 20 (23.5 %) did not show any. Likewise, 20 isolated actinomycetes (23.5 %) showed activity against Ralstonia solanacearum, and 14 (16.4 %) against Pectobacterium carotovorum (Figure 1). The isolate with the highest activity against R. solanacearum was the ACZII-35 strain (32 mm in diameter), while against P. carotovorum, the highest activity was shown by the AACI-5 strain (30 mm) (Table 2). In addition, the evaluation of actinomycetes from compost by their antagonism against fungi, indicated that 22 strains (25.8 %) have activity against Fusarium sp., 52 isolates (61.1 %) against Rhizoctonia solani and 36 strains (43.5 %) against Phytophthora infestans (Figure 1). Of the evaluated actinomycetes, 4 strains (4.7 %) (ACZI-24, ACZII-34, ACZII-52, ACZIII-69) showed a broad spectrum of antagonistic activity against the 5 phytopathogens, 10 strains showed antagonistic activity against 3 fungal pathogens, and 12 strains against the 2 bacterial pathogens (Table 2 and 3 ; Figure 2). The percentage of actinomycetes that inhibited at least one fungal pathogen was 72.9 %; in contrast, 43.5% inhibited chromista and 23.5 % solates with the highest inhibitory activity were ACZI-22, against R. solani (inhibition of 27 mm in distance) (Figure 3), ACZII-84 and ACZIII-88 against Fusarium sp. (18 mm in distance) (Figure 4) and AACI-5 against P. infestans (22 mm in distance) (Table 3 and Figure 5). Prapagdee et al. (2008) isolated 146 strains of actinomycetes from rhizosphere soil and confronted them with two pathogenic fungi (Colletotrichum gloeosporioides and Sclerotium rolfsii); only 10 isolates showed antagonistic capacity against both pathogens. Similarly, in the present work only 10 isolates showed such capacity against the three pathogenic fungi tested. Soil actinomycetes have proved Thus, Ningthoujam et al. (2009) evaluated 33 strains of soil actinomycetes, of which 4 showed activity against rice pathogens such as Curvularia oryzae, Pyricularia oryzae, Bipolaris oryzae and Fusarium oxysporum. Furthermore, Iznagas et al. (2004) reported that of 563 actinomycetes isolated from Cuban soils, 286 showed antifungal activity against plant pathogens. In this study, 23.5 % of the isolates showed antagonistic activity against R. solanacearum, a slightly lower percentage than that obtained by Bittencourt & Da Silva (1999), who evaluated 190 actinomycetes isolated from the soil and rhizoplane of a tomato crop, and found that 52 of them showed active against R. solanacearum. Toledo (2004) evaluated three strains of Bacillus sp. obtained from soils that showed biocontrol activity in vitro against the pathogen P. carotovorum. Likewise, Reinozo et al. (2006) isolated Bacillus sp. from soil and found 7 strains with inhibitory effect in vitro against P. carotovorum. Although bacteria of the genus Bacillus are generally use for the biocontrol of crop diseases, it is also important to note that actinomycetes play an important role in soil colonization. Oskay et al. (2004) tested 50 soil actinomycetes against plant pathogens and found 3 strains that were active against Pectobacterium amylovora, 5 against Pseudomonas viridiflava, 4 against Agrobacterium tumefaciens, and 5 against Clavibacter michiganensis. In this study, 13 actinomycetes were antagonistic against P. carotovorum, indicating that strains isolated from compost had a higher inhibitory capacity. These strains showed antibacterial and antifungal capacity.
Table 2 Size of the halos of inhibition caused by actinomycetes isolated from compost to combat pathogenic bacteria of Solanum tuberosum.
Inhibitory activity : Good ≥25mm ; Intermediate <25 and >15mm ; ≤15mm Scarce ; No activity (-).
Table 3 Actinomycete strains from compost selected for its greater antagonist activity to phytopathogenic fungi of Solanum tuberosum (Inhibition zone in mm).
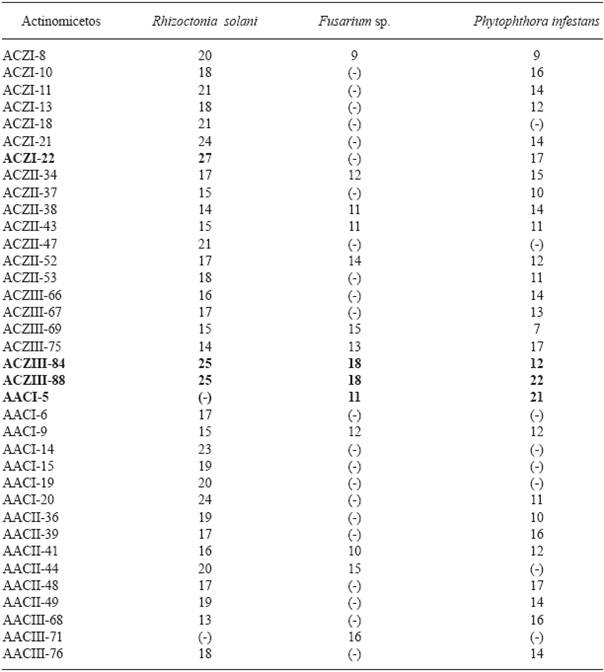
Inhibition activity: Good ≥20mm; intermediate ≤20mm >15mm; meagre <15mm; no activity (-).
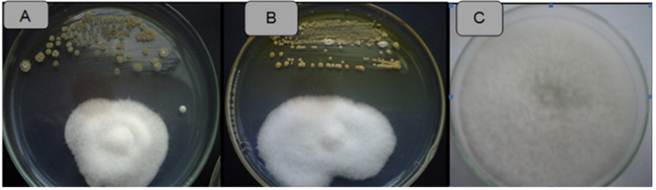
Figure 4 Antagonistic activity of Actinomycetes against Fusarium sp. Strain ACZIII-84 (A), strain ACZIII-88 (B), control (C).
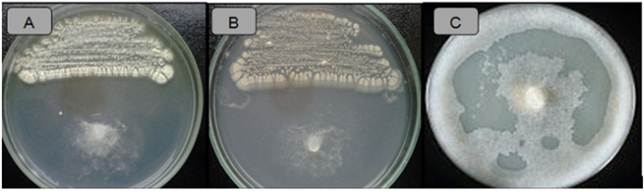
Figure 5 Antagonistic activity of Actinomycetes against Phytophthora infestans. Strain ACZIII-88 (A), strain AACI-5 (B), control (C).
Regarding Fusarium sp., this study revealed that 25.8 % of the isolates were antagonistic to the fungus, a considerably higher percentage compared to the studies by Oskay (2009), who evaluated the antifungal activity of Streptomyces isolated from soil against plant pathogens such as Penicillium sp., Alternaria alternata and Fusarium oxysporum; the latter was the least sensitive to the antagonistic activity of Streptomyces. De Boer et al. (1998) demonstrated that 12% of chitinolytic bacteria had inhibitory effects against F. oxysporum, while 56% inhibited F. culmorum. In addition, Ikeda et al. (2000) evaluated 4000 actinomycetes, of which only 27% were found to be antagonists against Fusarium sp. From these studies, it can be concluded that the susceptibility of Fusarium sp. to the active compounds of actinomycetes varies greatly.
In this study, 61.2 % of the isolates of actinomycetes showed antagonism against R. solani. Kathiresan et al. (2005) evaluated actinomycetes, though isolated from marine sediment, against different plant pathogens, reporting that 31% were effective against R. solani. That result is lower than the one found in this study; even though marine actinomycetes are consider greater producers of these metabolites. Meanwhile, Farfan and Gutierrez (2009) evaluated the antagonistic effect of 13 compost actinomycetes against Rhizoctonia sp. and Fusarium sp.; 3 of these isolates were found to have the highest percentages of inhibition against Rhizoctonia sp., and 5 against Fusarium sp.
Regarding the size of the inhibition zones, in the present study, actinomycetes inhibited up to 27 mm in distance against R. solani. Rothrock and Gottlieb (1981) reported inhibition zones of up to 15 mm, which indicates that our strains had higher antagonistic activity against this plant pathogen. Furthermore, P. infestans was inhibited by 43.5% of the actinomycete strains evaluated in this study; this value is lower compared to the results of Valois et al. (1996), who reported that Phytophthora sp. was inhibited by 72 % of the actinomycetes isolated from soils.
In the present study, Czapek agar (CZA) was used successfully in antagonism tests; however, Al-Zahrani (2007), who evaluated the antagonistic activity of Streptomyces sp. against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Salmonella typhi, Candida albicans and fungal phytopathogens such as Fusarium oxysporum and Rhizoctonia solani, reported better results in media starch casein agar media against bacteria, and in yeast-malt extract agar, Sabouraud dextrose agar and oatmeal agar against fungi. The antagonism tests against pathogenic fungi made in the current study were performed on PDA medium, which demonstrated its suitability for such tests. Other authors also used PDA in similar tests, even suggesting that it enhanced pathogen inhibition (Ezziyyani et al., 2004; Farfan and Gutiérrez, 2009). Oatmeal agar (recommended for growing Phytophthora infestans) was also used successfully in this work, unlike the findings of Duke and Quintana (2008) in similar tests of actinomycetes against F. oxysporum. These results indicate that the mode of action of the antagonists may vary according to the control pathogen. The antagonism varies not only between fungi of different genera, as is the case in this study, but also between fungi of the same genus but different species; one example is the case of De Boer et al. (1998), who reported that 12 % of chitinolytic bacteria inhibited the growth of F. oxysporum, while 56 % inhibited the growth of F. culmorum.
Macroscopic and microscopic analysis of selected strains. For the macroscopic and microscopic evaluation, we considered only the actinomycetes the showed the highest antagonism against both phytopathogenic bacteria and fungi. The selected actinomycetes were the strains ACZII-35 and AACI-5 (better antagonists against R. solanacearum and P. carotovorum, respectively), and strains ACZI-22, ACZIII-88 and ACZIII-84 (better antagonists against R. solani, Fusarium sp. and P. infestans, respectively). The results are shown in Table 4. The culture characteristics, the microscopic observations in microcultures and the culture behavior of the 5 selected strains of actinomycetes, indicated that all were potential members of the genus Streptomyces (Figure 6), but this must be confirmed by molecular testing.
Table 4 Phenotypic characteristics of Actinomycetes selected by the most inhibitory activity of pathogenic of Solanum tuberosum.

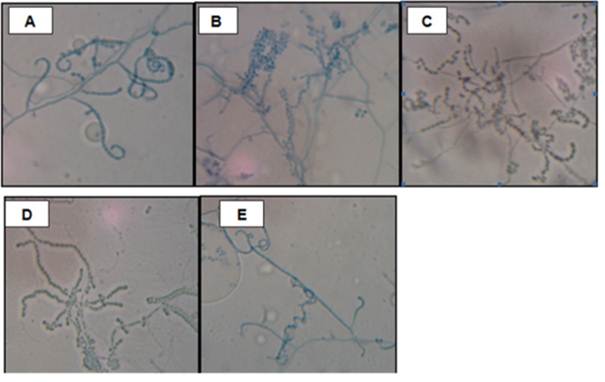
Figure 6 Microscopic observation of selected actinomycetes for the increased of antimicrobial activity against phytopathogenic of S. tuberosum. Strains AACI-5 (A); ACZI-22 (B); ACZII-35 (C); ACZIII-84 (D) y ACZIII-88 (E). Increase: 1000X.
According to the literature, the genus Streptomyces is the main responsible for the production of most bioactive metabolites. In particular, Streptomyces is part of an important group within the actinomycetes, as these are able to produce various types of secondary metabolites (Baltz, 2006).
But soil, including compost, is not only inhabited by Streptomyces; there are also other kinds of actinomycetes, as reported by Remya and Vijayakumar (2008), who in 64 colonies of actinomycetes, identified Streptomyces (30) (47 %), Galactomyces (10), Nocardiopsis (7), Nocardioides (4), Actinopolyspora (3), Nocardia (3), Kibdelosporangium (2), Actinosynnema (1), Kineosporia (1) and Saccharopolyspora (1). Similarly, Nakashima et al. (2009) isolated 800 strains of actinomycetes, and compared them with strains from the NBRC (National Biological Reserch Center); 64 of them showed similarity to 36 Streptomyces NRBC, specifically to the Streptomyces griseus group, which has activity against Kocuria rhizophila and Aspergillus oryzae, and produces compounds such as streptomycin and bafilomycin, with antibacterial and antifungal activity, respectively. Yuan and Crawford (1995) pointed to mechanisms of Streptomyces lydicus WYEC108 (isolated from the rhizosphere of plants) for controlling Pythium ultimum through antifungal compounds and extracellular chitinase, which destroy not only the spores, but also damage the hyphal cell wall; this showed that S. lydicus WYEC108 has biocontrol potential against P. ultimum.
Obtention of crude extract and antimicrobial activity. The extracts obtained after the culture with organic solvents were concentrated until obtaining the dry weight of each strain (Table 5). The extraction of active compounds was carried out using two solvents of different polarity: dichloromethane (DCM) and ethyl acetate (EtAc). We observed a slight variation in the recovery of crude extracts; in this case, the EtAC extracts showed a certain advantage over DCM extracts. Likewise, regarding the antimicrobial performance, the extract recovered with EtAc had better inhibitory activity than the DCM extract. Leiva et al. (2004) selected 13 strains out of a total of 31 actinomycetes in order to obtain extracts using 5 solvents of increasing polarity; these were petroleum ether, chloroform, ethyl methyl ketone, ethyl acetate and butanol; the most active extracts were those obtained with ethyl methyl ketone and ethyl acetate. In addition, Remya and Vijayakumar (2008) used extracts obtained with ethyl acetate, methanol, chloroform and ethanol against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Salmonella typhi, Cryptococcus neoformans and Candida albicans; the ethyl acetate extract showed the greatest inhibition capacity. Other authors such as Dhanasekaran et al. (2005) obtained different results with extracts obtained with aniline, chloroform, pyridine and ethyl acetate against E. coli, K. pneumoniae, Ps. aeruginosa, V. cholerae, S. typhi, Sh. dysenteriae, S. aureus, P. mirabilis and B. subtilis, but the ethyl acetate extracts had the best results. Narayana et al. (2008) separated 4 bioactive fractions from the crude ethyl acetate extract of Streptomyces sp. and confronted them with phytopathogenic fungi; Fusarium udum and Penicillium citrinum showed susceptibility to the 4 fractions, while Fusarium oxysporum showed resistance to one of the fractions. In this respect, our results confirm the EtAC as one of the best solvents for recovering bioactive metabolites from actinomycetes. The antimicrobial activity of the crude extracts obtained with the two solvents was evident both in bacterial and fungal pathogens of potato. Some results of these tests can be seen in Figure 7. Regarding the antimicrobial activity of actinomycetes, there are several studies that indicate its presence during the final exponential and stationary phases. Prapagdee et al. (2008) isolated S. hygroscopicus, which produces potent inhibitory compounds of plant pathogens; these compounds are an extracellular chitinase and a β-1,3-glucanase produced during the exponential and late exponential phases, respectively. According to Joo (2005), Streptomyces halstedii AJ-7 produces a cellular chitinase that causes abnormal morphologies in fungal hyphae, growth aberrations, hyphal swelling and cell wall lysis. Farfan and Gutierrez (2009) note that there may be synergy between chitinase-type enzymes and other antifungal metabolites in the inhibition process of fungal pathogens.
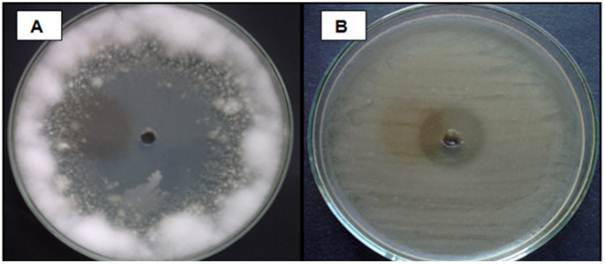
Figure 7 Inhibitory activity of the extract obtained with Ethyl Acetate from the strain ACZIII-88 compared to a Fusarium sp., (A) and strain ACZII-35 compared to Pectobacterium carotovorum (B).
Minimal Inhibitory Concentration (MIC) of extracts of actinomycetes against plant pathogens. The results of this test are show in Table 6. We used extracts of AACI-5 and ACZII-35 to determine the MIC of P. carotovorum and R. solanacearum. The initial concentration of the EtAC and DCM extracts was 2 mg.mL-1. Overall, we can say that the DCM extract of the ACZII-35 strain had a better performance, reaching a MIC of 0.125 mg.mL-1 against R. solanacearum, while the EtAC extract of the AACI-5 strain reached a MIC of 0.2 mg.mL-1 against P. carotovorum. To determine the MIC of Fusarium sp., we chose the extracts of the strains ACZIII-84 and ACZIII-88, with an initial concentration of 2 mg.mL-1. Both extracts showed low performance (Table 6). For the MIC of R. solani, we used the extracts of ACZI-22 and ACZIII-88 with an initial concentration of 2 mg.mL-1. The results indicate that inhibiting this pathogen required 0.125 mg.mL-1 of the DCM extract of the ACZIII-88 strain. The other extracts achieved poor results. Finally, the extracts of AACI-5 and ACZIII-88 were used to determine the MIC of P. infestans; both extracts had an initial concentration of 4 mg.mL-1. In this case, the extracts showed good performance at fairly low concentrations; only 0.0625 mg.mL-1 (62.5 µg.mL-1) of the EtCA extract of the AACI-5 strain was required to inhibit the growth of the oomycete P. infestans. Similar studies to this one were conducted by Narayana et al. (2008), who determined the MIC of 4 fractions of the bioactive compound of Streptomyces sp., ANU 6277, against F. udum, Penicillium citrinum and F. oxysporum, all of which were susceptible to the AF3 fraction at concentrations of 2, 5 and 10 μg.mL-1, respectively. Taechowisan et al. (2005) determined the MICs of two fractions, (i) and (ii), of the bioactive compound of Streptomyces aureofaciens, CMUAc 130, against Colletotrichum musae; the values were 120 and 150 μg.mL-1, respectively.
Table 6 Minimum Inhibitory Concentration (mg.ml-1) of crude extracts of actinomycetes.
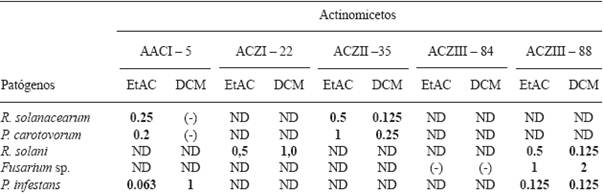
EtAC (Ethyl acetate); DCM (dichloromethane); (-) Negative; ND: undetermined.
Although in this study the MIC was evaluated from crude extract containing the bioactive compound, other authors, such as Taechowisan et al. (2005) and Narayana et al. (2008), evaluated the MIC from purified fractions, and thus the inhibitory activity was more efficient. Preliminary in vitro assays allow to know if the microorganisms are good antagonists, in order to evaluate their possible use in in vivo assays, as proposed by Bittencourt et al. (1998), who evaluated 190 actinomycetes isolated from rhizosphere of tomato against Ralstonia solanacearum, of which 18 were 100% effective; the best inoculation pathways were seeds and roots. Tuomi et al. (2001) applied antagonistic microorganisms through an irrigation system to control important pathogens such as F. oxysporum, Botrytis cinerea and Alternaria brassicicola.
Conclusion
This study demonstrates that actinomycete strains isolated from compost have significant antagonistic capacity against important fungal and bacterial phytopathogens of Solanum tuberosum spp. andigena. This allows us to affirm that they are possible candidates for use in biological control programs in the production of this crop as part of sustainable agriculture practices.
Acknowledgements
The authors thank the Vicerrectorado de Investigación (VRI) of the Universidad Nacional Mayor de San Marcos (UNMSM), Lima, Peru for funding through the program of work Undergraduate Thesis, 2010 (RR No. 01746-R-10).
REFERENCES
AL-Zaharani S. 2007. Studies on the antimicrobial activity of Streptomyces sp. isolated from Japan. Science Journal of King Abdulaziz University 19(1): 127-138. [ Links ]
Arios GN. 2005. Plant Pathology. Fifth Edition. Academic Press, New York, USA. 992p. [ Links ]
Arslan E, Oberk E, Kirbag R, Ipek U and TOPAL M. 2008. Determination of the effect of compost on soil microorganisms. International Journal of Science and Technology 3(2): 151-159. [ Links ]
Baltz R. 2006. Marcel Faber Roundtable: is our antibiotic pipeline unproductive because of starvation, constipation or lack of inspiration? Journal Indian Microbiology Biotechnology 33: 507-513. [ Links ]
Bittencourt A y Da Silva R. 1999. Avaliação in vitro de actinomicetos como antagonistas a Ralstonia solanacearum. Ciencia y Agrotecnología Lavras 23(2): 281-288. [ Links ]
Bittencourt A, Da Silva Rand Prata M. 1998. Bioensaio para avaliação massal de actinomicetos antagonistas a Ralstonia solanacearum, em tomateiro. Pesqueria Agropecuaria Brasilia 33 (12): 2065-2072. [ Links ]
Bobadilla C y Rincón S. 2008. Aislamiento y evaluación de bacterias fosfato solubilizadoras a partir de compost obtenido de residuos "plaza". Tesis de Grado, Pontificia Universidad Javeriana, Facultad de Ciencias, Bogotá, Colombia. [ Links ]
Caviedes L. Delgado J and Gilman R. 2002. Tetrazolium microplate assay as a rapid and inexpensive colorimetric method for determination of antibiotic susceptibility of Mycobacterium tuberculosis. American Society for Microbiology 40(5): 1873-1874. [ Links ]
Crowford D, Lynch J, Whipps J and Ousley M. 1993. Isolation and characterization of actinomycete antagonists of a fungal root pathogens. Applied and Environmental Microbiology 59: 3899-3905. [ Links ]
De Boer B, Gunnewiek K, Lafeber P, Janse J, Spit B and Woldendorp J. 1998. Antifungal properties of dune soil bacteria. Soil Biology and Biochemestry 30: 193-203. [ Links ]
Dhanasekaran D, Rajakumar G, Sivamani P, Selvamani R, Panneerselvam A and Thajuddin N. 2005. Screening of Salt Pans actinomycetes for antibacterial agents. The Internet Journal of Microbiology 1(2): 6-12. [ Links ]
Dopazo C, Lemos M, Lodeiros C, Bolinches J, Barja J and Toranzo A. 1988. Inhibitory activity of antibiotic producing marine bacteria against fish pathogens. Journal Applied Bacteriology 65(2): 97-101. [ Links ]
Duque C and Quintana M. 2008. Determinación preliminar de la actividad promotora de crecimiento vegetal de actinomicetos aislados del suelo. Tesis de Grado. Pontificia Universidad Javeriana, Bogotá, Colombia. [ Links ]
Elphinstone J. 1987. La pudrición blanda y la pierna negra de la papa. Erwinia sp. Boletín de información técnica 21. Lima: Centro Internacional de la Papa, 18p. [ Links ]
Ezziyyani M, Perez C, Requena M, Rubio L y Candela M. 2004. Biocontrol por Streptomyces rochei - ziyani, de la podredumbre del pimiento (Capsicum annum L.) causada por Phytophthora capsici. Anales de Biología 26: 69-78. [ Links ]
FAO (Food and Agricultural Organization). 2008. Material de propagación de calidad declarada. Estudio FAO. Producción y Protección Vegetal 195. p79. http://www.fao.org/news/archive/news-by-date/2014/en/ [ Links ]
Farfán D y Gutierrez C. 2009. Determinación de la actividad quitinolítica de cepas nativas de actinomicetos y su efecto antagónico sobre microorganismos fitopatógenos. Tesis de Grado, Pontificia Universidad Javeriana, Facultad de Ciencias, Bogotá, Colombia. [ Links ]
Franco-Correa M. 2008. Evaluación de caracteres PGPR en actinomicetos e interacciones de estas rizobacterias con hongos formadores de microrrizas. Tesis para optar al Grado de Doctor. Universidad de Granada, Facultad de Ciencias, Granada-España. [ Links ]
Franco-Correa M. 2009. Utilización de los actinomicetos en procesos de biofertilización. Revista Peruana de Biología 16(2): 239-242. [ Links ]
Gohel V, Singh A, Vimal M, Ashwini P and Chhatpar H. 2006. Bioprospecting and antifungal potential of chitinolytic microorganism. African Journal of Biotechnology 5: 54-72. [ Links ]
Gonzalez-Franco A, Deobald L, Spiak A and Crawford D. 2003. Actinobacterial chitinase-like enzymes: profiles of rhizosphere versus non-rhizosphere isolates. Canadian Journal of Microbiology 49: 683-698. [ Links ]
Goodfellow M and Williams S. 1983. Ecology of Actinomycetes. Annual Reviews Microbiology 37: 189-216. [ Links ]
Gurung T, Sherpa C, Prasad V and Lekhak B. 2009. Isolation and characterization of antibacterial actinomycetes from soil samples of Kalapatthar, Mount Everest Region. Nepal Journal of Science and Technology 10: 173-182. [ Links ]
He L, Sequeira L and Kelman A. 1983. Characteristics of strains of Pseudomonas solanacearum from China. Plant Disease 67:1357-1361. [ Links ]
Holt J, Krieg N, Sneath P, Staley J and Williams S. 1994. Bergey´s Manual of Determinative Bacteriology. 9ª ed. Maryland, Baltimore: Williams & Wilkins.. p 800. [ Links ]
Horna-Agueda J. 1996. Aislamiento, identificación y evaluación de la actividad antimicrobiana de Actinomicetos nativos de sedimentos marinos de Ancón y Pisco. Tesis de Grado, Facultad de Ciencias Biológicas, UNMSM, Lima-Perú. [ Links ]
Ikeda J, Saito H, Sato S and Kodama T. 2000. Growth-inhibition effect of Streptomyces sp. 39L40C to mulberry twig blight pathogen Fusarium lateritium. The Journal of Sericultural Science of Japan 69: 163-168. [ Links ]
INEI (Instituto Nacional de Estadística e Informática). 2014. Nota de prensa: Producción de papa creció en 45%. Informe de la Presidencia del Consejo de Ministros, Perú. http://www.inei.gob.pe/prensa/noticias/produccion-de-papa-crecio-45-7582/ [ Links ]
Iznaga Y, Lemus M, Gonzales L, Garmendia L, Nadal L and Vallin C. 2004. Antifungal activity of actinomycetes from Cuban soils. Phytopherapy Reserch 18: 494-496. [ Links ]
Joo G. 2005. Production of an anti-fungal substance for biological control of Phytophthora capsici causing phytophthora blight in red-peppers by Streptomyces haltedii. Biotechnology 27: 201-205. [ Links ]
Kathiresan K, Balagurunathan R and Masilamani M. 2005. Fungicidal activity of marine actinomycetes against Phytopathogenic fungi. Indian Journal of Biotechnology 4: 271-276. [ Links ]
Leiva S, Yañez M, Zaror L, Rodriguez H y García H. 2004. Actividad antimicrobiana de Actinomycetes aislados desde ambientes acuáticos del Sur de Chile. Revista Médica de Chile 132: 151-159. [ Links ]
León J, Liza L, Cuadra D, Patiño L y Zerpa R. 2007. Actinomycetes bioactivos de sedimento marino de la costa central del Perú. Revista Peruana Biología 14(2): 259-270. [ Links ]
Liu C, Westley J, Herman T, Prasser B, Palleroni N, Evans R and Miller P. 1986. Novel polyether antibiotics, X-14873 A, G and H produced by Streptomyces. Taxonomy of the producing culture, fermentation, biological and ionophores properties of the antibiotics. Journal of Antibiotics 39(12): 1712-1718. [ Links ]
Nakashima T, Anzai K, Suzuki R, Kuwahar N, Takeshita S, Kanamoto A and Ando K. 2009. Productivity of bioactive compounds in Streptomyces species isolated from Nagasaki marine environments. The Society for Actinomycetes Japan 23(1): 16-20. [ Links ]
Narayana K, Prabhakar P, Vijayalakshmi M, Venkateswarlu Y and Krishna S. 2008. Study on bioactive compounds from Streptomyces sp. ANU 6277. Polish Journal of Microbiology 57(1): 35-39. [ Links ]
Ningthoujam D, Sanasam S, Tamreihao K, Nimaichand S. 2009. Antagonistic activities of local Actinomycetes isolates against rice fungal pathogens. African Journal of Microbiology Research 3(11): 737-742. [ Links ]
Oskay M, Usame A and Azeri C. 2004. Antibacterial activity of some actinomycetes isolated from farming soils of Turkey. African Journal of Biotechnology 3(9): 441-446. [ Links ]
Oskay M. 2009. Antifungal and antibacterial compounds from Streptomyces strains. African Journal of Biotechnology 8(13): 3007-3017. [ Links ]
Pandey B, Ghimire P and Agarwal V. 2004. Studies on the antibacterial activity of the Actinomycetes isolated from the Khumbu Region of Nepal. Journal Biology Science 23: 44-53. [ Links ]
Park J, El-Tarabily K, Ghisalberti E and Sivasithamparam K. 2002. Pathogenesis of Streptoverticillium albireticuli on caenorhabdtis elegans and its antagonism to soilborne fungal pathogens. Applied Microbiology 35: 361-365. [ Links ]
Pérez N. 2004. Control biológico de patógenos vegetales. Manejo Ecológico de Plagas 7: 231-247. [ Links ]
Prapagdee B, Kuekulvong C and Mongkolsuk S. 2008. Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. International Journal of Biological Sciences 4: 330-337. [ Links ]
Reinozo Y, Casadeus L, García A, Gutierrez J y Pazos V. 2006. Aislamiento, selección e identificación de bacterias del género Bacillus antagonistas de Pectobacterium carotovorumFitosanidad 10(3): 187-191. [ Links ]
Remya M and Vijayakumar R. 2008. Isolation and characterization of marine antagonistic Actinomycetes from West Coast of India. Medicine and Biology 15(1): 13-19. [ Links ]
Rothrock C and Gottlieb D. 1981. Importance of antibiotics production antagonism of selected Streptomyces strains to two soilborne plant pathogens. The Journal of Antibiotics 34:830-835. [ Links ]
Serrano L y Galindo E. 2007. Control biológico de organismos fitopatógenos: un reto multidisciplinario. Revista de la Academia Mexicana de Ciencias 58(1): 77-88. [ Links ]
Sultan M, Khatune N, Sathi Z, Bluiyan S, Choudury M, Sadik G, Gafur M and Rahman A. 2002. In vitro antibacterial activity of an active metabolite isolated from Streptomyces species. Biotechnology Research 1(2): 100-106. [ Links ]
Taechowisan T, Lu C and Shen Y. 2005. Secundary metabolites from endophytic Streptomyces aureofaciens CMUAc 130 and their antifungal activity. Microbiology Reserch 151: 1691-1695. [ Links ]
Toledo D. 2004. Evaluación in vitro del efecto de cepas nativas de la bacteria Bacillus sp. en el biocontrol de la bacteria Erwinia carotovora. Tesis de Grado. Universidad de Talca. Chile. [ Links ]
Torres H. 2002. Manual de enfermedades más importantes de la papa en el Perú. Centro Internacional de la Papa. Lima, Perú, p 59. [ Links ]
Tuomi T, Heino M, Nordstrom K and Laakso S. 2001. Fiber fractions from processing of barley in production and conservation of a biology control agent. Applied Biochemical Biotechnology 94(2): 135-145. [ Links ]
Valois D, Fayad K, Barasubiye T, Garon M, Dery C, Brzezinski R, Singh P, Shin Y, Park C and Chung Y. 1996. Biological control of Fusarium wilt of Cucumber by chinolytic bacteria. Phytopathology 89(1): 92-99. [ Links ]
Whipps J. 2001. Microbial interaction and biocontrol in the rhizosphere. Journal of Experimental Botany 52(1): 487-511. [ Links ]
Yabuuchi E, Kosato Y, Yano I, Hotta H and Nishiuchi Y. 1995. Transfer of Two Burkholderia and Alcaligenes species to Ralstonia gen. nov.: Proposal of Ralstonia picketii (Ralton, Palleronii and Douderoff 1973) comb. nov., Ralstonia solanacearum (Smith 1986) comb.nov.and Ralstonia eutropha (Davis 1969). Microbiology and Inmunology 39(11): 897-904. [ Links ]
Yuan W and. Crawford D 1995. Characterization of Streptomyces lydicus WYEC108. As a potential biocontrol agent against fungal root and seed rots. Applied Environment Microbiology 61(8): 3119-3128. [ Links ]
Received: November 05, 2014; Accepted: June 11, 2015











 text in
text in