Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.33 n.1 Texcoco 2015
Scientific articles
Etiology and evaluation of control alternatives for wilt in chile de arbol (Capsicum annuum L.) in La Vega, Metzitlán, Hidalgo, México
1Programa de Postgrado en Fitosanidad-Fitopatología y Colegio de Postgraduados-Campus Montecillo. Km. 36.5 Carretera México-Texcoco. CP 56230, Montecillo, Texcoco, Estado de México. México.
2Programa de Postgrado en Recursos Genéticos y Productividad, Colegio de Postgraduados-Campus Montecillo. Km. 36.5 Carretera México-Texcoco. CP 56230, Montecillo, Texcoco, Estado de México. México.
Wilt and death of chile de árbol plants in "La Vega de Metztiltán", Hidalgo (VMH) are causing important yield losses in the region. In this work, the etiology of this disease was determined and immediate available control alternatives were evaluated (resistance of ten chile de árbol cultivars and efficiency of chemical and biological products available in the national market) as the first step to the integrated management of the problem. Fusarium oxysporum and Rhizoctonia solani were isolated from diseased plants; however, only Phytophthora capsici duplicated the symptoms of the disease. The CP 1261, CP 1264 and CP 1305 cultivars showed the highest resistance levels to the disease. The repeated application of metalaxyl, fosetyl-aluminum or propamocarb, metalaxyl alternated with fosetyl-aluminum or with propamocarb, exerted total wilt control. Even though metam sodium controlled the disease in the majority of the cases, it proved to be phytotoxic. Among the natural alternatives, Baktillis (Bacillus subtilis), administered on its own or as the combination of Fus Out (Trichoderma harzianum) with Probac (Bacillus subtilis), both reduced the severity of the disease, but did not prevent the infection by the pathogen.
Key words: Resistance; chemical control; biological control of Phytophthora capsici
La marchitez y muerte de plantas de chile de árbol en la Vega de Metztitlán, Hidalgo (VMH), está causando grandes pérdidas de rendimiento en la región. En el presente trabajo se determinó la etiología de esta enfermedad y se evaluaron alternativas de control de disponibilidad inmediata (resistencia de diez cultivares de chile de árbol y eficacia de productos químicos y biológicos disponibles en el mercado nacional), como un primer paso hacia el manejo integrado del problema. Aunque Fusarium oxysporum y Rhizoctonia solani fueron también aislados de plantas enfermas, sólo Phytophthora capsici reprodujo los síntomas de la enfermedad. Los cultivares CP 1261, CP 1264 y CP 1305 mostraron los mayores niveles de resistencia a la enfermedad. Aplicaciones recurrentes de me talaxil, fosetil aluminio o propamocarb, metalaxil alternado con fosetil aluminio o con propamocarb tuvieron un control total de la marchitez. El metam sodio aunque controló la enfermedad en la mayoría de los casos, resultó fitotóxico. De los biológicos, Baktillis (Bacillus subtilis) aplicado sólo o la combinación de Fus Out (Trichoderma harzianum) con Probac (Bacillus subtilis) redujeron la severidad de la enfermedad, pero no impidieron la infección por el patógeno.
Palabras clave: Resistencia; control químico; control biológico de Phytophthora capsici
The chile de árbol (Capsicum annuum L) is a recently introduced species in Vega, Metztitlán, Hidalgo (VMH), where it is affected by wilt and plant death problems, the incidence-severity of which is increasingly higher, to the point of having caused a yield loss of up to 100 %. To this date, there are no published reports about the etiology of this disease in the region, or the strategies for its management. Farmer of the region indiscriminately use chemical products that have been suggested by the local pesticide sellers; in most cases these are unhelpful and result in the abandonment of the crops. The wilt and death of chili plants are generally associated with the root fungal infection of Pythium, Phytophthora, Rhizoctonia and Fusarium genera (Velásquez et al., 2001). Even though cultural, genetic, chemical and biological control alternatives have been tried, the success in the management of the disease is usually variable, depending on the conditions of the crop, cultivar genotype and the pathogens involved and the characteristics of the soil, among other factors (Ristaino and Johnston, 1999; Granke et al., 2012), which makes it necessary to define specific control measures for each region. Once the etiology of a plant health problem has been determined, it is necessary to access the most easily available control resources while an integral management program is designed, in order to offer immediate alternatives to the producers. Generally, the first choice resource is the evaluation of the resistance or tolerance of crop genotypes and of the available and authorized chemical and biological commercial products in the market. In this work, in addition to determining the etiology of the causative agent of wilt and death of the chile in VMH, an evaluation was performed, in VMH soil, of the wilt resistance of chile de árbol cultivars collected from different parts of the Mexican Republic and of the efficiency of the chemical and biological products available in the national market, as well as of different alternation patterns focused on the reduction of resistance risks.
Materials and Methods
This investigation was carried out under greenhouse conditions, with the objective of reducing the problems associated with the variability of the soil and the spatial distribution of the pathogen, frequently associated to soil pathogens (Larkin et al., 1995; Gumpertz et al., 1997), particularly when a high number of treatments are being handled.
Isolation, identification and pathogenicity tests of the organisms associated with chile wilt. Rhizospheric soil and chile de árbol plants (Capsicum annuum L) with wilt symptoms were collected from three locations in VMH. The stems and roots were superficially disinfected according to the description of Silva-Rojas et al. (2009) and González-Pérez et al. (2004). They were sectioned in 1 cm segments to be cultivated in potato dextrose agar (PDA) and were incubated at room temperature (25°C). The resulting fungal isolates, or oomycetes, were purified in PDA or V8 medium, according to the specific case. The genus identification was done based on Barnett and Hunter (1998) and Erwin and Ribeiro (1996) keys. On a species level, Fusarium was identified through Booth's (1977) taxonomic keys and Rhizoctonia based on Sneh et al. (1991). For Phytophthora, the Gallegly and Hong (2008) keys were used. The reproductive keys were measured using a digital camera (Motic 2300, USA) connected to a Dell computer.
The pathogenicity tests were done in a growth chamber with controlled temperature and light using chile de árbol seeds from the state of Jalisco. The seed were germinated in Petri dishes with filter paper and distilled water. Once the seeds germinated, they were transferred to Polyurethane trays with 200 cavities with sterile substrate made of peat and agrolite (1:1). Seedlings with 3 to 4 pairs of real leaves were transplanted to 1 L polyurethane vessels with previously sterilized soil from VMH. The pathogenicity test was performed twice. In each test, four plants were inoculated for each fungal or oomycetes genus, with a non-inoculated control in each case.
For the Fusarium isolates, 1 ml of the conidial suspension was administered to the stem of each plant (Silva-Rojas et al., 2009) with 1 x 106 spores/plant. In the case of Rhizoctonia sp., a suspension of mycelium was inoculated (Singh et al., 2002; Pineda et al., 2005) having 1x105 colony forming units/plant. In the case of Phytophthora sp., 1x105 zoospores/plant (Morán- Bañuelos et al., 2010) were inoculated. In each experimental unit the presence or absence of symptoms was evaluated. The evaluations were done every 2 days during 30 days and the causative agent was isolated once again in order to comply with Kock's postulates (Agrios, 2005).
The identification of the isolated organisms was confirmed through the sequencing of fragments of the ITS region, amplified by means of the PCR technique. The extraction of DNA was done according to Sambrook and Russell (2001). For the PCR test, 2.5 µL of reaction buffer were used, 1.25 µL MgCL2, 0.5 µL dNTP´s, 1 µL of primer ITS4, 1 µL of primer ITS5, 0.5µL of Taq DNApol, 2 µL of DNA and 16.25 µL of water for injection (White et al., 1990). The primers were synthetized in the Instituto de Biotecnología of the UNAM (IBT-UNAM). The amplification was done in a Techme (r) thermal cycler, model TC-512, according to the procedure described by Silva-Rojas et al. (2009). In order to verify the product of the amplification, electrophoresis was carried out at 90 V for 30 minutes in the agarose gel at 1 % with 1 µL of ethidium bromide. The sequencing was carried out by the Macrogen Company (Korea). The sequence obtained was aligned with those entered into the database of the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Resistance evaluation of chile de árbol cultivars to Phytophthora. Due to the fact that only P. capsici caused wilt and plant death symptoms in the pathogenicity tests, the resistance to this pathogen was evaluated in ten chile de árbol accessions collected from the states of Jalisco, Nayarit and Puebla, cultivated in natural soil and in substrate made of peat-agrolite (1:1). The seed was superficially disinfected through immersion in a solution of sodium hypochlorite at 1.5 % (V/V) and two subsequent rinses with distilled water. Seedlings from each accession with 3 or 4 pairs of real leaves were transplanted to 250 mL pots with peat-agrolite (1:1) or non-sterilized soil from VMH, La Paila locality, and were kept in a greenhouse at the Colegio de Postgraduados, Campus Montecillo. The production of the inoculum and inoculation was done through the same procedure described for the pathogenicity tests. The inoculation was done 72 hours after the transplant. Before the inoculation, saturated irrigation was applied in order to create optimal conditions for the infection by P. capsici. A re-inoculation was done 15 days after the transplant in order to increase the possibility of infection (Andrés et al., 2005; Morán-Bañuelos et al., 2010). A completely random design was used with four repetitions of each treatment. The experimental unit was comprised of two plants for repetition. The experiment was performed twice. The severity of the disease was evaluated every three days according to the Sanogo scale (2006), and from this information the area under the severity progress curve was calculated (Campbell and Madden, 1990). The dry weight of the aerial parts of the plants was registered after subjecting them to desiccation at 70 °C during 72 hours. The analysis of variance was performed through the ANOVA procedure of the SAS (Statistical Analysis System) v 9.3 program. Each experiment was analyzed separately. The comparisons among averages were done according to the Tukey test (Steel et al., 1997).
Effectiveness of chemical products available in the Mexican market for the control of wilt caused by P. capsici. Chile de árbol seeds from the state of Jalisco were used. A seedling with 5 to 8 pairs of real leaves was transplanted to 250 mL pots with non-treated soil from the localities of San Pedro Tlatemalco, Tres Cruces and la Paila, VMH, where chile de árbol is usually cultivated. The pots were placed in a greenhouse at the Colegio de Postgraduados, Campus Montecillo. Before the transplant, 1 x 105 encysted zoospores were mixed in the soil of each pot.
The following chemical products were evaluated: metalaxyl at 45.28 % (Ridomild Gold 480 SL, soluble concentrate, Syngenta) 1.5 L∙ha-1; fosetyl aluminum 80 % (Aliette wdg, dispersible granules, Bayer Crop science) 2.5 kg∙ha-1; propamocarb 64 % (Previcur N, aqueous solution, Bayer Crop Science) 1.5 L∙ha-1 and metam sodium 42.5 % (Lucafum, aqueous solution, Lucava chemical) 0.1 L∙L-1, for 43 hours and 45 days aeration. Parallel to these treatments, three control were evaluated: 1) plants without fungicides and without inoculation, cultivated in sterilized soil from each VMH location; 2) non-inoculated cultivated plants in non-sterilized soil; and 3) inoculated plants (1 x 105 zoospores/plant) in sterilized soil from each location. The evaluated combinations of chemical products were defined based on a factorial arrangement 2 x 2 x 2 x 2 (with and without) of the metam sodium, metalaxyl, fosetyl aluminum and propamocarb fungicides. According to the resulting combinations, simple, double, triple and quadruple applications of fungicide were done in an alternated manner. In this manner, a total of 18 treatments, including the control, was evaluated. The first application of metalaxyl, fosetyl aluminum or propamocarb was administered to the substrate in the greenhouse stage (1 to 30 days after cultivation). Subsequently, metalaxyl and fosetyl aluminum were applied every 8 days and propamocarb every 10 days. The products were only administered once more when they were alternated, according to the corresponding order of each. In order to determine the quantity of applied product in each pot, the quantity of necessary water to irrigate them was calculated and the volume for one hectare was estimated considering a population density of 20,000 plants (Soria 1993). The plants were watered in an alternated manner with water and nutritive solution (630 g of Nitrofoska 12-12-12 in 20 L of water: dilution 1:10 (Nitrofoska; water)) (Villar-Luna et al., 2009).
The experiment had an entirely random experimental design, with four repetitions and was performed twice. The same variables of the resistance experiment to P. capsici were evaluated and the same statistical analysis approach was applied.
Effectiveness of biological products available in the Mexican market for the control of P. capsici. The genotype used, the handling of the seeds and seedlings, the procedure of soil inoculation, control nutrition, dose calculation methods, experimental design, evaluated variables and statistical analysis methodologies in this part of the work were the same as those described for the chemical control experiments. The only four biological products available in the national market were evaluated: Natucontrol(r) (Trichoderma harzianum) at 400 g∙ha-1; Fus out(r) (T. harzianum) at 1 L∙ha-1; Baktillis(r) (Bacillus subtilis) at 1 L∙ha-1; Probac BS 10(r) (B. subtilis) at 1 L∙ha-1. Regarding treatments, the individual evaluation of the four products was included. In addition, the combination of Natucontrol or Fus out mixed with Baktillis or with Probac was compared with the objective of using a mixture of organisms with different activity, colonization strategies, and suppression mechanisms that could cause a synergistic effect (Raupach and Kloepper, 1998; Mathre et al., 1999; Akgül and Mirik, 2008). The first application was done 48 hours prior to the transplant; the second one was done at the moment of the transplant, and the third one 15 days after. 48 hours after the transplant, 1 x 105 zoospores of P. capsici were applied to each pot. All treatments were applied under a factorial design (8x3) combined with soil from San Pedro Tlatemalco, Tres Cruces and La Paila, of VMH. The experiment was done twice.
Results
Identification and pathogenicity tests. From the roots and stems of the chile de árbol, nine strains from the Fusarium genus, three from the Rhizoctonia genus and one from the Phytophthora genus were isolated. Their correspondence to the Fusarium oxysporum Schlechtend.:Fr., Rhizoctonia solani Kühn and Phytophthora capsici Leonian species was determined through morphological and molecular analyses. The latter had a homology of 100 % with the KJ855326 strain of the GenBank, National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov/Blast.cgi), which corresponds to the same species. From these pathogens, only P. capsici caused yellowing, wilt, and necrosis of the stems and the death of the plant, within 7 days after inoculation, in both bioassays conducted.
Resistance evaluation of chile de árbol cultivars to Phytophthora capsici. According to the analysis of variance of the data from both experiments, the area under the severity progress curve (AUSPC) in collections of chile inoculated with the oomycete varied according to the type of cultivar (P<0.0001) and substrate (P<0.0001); there was also a highly significant interaction between the cultivar and the substrate (P<0.0001). The AUSPC was higher when the plants were cultivated in peat-agrolite compared to those cultivated in non-treated soil from VMH. The CP 1264 and 1261 accesions had the smallest AUSPC in peat-agrolite, according to the experiment, and in normal soil they had even smaller values (Figure 1). The CP 1305, CP Compuesto Jalisco 1, CP Compuesto Jalisco 2, CP 1306, CP 1261 and CP 1205 cultivars had average values of around 80 to 100 units in peat-agrolite, but once they were cultivated in soil the AUSPC decreased to values of around 40 units (Figure 1A). In the case of CP Compuesto Nayarit, CP Compuesto Puebla 1 and CP Compuesto Puebla 2 cultivars the AUSPC was consistently higher than the rest of the collections, regardless of the substrate in which the plants were cultivated. The AUSPC values in the non-inoculated plants were zero in all cases, with the exception of CP Compuesto Puebla 2, cultivated in soil, which was the only one that showed symptoms of disease under these conditions but with values that were very close to zero.

Figure 1 Area under the curve of the severity progress of wilt of different accesions of chile de árbol (Capsicum annuum) inoculated with a strain of Phytophthora capsici isolated from chile de árbol in soil from Vega de Metztitlán, Hidalgo (VMH). Plants cultivated in sterile peat-agrolite, 1:1 and plants cultivated in non-treated soil collected in VMH. Experiment 1(A) and 2 (B). The numbers in axis X indicate the cultivar: 1) CP 1305, 2) CP Compuesto Nayarit, 3) CP Compuesto Puebla 1, 4) CP Compuesto Puebla 2, 5) CP Compuesto Jalisco 1, 6) CP Compuesto Jalisco 2, 7) CP 1306, 8) CP 1261, 9) CP 1264, 10) CP 1265. Each value is the average of four repetitions. Means with the same letter are statistically equal (Tukey α = 0.05).
In experiment 1, the analysis of variance indicates that the dry weight of the plants varied only in relation to the type of substrate (P<0.0001) and the genotype (P= 0.034), but there was no interaction effect between both factors (P= 0.48). The non-inoculated plants in VMH soil, produced an average of 0.44 g/plant, while those cultivated without inoculation in peat-agrolite produced 0.2 g/plant. In the case of the inoculated plants the values were 0.12 and 0.09 g/plant, respectively and were statistically different (Tukey α=0.05) compared to the non-inoculated plants. In the case of the cultivars the values varied from 0.33 g/plant, in the CP 1306 cultivar, to 0.15 g/plant in the Compuesto Puebla 1 cultivar. The differences between these two cultivars were the only significant ones (Tukey α=0.05). The remaining genotypes had intermediate and statistically equal values to both averages.
In experiment 2, a similar pattern in the accumulation of dry matter in the aerial part was observed in the majority of the cases, with the exception of collections: CP 1305, CP 1261 and Compuesto Jalisco 1 that showed the highest values of their inoculation groups (Figure 2).

Figure 2 Experiment 2. Dry weight of the aerial part of different accesions of chile de árbol (Capsicum annuum) cultivated in peat-agrolite, 1:1 and in soil from Vega Metztitlán, Hidalgo (VMH), with and without inoculation with Phytophthora capsici, isolated from chile de árbol plants from VMH. The numbers in axis X indicate the collections: 1) CP 1305, 2) CP Compuesto Nayarit, 3) CP Compuesto Puebla 1, 4) CP Compuesto Puebla 2, 5) CP Compuesto Jalisco 1, 6) CP Compuesto Jalisco 2, 7) CP 1306, 8) CP 1261, 9) CP 1264, 10) CP 1265. Average of four repetitions. Means with the same letter are statistically equal (Tukey α = 0.05).
Effectiveness of chemical products available in the Mexican market for the control of wilt caused by P. capsici. The results of the analysis of variance of the AUSPC indicate that the plants cultivated in the three soils from VMH (San Pedro Tlatemalco, La Paila and Tres Cruces) there was a significant interaction between the four chemical products evaluated (P<0.0001). The inoculated plants that were not treated with chemical products had considerably higher AUSPC values than the rest of the treatments, while the plants cultivated in non-inoculated soil did not show disease symptoms, regardless of the sterilization or lack thereof of the soil. Metalaxyl, fosetyl-aluminum or propamocarb, applied alone, and the alternation of metalaxyl with fosetyl-aluminum or with propamocarb, presented a total disease control in both experiments carried out (Figure 3). The remaining treatments showed variations in the level of disease control, with total control cases as well as low levels of AUSPC, that varied between 0 and 125 units (Figure 3). The alternated applications with three or four products did not present a total control of the disease in all cases, but the maximum areas calculated did not exceed 90 units.
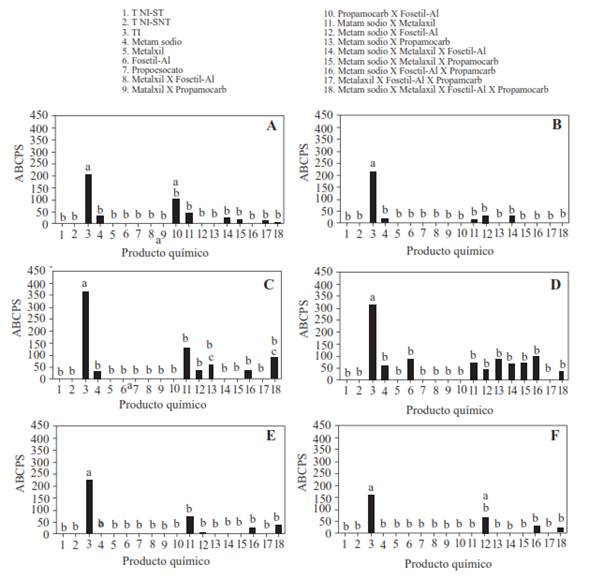
Figure 3 Area under the curve of severity progress of the wilt of chile de árbol (Capsicum annuum L) of plants cultivated in soils with and without inoculation with Phytophthora capsici and treated with metam sodium, metalaxyl, propamocarb and fosetyl aluminum alone or alternated. Experiment 1 (A) and 2(B) in soils from San Pedro Tlatemalco de la Vega de Meztitlán, Hidalgo (VMH). Experiment 1(C) and 2 (D) in soil from Tres Cruces, VMH. Experiment 1 (E) and 2 (F) in soil from la Paila. The strain of P. capsici was isolated from the chile de árbol plant from VMH. The inoculation was done with 105 zoospores per plant. Averages of 4 repetitions. TNI-S: Non-inoculated control sterilized soil; T NI-SNT: Non-inoculated control solution- non-sterilized soil; ICS: Inoculated control, sterilized soil. Means with the same letter are statistically equal (Tukey α = 0.05).
When comparing the effect of the soil on the AUSPC, it can be observed in Figure 3 that in the soil from Tres Cruces there were more symptoms of the disease than in the soils from San Pedro Tlatemalco and La Paila, as well as a decreased consistency in the reproducibility of the results among experiments; however, even in this scenario, the recurring treatments of metalaxyl or propamocarb, or the alternated application of metalaxyl with fosetyl aluminum or with propamocarb and propamocarb with fosetyl aluminum, presented a total control of the disease in both experiments.
According to the analysis of variance, in experiment 1 with soil from San Pedro Tlatemalco, only the applications of metam sodium alternated with metalaxyl had a significant effect (P=0.037) over the dry weight of the aerial part (DWAP). In this experiment, when the metalaxyl was applied alone, the DWAP was slightly higher (1.24 g/plant) than in the non-treated plants (1.05 g/plant); when metam sodium was applied alone or alternated with metalaxyl the DWAP was considerably lower (0.36 and 0.23 g/plant) than in the two previous treatments. In experiment 2, only the metam sodium interactions X fosetyl aluminum (P= 0.01) and metalaxyl X fosetyl aluminum X propamocarb (P= 0.05) were significant. With the application of fosetyl-aluminum, a higher DWAP than that of the inoculated control and without chemical treatment (1.56 vs 0.98 g/plant) was obtained; when metam sodium alone or combined with fosetyl aluminum was applied, the DWAP was reduced to 0.26 and 0.32 g/plant, respectively. Conversely, when metalaxyl alternated with fosetyl-aluminum and propamocarb was applied, almost three times the value of DWAP (1.28 g/plant) compared to that of the inoculated control (0.45 g/plant) was obtained. The administration of any of the three products in an individual manner or in simple alternation, even though it incremented the DWAP in comparison to the control, produced inferior values to the treatment with the three alternated fungicides, but which were considerably higher than the inoculated control (0.64 to 1 g/plant).In Tres Cruces soil, the analysis of variance of the DWAP in experiment 1 reported significant interaction among the four chemical products (P= 0.012), while in experiment 2 only the metam sodium X metalaxyl interaction was significant (P=0.004). In experiment 1, the DWAP of the cultivated plants in non-sterilized and non-inoculated plants (1.12 g/plant) was significantly lower than that of the non-inoculated plants that were cultivated in sterilized soil (1.93 g/plant) and their average and range of variation were similar to those observed with the recurring treatments of metalaxyl, fosetyl aluminum or propamocarb alone, or metalaxyl alternated with fosetyl aluminum or with propamocarb, or propamocarb alternated with fosetyl aluminum (1.25, 1.63, 1.01, 0.9, 0.92 and 1.04 g/plant, respectively) because their variation ranges overlapped (data not shown). The cultivated plants in non-sterilized but inoculated soil with the oomycete had a considerably lower DWAP (0.26 g/plant) than that in the non-inoculated and non-sterilized, inoculated control that were treated with the aforementioned chemicals. When fumigating with metam sodium alone or when alternating this product with the remaining chemicals, the DWAP was even lower (0.039 to 0.25 g/plant) than the one observed in the control without any chemical treatment. In the alternated treatment with metalaxyl, fosetyl- aluminum and propamocarb, twice the DWAP was produced (0.73 g/plant) than in the inoculated control and in the treatments where metam sodium was involved. Similarly, in experiment 2, when metam sodium was applied there was only one significant decrease in the DWAP (0.24 g/plant) compared to the inoculated control (0.72 g/plant); when applying metalaxyl alone, the average of this variable numerically exceeded that of the inoculated control (1.05 g/plant) and when both products were applied to the same plants the DWAP was 0.17 g/plant but was statistically equal to that observed for metam sodium alone.
In La Paila soil, VMH, no significant treatment effects were detected on the DWAP in the plants of experiment 1, but in experiment 2 a main significant effect of the recurring application of metalaxyl alone (P= 0.0025) was detected, or of the interaction metalaxyl X fosetyl aluminum (P= 0.0011). In this case, the plants treated with metalaxyl alone had a lower DWAP (0.5 g/plant) than the inoculated control (1.45 g/plant) or than that of the fosetyl aluminum alone treatments (1g/plant) or combined with metalaxyl (1.05/plant), but the variation ranges of the four averages had considerable overlaps (data not shown).
Effectiveness of biological products available in the Mexican market for the control of P. capsici. The results of the analysis of variance of the two experiments indicate that only the evaluated biological products had significant differences in the AUSPC (P<0.0001). The origin of the soil had no significance by itself or when interacting with the biological products. In both experiments the inoculated plants without any biological product application had higher AUSPC values than the rest of the treatments and the cultivated plants in non-inoculated soil had a value of zero (Figure 4A and B ). With the exception of Natu Control with Probac treatment, in experiment 2, the Tukey test (α=0.05) indicates significant differences between the biological treatments and the inoculated control without any control measures. All plants treated with some biological product showed an average low AUSPC value, without statistical differences among them as well as in regard to the non-inoculated control. The lowest AUSPC value was observed in the combination of Fus out with Probac (22.5 units) in experiment 1 and Baktillis (12.9 units) in experiment 2. The rest of the treatments showed variations in the disease level but they did not exceed 80 units in either of the experiments, while the inoculated control reached around 200.
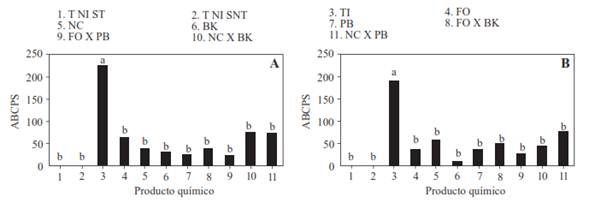
Figure 4 Area under the curve of severity progress of the wilt of chile de árbol (Capsicum annuum L.) of plants cultivated in soils with and without inoculation with Phytophthora capsici and treated with FO= Fus out(r) (Trichoderma harzianum), NC= Natucontrol(r) (Trichoderma harzianum), BK=Baktillis(r) (Bacillus subtilis) or PB=Probac(r) (Bacillus subtilis) alone or combined. Experiment 1 (A) and 2 (B). NICS-SS: Non-inoculated control sterilized soil; T NI-SNT: Non-inoculated control solution- non-sterilized soil; TI: Inoculated control solution, sterilized soil. The strain of P. capsici was isolated from chile de árbol plants from VMH. The inoculation was done with 105 zoospores per plant. Means with the same letter are statistically equal (Tukey α=0.05).
The results of the DWAP analysis of variance in experiments 1 and 2 indicated only significant differences in the effect of biological products (P= 0.0005 and P < 0.0001, respectively). In experiment 1, the plants cultivated in non-inoculated soil had the highest DWAP, while the plants inoculated with P. capsici, without control measure, had the lowest value (Figure 5A). Among the biological products, the treatments with Fus out plus Baktillis and Natu Control plus Baktillis had statistically equal weights as the ones observed in the non-inoculated control in sterilized soil, but their differences were not significant with regard to the rest of the treatments. The non-inoculated control in non-sterilized soil had the same behavior. The rest of the treatments with any biological product had dry weights that were statistically equal between them and with regard to the inoculated control solution.

Figure 5 Effect of the biological products on the dry weight of the aerial part of the chile de árbol plant (Capsicum annuum L.) cultivated in soils with and without inoculation with Phytophthora capsici and treated with FO= Fus out(r) (Trichoderma harzianum), NC= Natucontrol(r) (Trichoderma harzianum), BK=Baktillis(r) (Bacillus subtilis) or PB=Probac(r) (Bacillus subtilis) alone or combined in experiment 1 (A) and 2 (B). T NI-ST: Non-inoculated control- sterilized soil; T NI-SNT: Non-inoculated control- non-sterilized soil; TI: Inoculated control. The strain of P. capsici was isolated from chile de árbol plants from VMH. The inoculation was done with 105 zoospores per plant. Means with the same letter are statistically equal (Tukey α = 0.05).
In experiment 2, the non-inoculated plants, cultivated in sterilized or non-sterilized soil, had the highest DWAP, while the ones inoculated with P. capsici, without any control measures, had the lowest value (Figure 5B). All plants treated with any biological product, with the exception of the combination of Natucontrol plus Probac, even though they had a lower DWAP, were statistically equal to the non-inoculated control (Tukey a = 0.05). Conversely, the Fus out, Baktillis and the combination of both treatments had significantly higher averages than those of the inoculated control without control measures. Even though the treatments without biological products showed the aforementioned variations, the differences between their averages were not significant.
Discussion
This document is the first report about the etiology of the chile de árbol wilt in VMH and the effect of different control measures in the soils of the region. The results obtained in this work show that P. capsici is the causative agent of chile wilt in VMH. The pathogenicity tests and the morphological and molecular identification confirmed the identity of this oomycete. The wilt caused by P. capsici may be handled through the integration of several control methods (Granke et al., 2012). As a part of this, the CP 1264, CP 1261 and CP 1305 collections that showed the most resistance to the disease, can be considered good candidates to be included in genetic improvement programs or in the integration of a production system with complementary control measures, such as the ones evaluated in this research.
The results of this work show the effect of each chemical product, applied alone or alternated to VMH soils, but the disease was restricted to minimum levels and even total wilt control was also achieved with the individual and recurring applications of metalaxyl, fosetyl aluminum or propamocarb and the alternation of metalaxyl with fosetyl aluminum and metalaxyl with propamocarb. It has been reported that metalaxyl decreases the disease 83.3% (Fernández-Herrera et al., 2007), but in this research a reduction of 100% was obtained. Fosetyl aluminum controlled the disease 66.7% in one of our experiments, but in the rest of them it achieved a total control; meanwhile, propamocarb reduced the disease 100% in all cases. An 83.3% control of the disease with fosetyl aluminum has been reported in tomato plants (Fernández-Herrera et al., 2007) and of 60 to 100% with propamocarb in other cultivars (Hu et al., 2007). Factors such as the quantity of soil used in our experiments, variations in the genotypes of the pathogen and plant involved, and levels of inoculation, could explain the differences with respect to the aforementioned works. Conversely, the accumulation of dry matter in the aerial part, a preliminary indicator of the production potential of fruits by the plants (González-Real, 2008; García-Rodríguez, 2010), tended to be higher in the inoculated plants that were treated with these products than in the control witness. Such results could be considered first evidence that there are still low or null levels of resistance to the chemical products available in the Mexican market for the control of P. capsici in chile de árbol in VHM, because the recurring application worked better than the alternated application, within the same cycle. They are also an indicator to the possibility to increase or preserve the production potential of the cultivation in the region if used correctly.
Unlike the aforementioned products, under our experimental conditions, metam sodium, even though it prevented the disease in the majority of the cases, caused a negative effect in the accumulation of dry matter in the aerial part. This effect could have been caused by the impact of the agrochemical in the soil biota (Cao, 2004) and by phytotoxicity, because some plants transplanted in treated soil showed slight chlorosis, terminal necrosis in the leaves and in some cases necrosis in the stem that wasn't associated with the infection caused by P. capsici. Improvements in the management of the product, such as preliminary watering or a longer period of aeration could improve its effectiveness.
The four commercial biological products evaluated did not present total control of the wilt, however, there were important effects in the decrease of the disease with the treatments of Fus out, Natucontrol, Baktillis and Probac alone or combined. The treatments with Baktillis or Fus out with Probac stand out as the ones with low AUSPC values and increased the accumulation of dry matter compared to the inoculated control. The difficulty of introduced microorganisms to be established in the rhizosphere of the crops, is generally associated with high competitiveness and prior adaptation of the native microflora (Van Veen et al., 1997; Agrios, 2005), which could have been a determining factor of the limited effectiveness of the strains of Trichoderma sp. and Bacillus sp in the control of the pathogen (Schippers et al., 1987; Bais et al., 2004; Fernández-Herrera et al., 2007) that contain these products. However, the strains of Bacillus subtilis and Trichoderma harzianum could improve the growth of the plants whose rhizosphere they colonize (Benhamou et al., 1998; Kloepper et al., 2004; Ezziyyani et al., 2005). Therefore, in addition to their ability to reduce the levels of the disease, they could potentially contribute to the yield improvement of the crop.
The contrasts of the severity and production of biomass in plants cultivated in peat-agrolite were very evident in this work, especially in regards to the ones cultivated in the soil, which was clearly associated with the differences in the nutritional balance achieved in each substrate; however, it is possible that the native biota of the soils used in our study had a relevant part, given the absence of disease in non-inoculated soils and the evident decrease of AUSPC when the plants were cultivated in soil. Early reports have highlighted the importance of this nutritional balance (Duffy et al., 1997) and the importance of the organisms in the soil in the suppression of both oomycetes and other pathogens (Wardle, 2002; Yumusa and Newton, 2003). They are, therefore, factors worth considering in the process of integration of an integrated management system of the disease for the conditions of Vega de Metztitlán, Hidalgo.
The results of this research indicated that cultivars CP 1261, CP 1264 and CP 1305 were the most resistant to the pathogen and that the treatments with metalaxyl, fosetyl aluminum or propamocarb, metalaxyl alternated with fosetyl aluminum or with propamocarb completely controlled the wilt, whereas the treatments with Baktillis (Bacillus subtilis) alone or combined with Fus Out (Trichoderma harzianum) or Probac (B. subtilis) only reduced the severity of the disease.
REFERENCES
Agrios GN. 2005. Plant Pathology. Fifth Edition. Academic Press. New York, USA. 922p. [ Links ]
Akgül DS and Mirik, M. 2008. Biocontrol of Phytopththora capsici pepper plants by Bacillus megaterium strains. Plant Pathology 90:29-34. [ Links ]
Andrés A, JL., Rivera MA and Fernández PJ. 2005. Resistance of pepper germplasm to Phytophthora capsici isolates collected in northwest Spain. Spanish Journal of Agricultural Research. 3:429-436. [ Links ]
Bais HP, Fall R and Vivanco JM. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiology 134: 307-319. [ Links ]
Barnett HL and Hunter BB. 1998. Illustrated genera of imperfect fungi. 4a. ed. American Phytophathology Society, MN. 217 p. [ Links ]
Benhamou N, Kloepper JW and Tuzum, S. 1998. Induction of resistance against Fusarium wilt of tomato by combination of chitosan with an endophytic bacterial strain: ultrastructure and cytochemistry of the host response. Planta 204:153-168. [ Links ]
Booth C. 1977. Fusarium. Laboratory guide to the identification of the major species. Commonwealth Mycological Institute. Surrey, England. 58 p. [ Links ]
Campbell CL and Madden LV. 1990. Introduction to plant disease epidemiology. John Wiley and Sons, New York. 532 p. [ Links ]
Cao ZP, Yu YL, Chen GK and Dawson R. 2004. Impact of soil fumigation practices on soil nematodes and microbial biomass. Pedosphere 14:387-393. [ Links ]
Duffy BK, Ownley BH and Weller DM. 1997. Soil chemical and physical properties associated with suppression of take-all of wheat by Trichoderma koningii. Phytopathology 87:1118-1124. [ Links ]
Erwin DC and Ribeiro OK. 1996. Phytophthora diseases worldwide. American Phytopathology Society, MN. 562 p. [ Links ]
Ezziyyani M, Requena MA y Candela MA. 2005. Producción de proteínas-PR en la inducción de resistencia a Phytophthora capsici en plantas de pimiento. Anales de Biología. 27:143-154. [ Links ]
Fernández-Herrera E, Acosta-Ramos M, Ponce-González F y Manuel-Pinto V. 2007. Manejo biológico de Phytophthora capsici Leo., Fusarium oxysporum Schlechtend.:Fr. y Rhizoctonia solani Kühn en jitomate (Lycopersicon esculentum Mill.). Revista Mexicana de Fitopatología 25:35-42. [ Links ]
Gallegly ME and Hong M. 2008. Phytophthora identifying species by morphology and DNA fingerprints. American Phytopathology Society, MN. 158 p. [ Links ]
García-Rodríguez MR, Chiquito-Almanza E, Loeza-Lara D, Godoy-Hernández H., Villordo PE, Pons-Hernández JL, González-Chavira MM y Anaya-López L. 2010. Producción de chile ancho injertado sobre criollo de Morelos 334 para el control de Phytophthora capsici. Agrociencia. 44: 701-709. [ Links ]
González-Pérez E, Yañez-Morales MJ, Santiago-Santiago V y Montero-Pineda A. 2004. Biodiversidad fungosa en la marchitez del chile y algunos factores involucrados, en Tlacotepec de José Manzo, el Verde, Puebla. Agrociencia38:653-661. [ Links ]
González-Real MM, Baille A and Liu HQ. 2008. Influence of fruit load on dry matter and N-distribution in sweet pepper plants. Scientia Horticulturae 117: 307-315. [ Links ]
Granke LL, Quesada-Ocampo L, Lamour K and Hausbeck MK. 2012. Advances in research on Phytophthora capsici on vegetable crops in the United States. Plant Disease 96:1588-1600. [ Links ]
Gumpertz ML, Graham JM and Ristaino JB. 1997. Autologistic model of spatial pattern of Phytophthora epidemics in bell pepper: Effects of soil variables on disease presence. Journal of agricultural, biological and environmental statistics. 2:131-156. [ Links ]
Hu J, Hong C, Stromberg EL and Moorman GW. 2007. Effects of propamocarb hydrochloride on mycelial growth, sporulation, and infection by Phytophthora nicotianae isolates from Virginia nurseries. Plant Disease91:414-420. [ Links ]
Kloepper JW, Ryu CM and Zhang S. 2004. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology94:1259-1266. [ Links ]
Larkin RP, Gumpertz MLand Ristaino, J. B. 1995. Geostatistical analysis of Phytophthora epidemics in commercial bell pepper fields. Phytopathology84:191-203. [ Links ]
Mathre DF, Cook RJ and Callan NW. 1999. From discovery to use. Traversing the world of commercializing biocontrol agents for plant disease control. Plant Disease83:972-983. [ Links ]
Morán-Bañuelos SH, Aguilar-Rincón VH, Corona-Torres T y Zavaleta-Mejía, E. 2010. Resistencia a Phytopthora capsici LEO. de chiles nativos del sur de Puebla, México. Revista Fitotecnia Mexicana 33: 21-26. [ Links ]
National Center for Biotechnology Informatión (NCBI). 2014. Genbank. En: En: http://blast.ncbi.nlm.nih.gov/Blast.cgi .(Consulta marzo 2015). [ Links ]
Pineda J, Hernández A, González A, Barrientos V, Nass H, Gil E. 2005. Técnica de inoculación rápida y eficiente para la evaluación de materiales de maíz ante Rhizoctonia solani Kühn. Bioagro 17:93-98. [ Links ]
Raupach GS and. Kloepper JW 1998. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology88:1158-1164. [ Links ]
Ristaino BG and Johnston SA. 1999. Ecologically based approaches to management of Phytophthora blight on bell pepper. Plant Disease 83:1080-1089. [ Links ]
Sambrook J and Russell DW. 2001. Molecular cloning. A Laboratory Manual. 3era. ed. 1:1.32-1.34. Cold Spring Harbour Lab. Press, New York. [ Links ]
Sanogo S. 2003. Chile pepper and the threat of wilt diseases. Online. Plant Health Progress doi: 10.1094/PHP-2003-0430-01-RV. [ Links ]
Sanogo S. 2006. Predispositional effect of soil water saturation on infection of chile pepper by Phytophthora capsici. HortScience 41:172-175. [ Links ]
Schippers B, Baker AW and Bakker PAHM. 1987. Interactions of deleterious and beneficial rhizosphere microorganisms and the effect of cropping practices. Annual Review Phytopathology 25: 339-358. [ Links ]
Silva-Rojas HV, Fernández-Pavía SP, Góngora-Canul C, Macías-López BC y Ávila-Quezada, G.D. 2009. Distribución espacio temporal de la marchitez del chile (Capsicum annuum L.) en Chihuahua e identificación del agente causal Phytophthora capsici Leo. Revista Mexicana de Fitopatología27:134-147. [ Links ]
Singh A, Rohilla R, Singh US, Savary S, Willocquet L and Duveiller E. 2002. An improved inoculation technique for sheath bight of rice caused by Rhizoctonia solani. Canadian Journal of Plant Pathology 24:65-68. [ Links ]
Sneh B, Burpee L and Ogoshi A. 1991. Identification of Rhizoctonia Species. American Phytopathology Society, MN. 133 p. [ Links ]
Soria FMJ. 1993. Producción de hortalizas en la Península de Yucatán. ITA. Centro de investigaciones y Graduados Agropecuarios. Conkal, Yucatán. 303 p. [ Links ]
Steel RGD, Torrie JH, Dickey DA. 1997. Principles and procedures of statistics a biometric approach. 3rd ed. McGraw-Hill Series in probability and statistics. 666 p. [ Links ]
Van Veen JA, Van Overbeek LS and Van Elsas JD. 1997. Fate and activity of microorganisms introduced into soil. Microbiology Molecular Biology Reviews 61:121-135. [ Links ]
Velásquez VR, Medina AMM y Luna RJJ. 2001. Sintomatología y géneros de patógenos asociados con las pudriciones de la raíz del chile (Capsicum annuum L.) en el Norte-Centro de México. Revista Mexicana de Fitopatología19: 175-181. [ Links ]
Villar-Luna E, Reyes-Trejo B, Rojas-Martínez RI, Gómez-Rodríguez O, Hernández-Anguiano AM y Zavaleta-Mejía E. 2009. Hypersensitive response in foliage of chili pepper CM-334 resistant to Phytophthora capsici infected by Nacobbus aberransNematropica 39:143-155. [ Links ]
Wardle DA. 2002. Communities and ecosystems: Linking the aboveground and belowground components. Princeton University Press, Princeton, NJ, USA. 408 p. [ Links ]
White TJ, Bruns T, Lee S and Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. pp: 315-322 : Innis MA, Gelfand DF, Shinsky JJ,. White TJ (ed). PCR Protocols: A guide to methods and applications. Academic Press, San Diego. [ Links ]
Yunusa IAM and Newton PJ. 2003. Plants for amelioration of subsoil constraints and hydrological control: The primer-plant concept. Plant Soil 257:261-281. [ Links ]
Received: November 20, 2014; Accepted: December 13, 2014











 text in
text in 


