Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.26 n.2 Texcoco Jan. 2008
Artículos científicos
Disease Severity and Susceptibility of Sorghum [Sorghum bicolor (L.) Moench.] to Infection by Claviceps africana Frederickson, Mantle and de Milliano in Mexico and the United States of America
Severidad de la enfermedad y susceptibilidad del sorgo [Sorghum bicolor (L.) Moench.] a la infección por Claviceps africana Frederickson, Mantle y de Milliano en México y Los Estados Unidos de América
Noé Montes–García1, Héctor Williams–Alanís1, Louis K. Prom2, Thomas Isakeit3, Gary Odvody4, Jesús Narro–Sánchez5, y William L. Ronney6
1 INIFAP, Campo Experimental Río Bravo, Apdo. Postal 172, km 61 Carr. Matamoros–Reynosa, Río Bravo, Tamaulipas, México CP 88900. Correspondence to: nmontes62@hotmail.com
2 USDA–ARS, 2765 F&B Road, College Station, TX 77845, USA.
3 2132 TAMU, College Station, Texas 77845, USA.
4 TAMU Agricultural Research and Extension Center, Rt 2, Box 589, Corpus Christi, TX, 78406, USA.
5 INIFAP, Campo Experimental Bajío, km 6 Carr. Celaya–San Miguel de Allende, Apdo. Postal 112, Celaya, Guanajuato, Mexico CP 38000.
6 2132 TAMU, College Station, Texas 77845, USA.
Recibido: Enero 28, 2008
Aceptado: Febrero 25, 2008
Abstract
Experiments were carried out at College Station, USA, and Rio Bravo and Celaya, Mexico, under irrigated conditions during 2002 and 2003. Six sorghum hybrids and three male–sterile lines were planted at each location. Claviceps africana isolates were applied every other morning. Panicles were inoculated using a hand atomizer until runoff with a suspension of 1.6 x 106 conidia mL–1. Ergot severity was measured at milk stage. Bartlett's test was performed to determine homogeneity of variances among years. The results showed variability in susceptibility to ergot among genotypes at a single planting date, at planting dates within a year, locations and years. Ergot severity was statistically greater in 2002 in both hybrids and A–lines. Celaya had the greatest amount of ergot on hybrids, followed by College Station and Rio Bravo. In general, A–lines had the greatest severity. ATx2752 had the lowest ergot severity (22.8%), while ATx635 and ATx623, had 27.4% and 36.2%, respectively. Sorghum hybrid AP2233 was the most susceptible, and NC+8R18 showed the least amount of ergot severity.
Keywords: Ergot, hybrids, male–sterile lines.
Resumen
Los experimentos se llevaron a cabo en College Station, EUA, Río Bravo y Celaya, México, bajo condiciones de riego durante 2002 y 2003. Seis híbridos de sorgo y tres líneas andro–estériles se sembraron en cada localidad. los aislados de Claviceps africana se aplicaron cada dos días. Las panojas se inocularon usando un atomizador manual hasta escurrimiento total con una suspensión de 1.6 x 106 conidios mL–1. La severidad del cornezuelo fue evaluada en el estado lechoso. La prueba de Bartlett se utilizó para definir homogeneidad de varianzas entre años. Los resultados indicaron variabilidad en la susceptibilidad a la enfermedad entre genotipos en una misma fecha de siembra, entre fechas de siembra dentro de un mismo año, entre localidades y años. La severidad del cornezuelo fue estadísticamente mayor en 2002, tanto en híbridos como líneas andro–estériles. Celaya mostró el mayor valor de severidad en híbridos, seguida de College Station y Río Bravo. En general, las líneas andro–estériles mostraron la mayor severidad de la enfermedad. ATx2752 presentó la menor severidad (22.8%), mientras que ATx635 y ATx623, mostraron 27.4 y 36.2%, respectivamente. El híbrido AP2233 fue el más susceptible y NC+8R18 mostró la menor severidad a la enfermedad.
Palabras claves: Cornezuelo, híbridos, líneas androestériles.
Ergot has been observed in the majority of sorghum [Sorghum bicolor (L.) Moench.] production areas in the world. In Asia, sorghum ergot caused by Claviceps sorghi B.G.P. Kulk. (Kulkarni et al, 1976) was observed for the first time in India around 1915 (McRae, 1917). In Africa, the disease was first observed in Kenya in 1924, and later, in other countries (De Milliano et al., 1991). It was in Africa where Frederickson et al. (1991) identified a different species, Claviceps africana Frederickson, Mantle and de Milliano. In 1988, ergot was observed in Thailand (Boon–Long, 1992) and in 1991 in Taiwan (Cheng et al., 1991), although the pathogen was not identified to species. In Japan, another sorghum ergot species, Claviceps sorghicola Tsukib, was discovered (Tsukiboshi et al., 1999). Since early 1995, sorghum ergot caused by C. africana was observed for the first time outside Africa and Asia, in Brazil (Reis et al., 1996), Australia (Ryley et al, 1996), and in NorthAmerica (Aguirre et al, 1997; Isakeit et al, 1998). Losses due to ergot in seed production fields can be high. In India, losses up to 80% have been reported, whereas in Zimbabwe annual losses are between 12 and 25% and sometimes up to 100% (Bandyopadhyay et al., 1998). In 1997, nearly 45% of the hybrid seed production fields in the Texas Panhandle had ergot with varying degrees of severity (Workneh and Rush, 2003). In Mexico, losses have been reported up to 100% in seed production and 30% in grain production fields. Losses of seed quality can be an issue, because of honeydew contamination of healthy sorghum grain, increasing colonization by saprophytic fungi. McLaren (1992) found that such seed had reduced germination. In addition, honeydew stickiness can interfere with harvest. Genetic diversity among isolates of C. africana (Komolong et al., 2002) has been analyzed by random amplified microsatellite (RAM) and amplified fragment length polymorphism (AFLP) (Pazoutová et al., 2000; Pecina et al., 2007; Tooley et al, 2000; Tooley et al, 2002). Isolates from Australia, India, and Japan were grouped into one cluster, and isolates from the USA, Mexico, and Africa into another (Tooley et al., 2002). This supports the results obtained by Pazoutová et al. (2000), who found american isolates to be similar to isolates from South Africa. Pecina et al. (2007), using AFLP analysis found that isolates from Texas and Tamaulipas, Mexico (northern Mexico) were similar, and they were different from another group from Celaya and Ocotlan, Jalisco, Mexico (central Mexico), suggesting that maybe the pathogen has evolved to adapt to different weather conditions in those areas. According to Frederickson et al. (1993), spread by wind–borne secondary conidia is a factor in long distance dispersal. The initial ergot observation in Mexico was on sorghum plants that were planted out of the 1996 normal fall planting dates in San Fernando, Tamaulipas, and flowered in January–February period in 1997. During September 1998, high amounts of rainfall throughout south Texas supported ratooning of plants, which were exposed to low temperatures from October to December. The extended flowering period associated with a cool and wet environment caused a fast development and spread of the pathogen on forage sorghum, grain sorghum along roads and johnsongrass [Sorghum halepense (L.) Pers.] plants (Odvody et al, 1999b). During 1999 ergot was observed in several sorghum winter nurseries located on the west coast of Mexico and Puerto Rico, and south Texas. During November–December, the disease was observed in the Bajio area and Tamaulipas, and in early 2000 in Puerto Vallarta, Mexico, with disease incidence up to 35% and severity up to 100% in commercial hybrids. The epidemic in 2000 showed that C. africana is well established in the major sorghum production areas of Mexico and the United States, and has shown its capability to overwinter and survive hot and dry weather conditions. The objective of this study was to determine ergot susceptibility among sorghum hybrids and three widely–utilized male–sterile lines exposed to variable environments at multiple locations.
MATERIALS AND METHODS
The experiments were carried out in College Station, USA (30° 25' N, 96° 40' W), and Mexico (25° 58' N, 98° 00' W and 20° 34' N, 100° 50' W) under irrigated conditions 2002 and 2003. At all locations, population density used was around 125,000 plants ha–1. Weeds were controlled by hand hooded sprayer with Roundup® (Glyphosate) at 25 mL ha–1 per 12 L of water. Sorghum midge (Contarinia sorghicola Coquillett) was controlled as needed with Asana XL (70 mL ha–1 ). Sorghum hybrids AP 2233 (Syngenta®), KS 310 (Sorghum Partners®), NC+7W97 (NC+ seeds®), GARST 5664 (Syngenta®), ATx399 x Tx430 (Texas A&M University), NC+8R18 (NC+ seeds®), and A–lines ATx635, ATx2752, and ATx623 (all Texas A&M University) were planted every month at each location. Hybrids were chosen based on previous ergot reaction studies conducted during the fall in Weslaco, Texas (Isakeit et al, 1999), and similarities in flowering pattern. The number and planting dates (on the figures, Julian day is the planting date) at each location depended upon weather conditions. At Rio Bravo (RB), there were ten planting dates, at College Station (CS) six, and five at Celaya (CEL). Plants were grown at each location in a completely randomized block design with four replications. The experimental unit was one row of 5 m length, with a row spacing of 0.81 to 0.91 m (32" to 36"). In each row, five panicles of similar maturity were selected and tagged (total genotype sample = 20) at bloom initiation. Inoculation was conducted using local C. africana isolates at each location. Inoculum was increased under greenhouse conditions and applied every other morning between 8:00 am and 10:00 am. Targeted panicles were marked and inoculated using a hand atomizer until runoff with a suspension of 1.6 x 106 conidia mL–1. The period from initial and final bloom dates for each inoculated panicle was recorded (the period ranged from 4 to 13 days depending on weather conditions and type of plant, i.e. A–lines showed a wider period than hybrids), as well as ergot severity (percentage of infected florets observed in each inoculated plant) measured at milk stage (10–12 days after 50% flowering). Disease severity was transformed using arcsine of the square root of ergot severity to satisfy assumptions of normality. Data were analyzed taking into consideration the chronological variation (dates) nested in location by year (loc x year), instead of the spatial variation due to replications (blocks) in each planting date. Because male–sterile sorghum lines are highly susceptible to C. africana in comparison with hybrids, data were analyzed in two different groups (A–lines and hybrids). Bartlett's (X2) test of homogeneity was performed to determine homogeneity of variances among years. If the variances were homogeneous, data were combined. Analysis of variance was conducted for each location considering genotypes and locations as fixed effects, and years and dates as random effects. PROC GLM (SAS Institute, Cary, NC) procedure was used to estimate total variance, means of genotypes, dates and years. Genotype and planting date means were compared using Tukey's (P < 0.01) at each year and location.
RESULTS
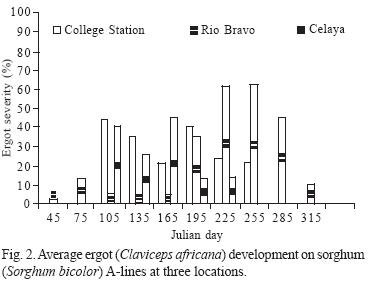
In general, sorghum ergot severity was statistically greater (P < 0.01) in 2002 in both hybrids and A–lines, compared with 2003. The CEL location had the greatest amount of ergot severity on hybrids (12.5%), followed by CS and RB, which was 9.6% and 7.2% respectively. Locations as CEL and CS had greater (P < 0.01) ergot severity in A–lines compared with RB. In general, A–lines had the greatest ergot severity that was 66% more than the average severity observed in hybrids (Table 1). According to the average severity presented across years, locations, and dates, male–sterile line ATx2752 had the lowest ergot (22.8%), while ATx635 and ATx623 had 27.4% and 36.2%, respectively. Sorghum hybrid AP2233 was the most susceptible to ergot followed by GARST 5664, ATx399 x RTx430, and NC+7W97. In general, hybrid NC+8R18 showed the least amount of ergot (4.7%), which was 32% of the severity of the most susceptible one (Table 1). The ANOVA for hybrids showed a highly significant (P < 0.01) difference among genotypes and dates at all locations (Table 2). The ANOVA for A–lines showed a highly significant effect of genotype, date and the interaction (genotype x date) in all the locations (Table 3) with the exception of CEL, where date and interaction were not significant. The differences in hybrid response at each location are shown in Figure 1. At RB and CS disease had similar trends, ranging from almost zero infection during the spring and summer, to almost 50% (P < 0.1) infection during the fall. Meanwhile, the CEL location showed an inverse relationship, where the highest ergot level was during the summer and the lowest during the fall, maybe due to complete sterility including the ovaries and/or poor pathogen development. With A–lines, ergot was almost identical at CS and CEL with 95 to 98% more infection than RB during the spring (Fig. 2). The reverse was seen at RB, where the highest infection (66% more than CS) was during the fall.
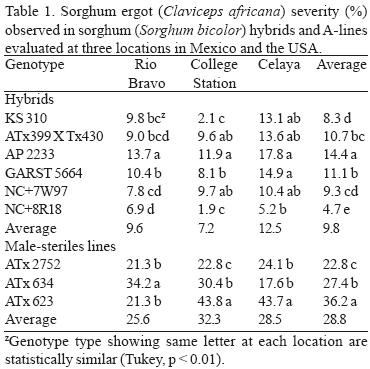
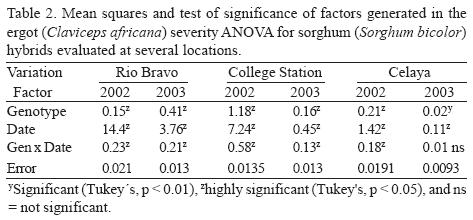
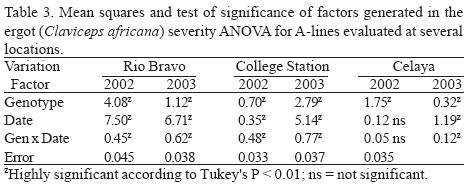
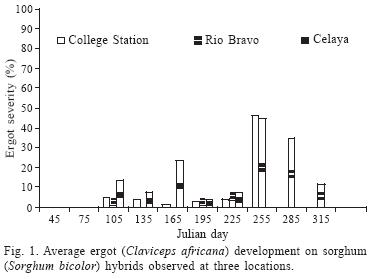
DISCUSSION
The results show great variability in susceptibility to sorghum ergot among genotypes that are evaluated at a single planting date, and also the variation in susceptibility of a single genotype evaluated at different planting dates within a year, locations and years. Therefore, sorghum ergot resistance studies should use all these components to assure that the disease resistance is genetic and not a type of avoidance mechanism. These results corroborate McLaren (1992), who found that ergot reaction of a specific line is quantified by comparing observed ergot severities associated with different flowering dates with the ergot potential of those dates. The hybrid NC+8R18 showed the least amount of ergot over planting dates, years and locations. However, data showed that none of the hybrids and A–line genotypes had genetic resistance. This confirms the observations of Bandyopadhyay et al. (1996), who found that variations in ergot incidence and severity in sorghum lines is related to partial genetic resistance (Hernández et al., 2005), tolerance of lines to ergot favorable conditions (McLaren and Flett, 1998; Montes et al., 2003b; Montes–Belmont et al., 2002; Workneh and Rush, 2002; 2006) such as pre–flowering cold stress (Brooking, 1976; McLaren and Wenher, 1992; Montes et al., 2003a), and the ability to escape infection by ensuring rapid and effective pollination or reducing the stigma receptivity period (Moran, 2000). It is clear that the hybrid seed production system is at high risk for ergot, because it can develop on A–lines at all locations during the normal growing season. Ergot infection in CS and CEL locations during the fall season did not occur due to frost that reduced flower development and cool temperatures that inhibited the pathogen. Situations like this and that at RB, where there are no commercial sorghum fields planted after September, result in no new infections during the winter, so new infections in the next growing season could arise from overwintering of inoculum (Odvody et al., 1999a; 2003; Prom et al., 2005b) or secondary conidia coming from infected hosts elsewhere or transmitted by insects that have been feeding in infected plants (Prom and López, 2004). The observed ergot severity values were attributable to the C. africana inoculum and the inoculation method that were used. Inoculum used at these locations came from fresh infected panicles from which the spore suspension was prepared. The hand sprayer inoculation technique has shown to be the best method in evaluating disease resistance (Prom et al., 2005a) or in chemical control studies (Montes et al., 2003c). The recommendation to farmers will be to plant sorghum hybrids in periods in which plants can avoid exposure to low minimum temperatures before flowering (Brooking, 1976; McLaren and Wenher, 1992; Montes et al., 2003a). For seed production fields, the recommendation is to apply chemical control (Montes et al., 2005) and crop management (Komolong et al., 2003) to reduce ergot. The analysis of historical weather data can give a better idea of the possible C. africana impact in sorghum hybrids and male–sterile lines.
CONCLUSIONS
A–lines ATx2752, ATx634, and ATx623 had greater ergot susceptibility than hybrids KS310, ATx399 x Tx430, AP 2233, NC+7W97, GARST 5664 and NC+8R18. Nevertheless, differences between A–lines suggests that breeding for resistance is a possible control option. Sorghum hybrids are at great risk for ergot at Celaya, due to the low minimum temperatures that are present throughout the year that can cause pollen sterility.
ACKNOWLEDGEMENTS
This research was developed between INIFAP, TAMU, and USDA–ARS, and it was funded in part by the USDA/FAS/ICD/RSED grants 58–3148–7–026 and 586202–1–F151 to develop research on sorghum ergot.
LITERATURE CITED
Aguirre, R.J., Williams, A.H., Montes, G.N., and Cortinas, H.M. 1997. First report of sorghum ergot caused by Sphacelia sorghi in Mexico. Plant Disease 81:31. [ Links ]
Bandyopadhyay, R., Frederickson, D.E., McLaren, N.W., and Odvody, G.N. 1996. Ergot– A global threat to sorghum. International Sorghum and Millets Newsletter 37:1–32. [ Links ]
Bandyopadhyay, R., Frederickson, D.E., McLaren, N.W., Odvody, G.N., and Ryley, M.J. 1998. Ergot: A new disease threat to sorghum in the Americas and Australia. Plant Disease 82:356–367. [ Links ]
Boon–Long, T. 1992. Sorghum diseases in Thailand. pp. 4143. In: W.A. J. de Milliano, R.A. Fredericksen, and G.D. Bengston (eds). Sorghum and Millets Diseases: A Second World Review. International Crops Research Institute for the Semi–Arid Tropics. Patancheru, India. 370 p. [ Links ]
Brooking, I.R. 1976. Male sterility in Sorghum bicolor (L.) Moench induced by low night temperature. Timing of the stage of sensitivity. Australian Journal of Plant Physiology 3:589–596. [ Links ]
Cheng, G.M., Cheng, Q.H., and Yeh, C.C. 1991. Ergot disease of sorghum in Taiwan. Plant Protection Bulletin 33:223–226. [ Links ]
De Milliano, W.A.J., Tavares–Nogueira, M.F.R., Pomela, L.M., Msiska, F.S., Kunene, S., Matalaote, B., Mbwaga, A.M., Kaula, G.M., and Mtisi, E. 1991. New records of ergot of sorghum caused by Sphacelia sorghi in southern Africa. Plant Disease 75:215. [ Links ]
Frederickson, D.E., Mantle, P.G., and De Milliano, W.A.J. 1991. Claviceps africana sp. nov.; the distinctive ergot pathogen of sorghum in Africa. Mycological Research 95:1101–1107. [ Links ]
Frederickson, D.E., Mantle, P.G., and De Milliano, W.A.J. 1993. Windborne spread of ergot disease (Claviceps africana) in sorghum A–lines in Zimbabwe. Plant Pathology 42:368–377. [ Links ]
Hernández, M.M., Mendoza, L.E., Solis, M.E., Aguilar, A.J.L. y Grageda, C.O.A. 2005. Caracterizacion epidemiological de la tolerancia al cornezuelo (Claviceps africana Frederickson, Mantle and de Milliano) en diez líneas de sorgo [Sorghum bicolor (L.) Moench.]. Revista Mexicana de Fitopatología 23:42–48. [ Links ]
Isakeit, T., Odvody, G.N., and Shelby, R.A. 1998. First report of sorghum ergot caused by Claviceps africana in the United States. Plant Disease 82:592. [ Links ]
Isakeit, T., Barroso, M., and Garza, B. 1999. Ergot reaction of grain sorghum hybrids, 1998. Biological and Cultural Tests for Control of Plant Diseases 14:19. [ Links ]
Komolong, B., Chakraborty, S., Ryley, M., and Yates, D. 2002. Identity and genetic diversity of the sorghum ergot pathogen in Australia. Australian Journal of Agricultural Research 53:621–628. [ Links ]
Komolong, B., Chakraborty, S., Ryley, M., and Yates, D. 2003. Ovary colonization by Claviceps africana is related to sorghum resistance in male–sterile sorghum lines. Plant Pathology 52:620–627. [ Links ]
Kulkarni, B.G.P., Seshadri, V.S., and Hedge, R.K. 1976. The perfect stage of Sphacelia sorghi McRae. Mysore Journal of Agriculture Science 10:286–289. [ Links ]
McLaren, N.W. 1992. Quantifying resistance of sorghum genotypes to the sugary disease pathogen (Claviceps africana). Plant Disease 76:986–988. [ Links ]
McLaren, N.W., and Wenher, F.C. 1992. Pre–flowering low temperature predisposition of sorghum to sugary disease (Claviceps africana). Journal of Phytopathology 135:328–334. [ Links ]
McLaren, N.W., and Flett, B.C. 1998. Use of weather variables to quantify sorghum ergot potential in South Africa. Plant Disease 82:26–29. [ Links ]
McRae, W. 1917. Notes on some south Indian fungi. Madras Agriculture Yearbook 1917: 108–111. [ Links ]
Montes–Belmont, R., Mendez–Ramírez, I., and Flores–Moctezuma, E. 2002. Relationship between sorghum ergot, sowing dates, and climatic variables in Morelos, Mexico. Crop Protection 21:899–905. [ Links ]
Montes, G.N., Odvody, G.N., and Marin–Silva, M. 2003a. Effect of cold degree units on incidence of Claviceps africana in sorghum hybrids. pp. 103–104. In: J.F. Leslie (ed.). Sorghum and Millets Diseases. Iowa State Press. First Ed. Ames, Iowa, USA. 504 p. [ Links ]
Montes, G.N., Odvody, G.N., and Williams, A.H. 2003b. Relationship between climatic variables and Claviceps africana incidence on sorghum hybrids in northern Mexico. pp. 111–112. In: J.F. Leslie (ed.). Sorghum and Millets Diseases. Iowa State Press. First Ed. Ames, Iowa, USA. 504 p. [ Links ]
Montes, G.N., Odvody, G.N., and Williams, A.H. 2003c. Advances in Claviceps africana chemical control. pp. 105110. In: J.F. Leslie (ed.). Sorghum and Millets Diseases. Iowa State Press. First Ed. Ames, Iowa, USA. 504 p. [ Links ]
Montes, G.N., Williams, A.H., Odvody, G.N., Prom, L.K. 2005. Comparative efficacy of trizole fungicides for control of Claviceps africana Frederickson, Mantle and de Milliano in sorghum [Sorghum bicolor (L.) Moench.] seed production of northern Mexico. Revista Mexicana de Fitopatología 23:152–156. [ Links ]
Moran, M.J.L. 2000. Differences in ergot vulnerability among sorghum genotypes and the relationship between stigma receptivity and ergot vulnerability. M.Sc. Thesis. Texas A&M University. College Station, Texas, USA. 125 p. [ Links ]
Odvody, G.N., Frederickson, D.E., Isakeit, T., Dahlberg, J.A., and Peterson, GL. 1999a. The role of seedborne inoculum in sorghum ergot. pp. 136–140. In: J.W. Sheppard (ed.). Proc. 3rd. International Seed Testing Assoc. Seed Health Symposium. Ames, Iowa, USA. 160 p. [ Links ]
Odvody, G.N., Isakeit, T., Montes, N., Narro–Sanchez, J., and Kaufman. H. 1999b. Ocurrence of sorghum ergot in Mexico and Texas in 1998. p. 62. In: SICNA, National Grain Sorghum Producers (eds.). Proceedings of 21st. Biennial Grain Sorghum Res. & Utilization Conference. Feb. 21–23, 1999. Tucson, Arizona, USA. 289 p. [ Links ]
Odvody, G.N., Montes, N., Frederickson, D.E., and Narro–Sanchez, J. 2003. Detection of sclerotia of Claviceps africana in the western hemisphere. pp. 129–130. In: J.F. Leslie (ed.). Sorghum and Millets Diseases. Iowa State Press. First Ed. Ames, Iowa, USA. 289 p. [ Links ]
Pazoutová, S., Bandyopadhyay, R., Frederickson, D.E., Mantle, P.G, and Frederiksen, R.A. 2000. Relations among sorghum ergot isolates from the Americas, Africa, India and Australia. Plant Disease 84:437–442. [ Links ]
Pecina, VQ., Montes, G.N., Williams, A.H., Hernández, D.S., Mayek, P.N., and Prom, L.K. 2007. Diversidad genética de aislamientos de cornezuelo (Claviceps africana Frederickson, Mantle y de Milliano) de sorgo [Sorghum bicolor (L.) Moench.] en México. Revista Mexicana de Fitopatología 25:43–47. [ Links ]
Prom, L.K., and López, Jr. J.D. 2004. Viability of Claviceps africana spores ingested by adult corn earworm moths, Helicoverpa zea (Boddie) (Lepidoptera:Noctuidae). Journal of Economic Entomology 97:764–767. [ Links ]
Prom, L.K., Erpealding, J., Isakeit, T., and Montes, N. 2005a. Inoculation techniques for identifying resistance in sorghum genotypes to sorghum ergot. Journal of New Seeds 7:9–22. [ Links ]
Prom, L.K., Isakeit, T., Odvody, G.N., Rush, C.M., Kaufman, H.W,, and Montes, N. 2005b. Survival of C. africana within sorghum panicles at several Texas locations. Plant Disease 89:39–43. [ Links ]
Reis, E.M., Mantle, P.G, and Hassan, H.A.G. 1996. First report in the Americas of sorghum ergot disease, caused by a pathogen diagnosed as Claviceps africana. Plant Disease 80:463. [ Links ]
Ryley, M.J., Alcorn, J.L., Kochman, J.K., Kong, G.A., and Thompson, S.M. 1996. Ergot on sorghum sp. in Australia. Australian Plant Pathology 25:214. [ Links ]
Tooley, P.W., O'Neill, N.R,, Goley, E.D., and Carras, M.M. 2000. Assessment diversity in Claviceps africana species by RAM and AFLP analyses. Phytopathology 90:1126–1130. [ Links ]
Tooley, P.W., Goley E.D., and Carras, M.M. 2002. AFLP comparisons among Claviceps africana isolates from the United States, Mexico, Africa, Australia, India and Japan. Plant Disease 86:1247–1252 [ Links ]
Tsukiboshi, T., Shimanuki, T., and Uematsu, T. 1999. Claviceps sorghicola sp. Nov., a destructive ergot pathogen of sorghum in Japan. Mycological Research 103:1403–1408. [ Links ]
Workneh, F., and Rush, C.M. 2002. Evaluation of relationships between weather patterns and prevalence of sorghum ergot in the Texas Panhandle. Phytopathology 92:659–666. [ Links ]
Workneh, F., and Rush, C.M. 2003. Status of sorghum ergot in the Texas Panhandle and efforts towards development of a risk forecasting model. p 47. In: J.A. Dahlberg, R. Kochenower, R. Klein, B. Rooney, S. Bean, B. Pendleton, J. Stack, and B. Maunder (eds). Proceedings of the 23rd. Biennial Sorghum Industry Conference. Sorghum Research and Utilization Conference. Alburquerque, New Mexico, USA. (CD ROM). [ Links ]
Workneh, F., and Rush, C.M. 2006. Weather factors associated with development of sorghum ergot in the Texas Panhandle. Plant Disease 90:717–722. [ Links ]














