Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista mexicana de fitopatología
versão On-line ISSN 2007-8080versão impressa ISSN 0185-3309
Rev. mex. fitopatol vol.25 no.2 Texcoco 2007
Artículos de revisión
Development of the Volatile-Producing Fungus Muscodor albus Worapong, Strobel, and Hess as a Novel Antimicrobial Biofumigant
Desarrollo del hongo Muscodor albus Worapong, Strobel y Hess productor de compuestos volátiles, como un nuevo biofumigante antimicrobiano
Julien Mercier, Jorge Isaac Jiménez-Santamaría, and Patricia Tamez-Guerra*
AgraQuest Inc. 1530 Drew Avenue, Davis, California, 95618, USA.
* Current address: Universidad Autónoma de Nuevo León, Departamento de Microbiología e Inmunología, FCB, Apdo. Postal 46-F, San Nicolás de los Garza, Nuevo León, México CP 66450. Correspondence to: patamez@hotmail.com.
Received: April 27, 2007
Accepted: July 13, 2007
Abstract
Worldwide growers are seeking for safer alternatives to control soil-borne and postharvest diseases. Chemical fumigants, such as methyl bromide, are currently used because of their high efficacy and yield enhancement. Nevertheless, the adverse environmental and health effects of chemical fumigants have triggered the search for less hazardous options. Muscodor albus, a non-spore forming fungus, produces a volatile compound complex with broad-spectrum antimicrobial activity that could be used as alternative biofumigant to control plant pathogens. Unfortunately, production of biocontrol agents on solid-state fermentation is expensive and may limit the use of a biofumigant in the pesticide market. This may be overcome by using improved fermentation and formulation processes. This study reports the antimicrobial activity, potential use, and development of M. albus as an optimized product for controlling soil-borne, seed-borne and postharvest disease problems, as well as building molds. Its potential as biofumigant with lower use rates resulting from improved fermentation conditions and formulation processes is discussed.
Keywords: Fumigant, postharvest, damping-off, fermentation, formulation, soil.
Resumen
Productores agrícolas de todo el mundo buscan alternativas de menor riesgo para el control de patógenos de suelo y poscosecha. Los fumigantes químicos, tales como el bromuro de metilo, se emplean debido a su eficacia y mejora del rendimiento. Sin embargo, los efectos nocivos de los fumigantes químicos a la salud y al medio ambiente han estimulado la búsqueda de alternativas menos dañinas. Muscodor albus, un hongo filamentoso, produce un complejo de compuestos volátiles con un amplio espectro de actividad antimicrobiana que podría utilizarse como un biofumigante alternativo para el control de fitopatógenos. Desafortunadamente, la producción de agentes de biocontrol por fermentación sólida es cara, lo cual pudiera limitar el uso del biofumigante en el mercado de los pesticidas. Esto podría ser solucionado mejorando los procesos de fermentación y formulación. En este trabajo se reporta la actividad antimicrobiana, uso potencial y desarrollo de M. albus como producto dirigido al control de patógenos de suelo, semilla y poscosecha, al igual que mohos de edificios. Se discute su empleo potencial como biofumigante a dosis más bajas, gracias a la mejora en los procesos de fermentación y formulación.
Palabras clave: Fumigante, postcosecha, ahogamiento, fermentación, formulación.
INTRODUCTION
Muscodor albus Worapong, Strobel, and Hess is an extraordinary fungus that has the property of producing an array of low-molecular weight volatile organic compounds which have broad antimicrobial activity, inhibiting or killing most species of fungi and bacteria (Strobel et al., 2001). The original M. albus isolate 620 was isolated from a cinnamon (Cinnamomum zeylanicum Blume) tree growing in Honduras. The fungus was found to be closely related to the endophytic fungus Xylaria (family Xylariaceae, Ascomycetes) (Worapong et al., 2001). Since the discovery of the first isolate in Honduras, several more M. albus or Muscodor species were isolated from plant material in other tropical locations (Daisy et al., 2002; Ezra et al., 2004; Sopalun et al., 2003; Worapong et al., 2002). M. albus exists only as a sterile mycelium and has never been observed to sporulate or produce other survival structures. Due to these unusual antimicrobial volatile properties, the use of Muscodor species in agriculture has been patented (Strobel et al., 2005) andM. albus is being developed by AgraQuest Inc. as an antimicrobial biofumigant for the control of postharvest, soil-borne and seed-borne diseases as well as building mold problems. One challenge for M. albus to become a commercial product is reducing the amount required for the effective use in the field as a soil fumigant. For example, effective doses of some early formulations for soil application are in the range of 100 to 200 kg/ha (Grimme, 2004; Strobel et al., 2005). This paper reviews recent approaches to improve the production and formulation ofM. albus as a biofumigant for controlling soil-borne, seed-borne, and postharvest diseases, as well as building molds.
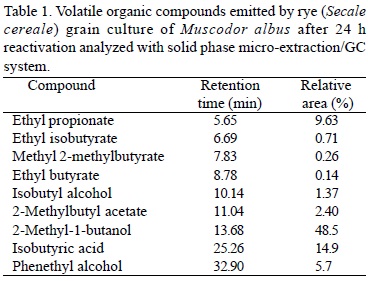
Production ofvolatile organic compounds by M. albus. Volatile organic compounds (VOCs) are chemicals with low molecular weight, high vapor pressure, and low water solubility which allow them to easily evaporate into the air or "off-gas". Many fungal species are known to emit low concentration of gaseous compounds, especially ones that have distinctive obnoxious odors, and this has prompted research on chemical analysis of fungal volatiles (Bjurman and Kristensson, 1992). Some of these volatile substances are common to many fungi, whereas others appear to be unique for one species (Rapior et al., 2000; Schnurer et al., 1999). Dennis and Webster (1971) reported that certain Trichoderma spp. produced volatile antibiotics which inhibited the growth of Rhizoctonia solani Kühn, Pythium ultimum Trow, and Fusarium oxysporum Schlecht. M. albus was found to produce some 28 volatile compounds which together inhibited and killed various species of fungi, and bacteria. This mixture of gases consisted primarily of various small molecular weight alcohols, acids, and esters (Strobel et al., 2001). Headspace analysis of M. albus grown on rye (Secale cereale L.) detected six major volatile compounds and three others were found in trace amounts (Fig. 1, Table 1). All of these were esters and alcohols, except for the aromatic compound, phenethyl alcohol. The most abundant compound was 2-methyl-1-butanol followed by isobutyric acid and ethyl propionate (Mercier and Jiménez, 2004). The three main VOCs (isobutyl alcohol, 2-methyl-1-butanol, and isobutyric acid) were captured by Solid Phase Micro-extraction (SPME) and analyzed using a HP 5890 adapted with an FID detector. The VOC were absorbed onto the SPME fiber for 30 min and injected into a 30 m x 0.25 mm ID x 0.5 mm ZB-wax capillary column, 31°C to 220°C over 42 min with Helium as a carrier. The identity of individual compounds was based on retention time of pure standards. Ezra and Strobel (2003) have also demonstrated that the composition of the medium used to support the growth of the M. albus greatly influences the quality and effectiveness of the volatiles emitted by this organism. A sucrose enriched medium primarily yielded ketones and esters as the primary volatiles, while more enriched media were more effective in inhibiting a suite of plant pathogens. Desiccated rye culture of M. albus was found equivalent to fresh culture for the control of blue mold of apple [Penicillium expansum (Link) Thom.] when used for 24 h fumigation at 21°C (Jiménez and Mercier, 2005). The effect of the re-hydration regime and temperature were investigated to optimize the production of volatile organic compounds (VOC); in particular, isobutyric acid was used as an indicator of fungicidal activity. Rye culture re-hydrated immediately before use produced the highest levels of isobutyric acid and killed all Penicillium expansum spores when used at a rate of 0.4-0.9 g/L (w/v) in closed plastic boxes. Dry rye culture does not reactivate without re-hydration, even when exposed to high humidity in closed containers. For postharvest fumigation at 4°C, a short incubation period at room temperature following re-hydration enhanced antifungal activity and VOC production, as opposed to re-hydration directly at 4°C or with a 24 h incubation at 21°C. During fumigation at 4°C, VOC production was highest during the first week and diminished gradually during storage. Levels of isobutyl alcohol, 2-methyl-1-butanol, and isobutyric acid at 7 days, with a 6 h pre-incubation time at 21°C, were 1.4, 2.2, and 7.6 µg/L and after 24 days at 0.8, 1.2, and 2.3 µg/L, respectively (Jiménez and Mercier, 2005).
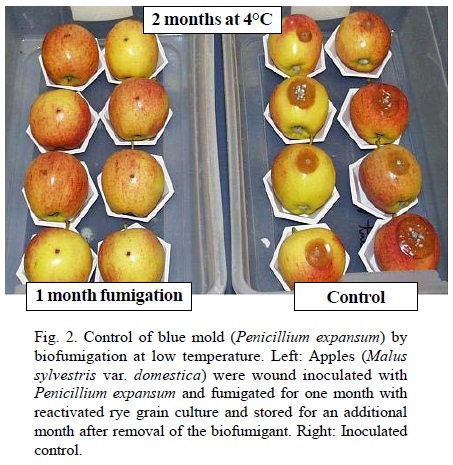
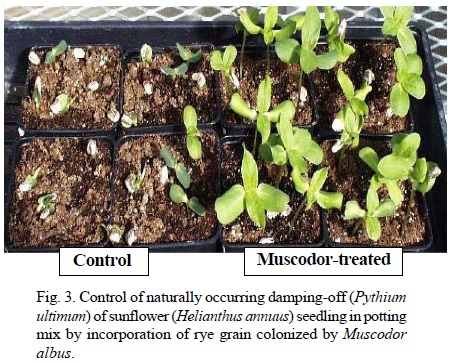
Potential uses of M. albus as biofumigant. Control of postharvest diseases. While low temperatures help slow down microbial decay of fruits and vegetables, postharvest fungicide applications are often used in commodities such as pome fruit, stone fruit, citrus and potatoes to minimize storage and shipping losses (Adaskaveg, 2002). Fumigation with sulfur dioxide, storage in modified atmospheres or rapid cooling and shipping are also used for commodities that are too fragile to withstand postharvest fungicide applications like grapes or strawberries. Also, there are few available treatments to extend the shelf-life of organically-grown commodities. The fact that volatiles from M. albus kill most storage pathogens exposed in vitro (Table 2) opens up new possibilities to develop biofumigation as a postharvest treatment for a range of commodities. Biofumigation for the control of fungal decay was first demonstrated by placing fresh rye culture of M. albus in closed plastic boxes with inoculated fruit (Mercier and Jiménez, 2004). With this system, biofumigation for periods of up to five days controlled blue mold and gray mold of apple (Botrytis cinerea Pers.:Fr.) (Mercier and Jiménez, 2004), brown rot of peaches (Monilinia fructicola (G. Wint.) Honey] (Mercier and Jiménez, 2004) and green mold [Penicillium digitatum (Pers.:Fr.) Sacc.] and sour rot (Geotrichum candidum Link) of lemons [Citrus limon (L.) N.L. Burm.] (Mercier and Smilanick, 2005). While these experiments were carried out at ambient temperature, it was also shown that M. albus could produce volatiles at cold storage temperature (Jiménez and Mercier, 2005) and control diseases such as blue mold of apples (Fig. 2) and gray mold of grapes (Botrytis cinerea) (Mlikota-Gabler et al., 2006). On other commodities, bacterial soft rot [Erwinia carotovora subsp. carotovora (Jones) Bergey, Harrison, Breed, Hammer, and Huntoon] (Corcuff et al., 2006) and human pathogens such as Salmonella (Freitas et al., 2005) were also controlled by biofumigation with M. albus. Two main strategies have been attempted for using M. albus for postharvest decay control. One of them is to fumigate the entire storage room where the fruit is held. This was attempted successfully with lemons held in a storage room during the degreening process (i. e. artificial chlorophyll breakdown with ethylene for color enhancement) (Mercier and Smilanick, 2005). For this application, trays of reactivated rye M. albus culture were placed for in a storage room with a fan in the presence of wound-inoculated lemons. The biofumigation process was stopped after 48 h and disease incidence was measured after one week. Depending on the experiment, there was from 52 to 88% reduction of green mold incidence in lemons held in open bins. The biofumigation treatment did not interfere with color development. The other strategy is to use M. albus to fumigate individual fruit cartons or pallets. This strategy appears particularly suitable for very perishable commodities as the use of M. albus pads could help suppress mold development during shipping and does not require additional handling of the commodity, even if it is field-packed. As desiccated culture of M. albus can be readily activated by rehydration to produce volatiles (Jiménez and Mercier, 2005), a pad made of tea bag paper containing dry rye culture was designed for use in individual fruit cartons. The pad is activated by brief immersion in water shortly before use. This treatment concept was tested successfully under commercial or near-commercial situations in cartons held at low temperature storage with peaches [Prunus persica (L.) Batsch] (Schnabel and Mercier, 2006), grapes (Vitis vinifera L.) (Mercier et al., 2005) and cherries (Prunus cerasifera Ehrh.) (J. Mercier, unpublished data). In the case of peaches and cherries, the biofumigation treatment compared favorably with the fungicide standards. There was also a significant shelf-life extension of raspberries (Rubus strigosus Michx.) fumigated in plastic-wrapped pallets (J. Mercier, unpublished data) which could be very interesting for the shipment of this fragile commodity. Seed treatment. Fumigation of seed for decontamination is another interesting possibility for biofumigation with M. albus. Strobel et al. (2001) report of an experiment in which barley (Hordeum vulgare L.) seed contaminated with Ustilago hordei (Pers.) Lagerh. was fumigated for four days with potato-dextrose-agar cultures of M. albus. There was 100% control of covered smut in the plants grown from the fumigated seed. Considering the broad antimicrobial activity of M. albus, it is possible that its use could be extended against a number of fungal and bacterial seed contaminants. Control of soil-borne pathogens. Diseases caused by soil-borne pathogens affect greenhouse, nursery and field productions and often require the use of fungicide drench, crop rotation or soil fumigation for successful crop production. Volatiles from M. albus kill major soil-borne pathogens such as Pythium ultimum, Phytophtohora cinnamomi Rands, Rhizoctonia solani and Verticillium dahliae Kleb. (Strobel et al., 2001). The incorporation of solid-state culture of M. albus to artificially-infested soil or potting mix controlled Verticillium wilt of eggplants (Solanum melongena L.) (Stinson et al., 2003), Phytophthora root rot of pepper (Capsicum annuum L.) (Mercier and Manker, 2005) as well as various damping-off diseases of sugar beet (Beta vulgaris L.) and broccoli (Brassica oleracea L. var. italica L.) seedlings (Mercier and Manker, 2005; Stinson et al., 2003). The incorporation of M. albus to commercial potting mix also resulted in plant growth enhancement and control of naturally-occurring damping-off (Fig. 3), associated with the elimination of the background plant pathogens population (Mercier and Manker, 2005). Experiments conducted with fresh rye culture incorporated to potting mix infested with R. solani show that disease control by biofumigation is immediate and local (Mercier and Manker, 2005). Mixing M. albus-treated potting mix with pathogen-infested mix at different times showed that biofumigation activity of the fungus in soil substrates is shortlived. M. albus survives poorly in soil substrates and is increasingly difficult to re-isolate 24 h after incorporation (J. Mercier, unp ublished). While most soil biocontrol agents are used to colonize the rhizosphere and directly compete with pathogens, M. albus acts as a short-lived fumigant that reduces or eliminates pathogen populations. Control of building molds. Mold contamination of buildings has been associated with various odor and health problems (Li and Yang, 2004). Control measures include the removal of colonized building material, the control of moisture sources and the application of germicidal solutions. M. albus volatiles killed most species of fungi associated with building mold problems and significantly reduced the population of Cladosporium cladosporioides (Fresen) G.A. de Vries, Stachybotrys chartarum (Ehrenb.) S.J. Hughes and Aspergillus niger Van Tieghem on the surface of gypsum drywall (Mercier and Jiménez, 2007). It was also effective as a preventative treatment for killing molds on drywall before simulated water damage (Fig. 4). Such biofumigation application could be particularly useful for treating building material in hard-to-reach area prone to fungal colonization.


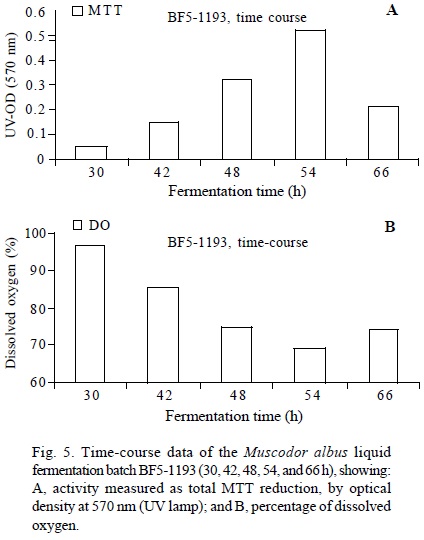
Production and formulation of biofumigant preparations of M. albus. Muscodor growth and production. M. albus production for use as biofumigant is commonly achieved in liquid or solid-state fermentation. It may involve a liquid fermentation in a basic culture medium, and a solid-state fermentation. For inoculum production, two growth conditions with potato-dextrose-broth in baffled flasks agitated with a magnetic stirrer or an orbital shaker were tested to increase the amount dissolved oxygen. It was found that the growth using magnetic stirring was detrimental and required a week to reach the optimum growth. On the other hand, growth on an orbital shaker required only 72 h and mycelial pellets were observed after 24 h incubation. Once the inoculum is ready, it is possible to scale up mycelium production in liquid fermentation. It is very important to monitor the growth of M. albus during the liquid or solid fermentation process to avoid the adverse effects metabolites may induce (Fig. 5). One technique for monitoring growth during fermentation is by using a colorimetric technique to evaluate microbial respiration. The tetrazolium salt (3-(4,5-dimethyltetrazolium-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO) is a colorimetric assay and was selected to monitor the viability and proliferation of the mycelium during liquid fermentation. It is based on the ability of viable cells to reduce the yellow tetrazolium salt to dark blue formazan crystals during mitochondrial electron transport activity (Gomez-Flores et al., 1995). The formazan crystals can then be released and dissolved in an extraction buffer containing sodium dodecyl sulfate (SDS) and dimethyl-formamide (DMF) and quantification can be accomplished by optical density readings of supernatant culture at 540, 570 or 620 nm. Large scale production of mycelium inoculum can be easily monitored using the MTT values and dissolved oxygen data to determine the optimal time for harvesting of tanks (Fig. 5). The MTT assay is also useful to monitor the course of solid-state fermentation and to discriminate and select formulation samples with the best shelf-life profile. Using the same medium for all liquid fermentation steps (inoculum train) helps reducing total fermentation time (unpublished data). Growth of M. albus in solid-state fermentation. The liquid mycelial broth is used to inoculate autoclaved grain in a scale-up production process. The quality of the biofumigant end-use product can be affected by a number of factors during the solid-state fermentation process. As for liquid fermentation, time-course monitoring is recommended to avoid the accumulation of metabolic waste compounds which could interfere with biomass production. Several types of grains and other starch or cellulose-based materials have been tested as nutritive source and carriers to grow M. albus by solid-state fermentation. M. albus grown on barley can be air-dried and delivered to the target area (Stinson et al., 2003; Strobel et al., 2005). However, grain-based formulations must have sufficient moisture to be properly reactivated in order to have efficacious volatile compound production. Whole or chopped rye grain have demonstrated to be excellent culture support choices to evaluate the antimicrobial activity using postharvest, damping-off, and building mold tests (Jiménez and Mercier, 2005; Mercier and Jiménez, 2007; Mlikota-Gabler et al., 2006). Under optimal growth conditions, solid fermentation may take a week to allow the fungus to fully colonize the solid substrate. The colonized grain must be air-dried to arrest growth and keep the fungus in a "suspended" state (Strobel et al., 2005). Formulation of M. albus. Muscodor spp. grown on rye and barley grain has been used in many post-harvest and soil fumigation trials (Mercier and Jiménez, 2005; Mercier and Jiménez, 2007; Mlikota-Gabler et al., 2005; Stinson et al., 2003; Strobel et al., 2005). It would be advantageous to develop a formulation with higher efficacy and longer shelf-life, and when possible, minimize or eliminate the solid-state fermentation step. Muscodor spp. can be grown or formulated on ground pasta (semolina flour and kaolin clay), alginate capsules (sodium alginate and kaolin clay) and "stabilize" (a formulation made of water absorbent starch, corn oil, sucrose, and fumed silica). Out of these formulations, the stabilize formulation showed the best results as soil biofumigant, but a stunting effect in sugar beet plants was noticed (Grimme, 2004; Stinson et al., 2003;). Using a mixture ofpre-gelatinized starch, cellulose and Proflo® oil (cotton seed oil, Traders Protein, Memphis, TN), it is possible to formulate M. albus into a pelletelized formulation. This formulation showed good efficacy in post-harvest tests, but it could cause plant stunting in soil tests (unpublished data). Sterilized paper pellets mixed with different nutrient sources such as casein, soy flour or potato flakes showed initial good results, but completely failed in long-term stability experiments (unpublished data). The use of paper or cellulose as carriers in combination with carbohydrates, vegetable or mineral oil, casein and nitrogen sources marginally increased the shelf-life and stability up to 5 months at room temperature. A possibly less expensive formulation was made using sawdust, rye grits and several nitrogen sources which required a shorter solid-state fermentation stage, but had inconsistent results in soil tests. Biocar®, a soybean product water-absorbent material, was also tested as a carrier, but these formulations had poor performance in soil tests. A formulation combining cellulose, potato flakes and mineral oil had the highest shelf-life at room temperature with good results in postharvest and soil tests (unpublished data). Biofumigant production and formulation lots are evaluated for activity in soil with a damping-off control assay. For this assay, potting mix is infested with Rhizoctonia solani and the biofumigant formulations are incorporated, followed by planting with broccoli seeds (Mercier and Manker, 2005). Activity for postharvest systems is evaluated with a box fumigation test where agar plates to which a spore suspension of Penicillium expansum has been applied are placed in the presence of the activated biofumigant for periods ranging from 24 to 48 h at room temperature. The plates are then taken out and incubated in ambient air and growth compared to the non-fumigated control. The killing of P. expansum spores in Petri plat es placed in closed plastic boxes in the presence of active grain culture of M. albus was found to correlate closely with the control of blue mold of apples in wound-inoculated fruit. Shelf-life at room (25°C) and cold (4°C) temperatures is also followed with viability test from MTT, viable cell counts and volatile production by gas chromatography.
CONCLUSIONS
The potential commercialization of microorganisms as biological control agents are based on their efficacy. To achieve efficacy, most important issues rest mainly on finding appropriate production and formulation methodologies. Since M. albus is a novel fungus that occurs only as a sterile mycelium, its production in a stable formulation is a challenging endeavor. Furthermore, the very unusual mode of action of this volatile-producing organism requires different assays than are normally used for biocontrol agents that rely on antibiotic production or competition to control diseases and pests. With continued improvement of our knowledge of this organism, it is likely that further advances can be achieved to develop it into biofumigant formulations with lower production cost, higher stability and activity.
LITERATURE CITED
Adaskaveg, J.E., Fórster, H., and Sommer, N.F. 2002. Principles of postharvest pathology and management of decays of edible horticultural crops. pp. 163-195. In: A.A. Kader (ed.). Postharvest Technology of Horticultural Crops. Publication 3311. University of California, Agriculture and Natural Resources. Oakland, California, USA. 32 p. [ Links ]
Bjurman, J., and Kristensson, J. 1992. Volatile production by Aspergillus versicolor as a possible cause of odor in houses affected by fungi. Mycopathologia 118:175-178. [ Links ]
Corcuff, R., Mercier, J., Marquet, X., and Arul, J. 2006. Biofumigation potential of Muscodor albus volatiles in the storage of potato tubers. Phytopathology 96:S26 (Abstract). [ Links ]
Daisy, B., Strobel, G., Ezra, D., Castillo, U.F., Baird, G., and Hess, W.M. 2002. Muscodor vitigenus anam. sp. nov., an endophyte from Paullinia paullinioides. Mycotaxon 84:39-50. [ Links ]
Dennis, C., and Webster, J. 1971. Antogonistic properties of species-groups of Trichoderma. 11. Production of volatile antibiotics. Transactions of the British Mycological Society 57:41-48. [ Links ]
Ezra, D., Hess, W.M., and Strobel, G.A. 2004. New endophytic isolates of Muscodor albus, a volatile-antibiotic-producing fungus. Microbiology 150:4023-4031. [ Links ]
Ezra, D., and Strobel, G.A. 2003. Effect of substrate on the bioactivity of volatile antimicrobials produced by Muscodor albus. Plant Science 165:1229-1238. [ Links ]
Freitas, P., Suslow, T., and Mercier, J. 2005. Biofumigation with Muscodor albus for postharvest control of gray mold rot and Salmonella contamination of tomatoes. Phytopathology 95:S31 (Abstract). [ Links ]
Gómez-Flores, R.S. Gupta, R., Tamez-Guerra, P., and Mehta, R.T. 1995. Determination of MICs for Mycobacterium avium-M. intracellulare complex in liquid medium by a colorimetric method. Journal of Clinical Microbiology 33:1842-1846. [ Links ]
Grimme, E. 2004. Effect ofmycofumigation using Muscodor albus and Muscodor roseus on diseases of sugar beet and Chrysanthemum. M.Sci. thesis. Montana State University. Bozeman, Montana, USA. 99 p. [ Links ]
Jiménez, J.I., and Mercier, J. 2005. Optimization of volatile organic compound production from rye grain culture of Muscodor albus for postharvest fumigation. Phytopathology 95:S48 (Abstract). [ Links ]
Li, D.W., and Yang, C.S. 2004. Fungal contamination as a major contributor to sick building syndrome. Advances in Applied Microbiology 55:31-112. [ Links ]
Mercier, J., and Jiménez, J.I. 2004. Control of fungal decay of apples and peaches by the biofumigant fungus Muscodor albus. Postharvest Biology and Technology 31:1-8. [ Links ]
Mercier, J., and Jiménez, J.I. 2007. Potential of the volatile-producing fungus, Muscodor albus, for control of building molds. Canadian Journal of Microbiology 53:404-410. [ Links ]
Mercier, J., and Manker, D.C. 2005. Biological control of soil-borne diseases and plant growth enhancement in greenhouse soilless mix by the volatile-producing fungus Muscodor albus. Crop Protection 24:355-362. [ Links ]
Mercier, J., and Smilanick, J.L. 2005. Control of green mold and sour rot of stored lemon by biofumigation with Muscodor albus. Biological Control 32:401-407. [ Links ]
Mercier, J., Walgenbach, P., and Jiménez, J.I., 2005. Biofumigation with Muscodor albus pads for controlling decay in commercial table grape cartons. HortScience 40:1144 (Abstract). [ Links ]
Mlikota-Gabler, F., Fassel, R., Mercier, J., and Smilanick, J.L. 2006. Influence of temperature, inoculation interval, and dosage on biofumigation with Muscodor albus to control postharvest gray mold on grapes. Plant Disease 90:1019-1025. [ Links ]
Rapior, S., Fons, F., and Bessiere, J. 2000. The fenugreek odor of Lactarius helvius. Mycologia 92:305-308. [ Links ]
Schnabel, G., and Mercier, J. 2006. Use of a Muscodor albus pad delivery system for the management of brown rot of peach in shipping cartons. Postharvest Biology and Technology 42:121-123. [ Links ]
Schnurer, S., Olsson, J., and Borjesson, T. 1999. Fungal volatiles as indicator of food and feeds spoilage. Fungal Genetics and Biology 27:209-217. [ Links ]
Sopalun, K., Strobel,G.A., Hess, W.M., and Worapong, J. 2003. A record of Muscodor albus, an endophyte from Myristica fragrans, in Thailand. Mycotaxon 88:239-247. [ Links ]
Stinson, A.M., Zidack, N.K., Strobel, G.A., and Jacobsen, B.J. 2003. Mycofumigation with Muscodor albus and Muscodor roseus for control of seedling diseases of sugar beet and Verticillium wilt of eggplant. Plant Disease 87:1349-1354. [ Links ]
Strobel, G.A., Dirkse, E., Sears, J., and Markworth, C. 2001. Volatile antimicrobials from Muscodor albus, a novel endophytic fungus. Microbiology 147:2943-2950. [ Links ]
Strobel, G.A., Manker, D.C., and Mercier, J. 2005. Endophytic fungi and methods of use. US Patent 6,911,338. 9 p. [ Links ]
Worapong, J., Strobel, G., Ford, E.J., Li, J.Y., Baird, G., and Hess, W.M. 2001. Muscodor albus anam. sp. nov., an endophyte from Cinnamomum zeylanicum. Mycotaxon 79:67-79. [ Links ]
Worapong, J., Strobel, G., Daisy, B., Castillo, U.F., Baird, G., and Hess, W.M. 2002. Muscodor roseus anam. nov. an endophyte from Grevilleapteridifolia. Mycotaxon 81:463-475. [ Links ]














