Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista mexicana de fitopatología
On-line version ISSN 2007-8080Print version ISSN 0185-3309
Rev. mex. fitopatol vol.25 n.2 Texcoco 2007
Artículos científicos
Antifungal Activity of Plant Methanolic Extracts Against Fusarium verticillioides (Sacc.) Nirenb. and Fumonisin B1 Production
Actividad antifúngica de extractos vegetales sobre Fusarium verticillioides (Sacc.) Nirenb. y producción de fumonisina B1
Guadalupe Miroslava Suárez-Jiménez, Mario Onofre Cortez-Rocha, Ema Carina Rosas-Burgos, Armando Burgos-Hernández, Maribel Plascencia-Jatomea, and Francisco Javier Cinco-Moroyoqui
Universidad de Sonora, Departamento de Investigación y Posgrado en Alimentos, Apdo. Postal 1658, Hermosillo, Sonora, México CP 83000. Correspondencia: mcortez@guayacan.uson.mx.
Received: June 21, 2007
Accepted: September 10, 2007
Abstract
The aim of this work was to determine the inhibitory effect of methanolic extracts of Ambrosia confertiflora, Azadirachta indica, Baccharis glutinosa, and Larrea tridentata on spore germination and mycelial growth of Fusarium verticillioides. The spore germination percentage, radial growth extension, and biomass production were evaluated on potato-dextrose-agar (PDA) amended with 5.6, 11.0, 14.0, and 16.7% (v/v) of the extracts at 25°C. PDA medium with or without methanol was used as a control. Results showed that extracts of B. glutinosa and L. tridentata had the highest inhibitory effect (P < 0.05) on spore germination (> 92%), radial growth extension (> 90%), and biomass production (> 95%) at 100, 336, and 336 h after inoculation, respectively. On the other hand, extracts from A. confertiflora and A. indica were not significantly different (P > 0.05) from the control. These results indicate that the extracts of B. glutinosa and L. tridentata might be natural and effective alternatives for control of F. verticillioides during its different growth phases, spores germination, and mycelial growth.
Keywords: Antifungal properties, natural compounds, Ambrosia confertiflora, Azadirachta indica, Baccharis glutinosa, Larrea tridentata.
Resumen
El objetivo de este trabajo fue determinar el efecto inhibitorio de extractos metanólicos de Ambrosia confertiflora, Azadirachta indica, Baccharis glutinosa y Larrea tridentata en la germinación de esporas y en el crecimiento micelial de Fusarium verticillioides. El porcentaje de germinación de esporas, el crecimiento radial y la producción de biomasa se evaluaron usando papa-dextrosa-agar (PDA) con 5.6, 11.0, 14.0 y 16.7% (v/v) de los extractos a 25°C. Se utilizó medio PDA con y sin metanol como testigo. Los resultados indicaron que los extractos de B. glutinosa y L. tridentata presentaron el mayor efecto inhibitorio (P < 0.05) en la germinación de las esporas (> 92%), en el crecimiento radial (> 90%) y en la producción de biomasa (> 95%) a las 100, 336 y 336 h después de la inoculación, respectivamente. Por otro lado, los extractos de A. confertiflora y A. indica no fueron significativamente diferentes (P > 0.05) al testigo. Estos resultados indican que los extractos de B. glutinosa y L. tridentata pueden ser una fuente de productos naturales alternativos y efectivos para el control de F. verticillioides en sus diferentes fases de crecimiento, en la germinación de esporas, así como en el crecimiento micelial.
Palabras clave: Propiedades antifúngicas, compuestos naturales, Ambrosia confertiflora, Azadirachta indica, Baccharis glutinosa, Larrea tridentata.
The genus Fusarium is one of the most important pathogens in corn (Zea mays L.), being F. graminearum Schwabe, F. verticillioides (Sacc.) Nirenberg, and F. subglutinans (Wollenweb and Reinking) Nelson, Toussoun and Marasas the most persistent species (Marasas et al., 1984; Nelson, 1992; Pitt and Hocking, 1999). F. verticillioides is however, one of the predominant pathogens associated with corn worldwide. Besides the economic impact of corn diseases caused by Fusarium, some species are able to produce highly potent mycotoxins such as zearalenone, T-2 toxin, tricothecenes, vomitoxin and fumonisins, which may affect both animal and human health (Marasas et al., 1984; Moreno, 1988; Nelson et al., 1993). F. verticillioides is able to produce toxins such as fumonisins most frequently in corn (Blackwell et al., 1996; Marasas, 1996; Marasas et al., 2000; Nelson, 1992), sorghum [Sorghum bicolor (L.) Moench.] and oat (Avena sativa L.) (Acuña et al., 2005; Bacon and Nelson, 1994; Leslie et al., 1990), rice (Oryza sativa L.) (Abbas et al., 1998), and wheat (Triticum aestivum L.) (Castoria et al., 2005; Shephard et al., 2005). Fumonisins are stable during food processing: they are not degraded during corn fermentation (Scott and Lawrence, 1995); they are heat stable (Marasas et al., 2000) and resistant to canning and baking processes (Castelo et al., 1998), although in corn the nixtamalization process reduces fumonisin B1 levels, a five-fold more toxic product with respect to the original level (Bullerman et al., 2002; Hendrich et al., 1993; Voss et al., 1996). To avoid effectively the associated problems with Fusarium and their toxins, it is necessary to prevent fungal growth on the substrate, which can be achieved by the use of chemical inhibitors (Moreno and Ramírez, 1985). Organic acids and their salts, like propionic and sorbic acids, have been added in corn destined for animal consumption (Marín et al., 1999a; 1999b). Recent studies have shown that growth of Fusarium species can be controlled by propionic acid and their commercial formulas, depending on water availability and temperature (Marín et al., 2000). The use of natural bioactive substances for control of postharvest fungal infections has gained attention due to problems associated with chemical agents. These include the development of fungal species resistant to chemical treatments, which increases food-borne pathogenic microorganisms, in addition to increasing the number of pesticides under observation or regulation (Rabea et al., 2003). Natural compounds such as essential oils of oregano (Origanum vulgare L.), lemon [Citrus limon (L.) N.L. Burm.], and palmarose [Cymbopogon martinii (Roxb.) J.F. Watson] (Vellunti et al., 2003), may constitute potential preservative compounds due to their capacity to inhibit fungal growth and toxin production of Fusarium. Also, essential oils of cinnamon (Cinnamomum zeylanicum Blume) and oregano have shown fungicidal activity in vitro against Aspergillus flavus Link: Fr. (García-Camarillo et al., 2006). Aqueous plant extracts from garlic (Allium sativum L.), creosote bush [Larrea tridentata (Seesé and Moc. ex D.C.) Coville], and clove [Sycygium aromaticum (L.) Merr. and Perry] inhibited the growth of Fusarium oxysporum Schlechtend:Fr. f. sp. lycopersici (Sacc.) Snyder and Hansen, Rhizoctonia solani Kühn, and Verticillium dahliae Kleb. (López-Benítez et al., 2005). According to Verastegui et al. (1996), alcoholic extracts from natural desert plants like Baccharis glutinosa William and Wilma and Larrea tridentata, may act against the growth of fungi, yeast, and bacteria. In addition, Sánchez et al. (2005) reported the inhibition of both growth and mycotoxin production by Aspergillus flavus and A. parasiticus Speare when exposed to ethanolic, methanolic, and aqueous extracts of Agave species. For that reason, it is possible that native plants such as L. tridentata, Baccharis glutinosa, Ambrosia confertiflora DC, and Azadirachta indica A. Juss. can be used as source of natural preservative compounds for the control of filamentous fungi like Fusarium verticillioides. The aim of this work was to study the inhibitory effects of methanolic crude plant extracts (Larrea tridentata, Baccharis glutinosa, Ambrosia confertiflora, and Azadirachta indica), on spore germination, biomass production, and radial growth of Fusarium verticillioides. Also, to evaluate the effect of the extracts on fumonisin B1 production.
MATERIALS AND METHODS
Plant extracts and media preparation. Stems and leaves of Larrea tridentata, Baccharis glutinosa, and Ambrosia confertiflora were collected from several regions of Sonora, Mexico. Azadirachta indica was obtained from Ciudad Obregon, Sonora. They were sun dried for 24 h and milled (Willey Laboratory Mill model 4, USA) to a particle size of 0.5-1.0 mm. Extracts were prepared by mixing 6 g of each plant powder with 94 mL of a 70% (v/v) methanol solution, with 15 min of stirring and incubated at 25 ± 2°C. Extracts were filtered through a Whatman No. 1 filter paper, then through a micropore glass fiber paper, and stored at 5°C in darkness until further use (Tequida-Meneses et al., 2002). Each methanolic plant extract was added to potato-dextrose-agar (PDA) nutrient medium (DIFCO, USA) at concentrations of 5.6, 11.1, 13.9, and 16.7% (v/v). PDA plates with 30% methanol and without methanol were used as controls.
Microorganism and growth conditions. A strain ofFusarium verticillioides isolated from natural contaminated corn, was selected due to its high fumonisin B1 production (763.75 |ig/ g, detected by HPLC analysis) (Gallardo-Reyes et al., 2006). The strain was activated in PDA and incubated at 25 ± 2°C for 10 days. Spores were collected by pouring a sterile solution of 0.1% (v/v) Tween 20 into the flask and stirring with a magnetic bar for 5 min. The spore concentration of 1 x 105 was determined using a Neubauer chamber.
Spore germination assays. Agar plates were inoculated by spreading the spore suspension onto the agar surface, and incubated at 25°C using a 12 h light/dark cycle (Precision low temperature illuminated incubator 818, USA). The number of germinated spores per plate was determined by taking at random 200 spores (germinated and non-germinated) at 4, 8, 12, 16, 20, 28, 48, 72, and 100 h after plating and using a light microscope. A spore was considered germinated when the length of its germinal tube reached one-half of the spore diameter (Paul et al., 1993; Plascencia-Jatomea et al., 2003; Smilanick et al., 1990). Each germination experiment was made in duplicate and the concentration that delayed 50% of spore germination (CC50) was determined at 95% of confidence intervals using Probit analysis in the NCSS 2001 program (NCSS Inc., USA) (Finney, 1952; Infante and Calderón, 1994). For each inhibitor compound, the percentage of germinated spores of F. verticillioides was fitted to the logistic expression (Equation 1), where S was the percentage of germinated spores after time (t), S0 was the initial percentage of germinated spores, Smax was the highest percentage of germinated spores as the time increases, and k was the spore germination rate. The S0 , Smax and k values were estimated using the NCSS 2001 program (NCSS Inc., USA). The inhibition of spore germination was calculated as a percentage of the control (Equation 2), in which Sc represented the percentage of spores germinating in the treated samples and Sc was the percentage of spores germinating in the control. The percentage of spore germination inhibition was calculated from data of 100 h old cultures.


Radial extension growth. Puncture inoculation of F. verticillioides carried out by a point wise deposition of the inoculum in the center of the plate, was used to measure the radial growth of the colony which was measured daily and compared to the control media. The radial inhibition percentages were calculated using Equation 3: where Rc was the mean value of colony radius of control media and Ri was the colony radius of the inhibitor amended media (Holmes and Eckert, 1999; Plascencia-Jatomea et al., 2003). The radial extension rate of the colony, U (µm/h), was determined from the slope resulting from the radio versus time graph. The extract concentration that delayed 50% of colony radial extension (CI50) was determined at 95% of confidence intervals, using a Probit analysis with NCSS 2001 statistical program (NCSS Inc., USA). All the measurements or analysis were carried out in triplicate.

Biomass production. The biomass production was quantified daily as the mycelium dry weight during 2 weeks. The agar gel with the produced biomass was separated from the plate, poured into a glass beaker containing 200 mL of distilled water, and heated until complete dissolution of the agar. The solution was vacuum filtered using a previously weighted Whatman No. 40 filter paper and washed once with distilled water. Finally, the filter containing the mycelium was dried at 105°C for 2 h and the colony dry weight was expressed in mg/cm2, corresponding to mg of mycelium per plate area (Larralde et al., 1997; López et al., 1997). All determinations were carried out in triplicate. Growth curves were adjusted to the logistic expression (Equation 4), where X was the biomass density (mg/cm2), Xmax was the highest biomass production (when dX/dt = 0) and max was the specific growth rate (when X<Xmax). Growth parameters were estimated using the Statistica 6.0 program (Stat Soft, Inc., USA), which minimized the sum of the square of the error by comparing the experimental data to predicted data obtained by the resolution of Equation 4, given by Equation 5, where X0 was the initial value of biomass density.

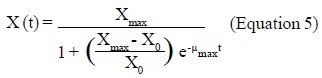
Fumonisin B1 production. The most effective extracts against fungal growth were selected to study their possible effects on fumonisin B1 production in corn grain inoculated with F. verticillioides, using their CI50 on the radial growth. The extracts tested were Baccharis glutinosa and Larrea tridentata at concentrations of 7.4 and 4.0% (v/v), respectively. The FB1 production was determined according to Castellá et al. (1999) using healthy maize as substratum. Corn grain (50 g) portions, free from FB1, were placed in 500 mL Erlenmeyer flasks, adjusted at 40% humidity, and sterilized for two consecutive days in an autoclave for 15 min at 121°C. Autoclaved maize was separately treated with extracts from B. glutinosa and L. tridentata at 7.4 and 4.0% (v/v), respectively. Control flasks were prepared following the same procedure with no extract added or methanol alone. Each treatment was inoculated with 1 x 107 spores of F. verticillioides. Flasks were incubated for 30 days at 25 ± 2°C using a 12 h light/dark cycle (Precision low temperature illuminated incubator 818, USA). Three replicates for each treatment were performed. Separation and purification of FB1 was carried out according to Etcheverry et al. (2002). The cultures were oven-dried overnight at 50°C and the FB1 was extracted with 150 mL of acetonitrile:water (1:1 v/v) by shaking the culture media and mycelia with the solvent for 30 min on an orbital shaker (150 rev min-1). The extracts were filtered through a Whatman No. 4 filter paper (International Ltd, Maidstone, Kent, UK). An aliquot (1000 µL) was taken from the filtrate and diluted with 9 mL of acetonitrile:water. Diluted extracts were purified using a pre-conditioned strong anion exchange column (SAX) SPE (Bond Elut Sax, Varian, Inc., USA). FB1 was eluted with 15 mL of methanol acetic acid 1% and evaporated to dryness under air flow.
Fumonisin B1 quantification. The fumonisin B1 content was determined by HPLC as described by Shephard et al. (1990). The dried eluate was re-dissolved with acetonitrile: water (1:1 v/v). For the analysis, 50 µL were transferred into a test tube, combined with 200 µL of o-phtaldialdehyde solution (OPA), shaken for 30 s, and a 20 µL aliquot was injected into the chromatography system consisting of a pump (9012, Varian, USA) connected to a fluorescence detector (9070, Varian, USA). Chromatographic separation was performed on a stainless steel reversed-phase column (Supercosil LC-ABZ, C18; 150 x 4.6 mm I.D., 5 µm particle size; Supelco, Sigma-Aldrich). Methanol:0.1 mol l-1 sodium dihydrogen phosphate (75:25) solution, adjusted to pH 3.35 with orthophosphoric acid, was used as the mobile phase at a flow rate of 1.5 mL min-1. The fluorescence of the fumonisin B1-OPA derivative was recorded at excitation and emission wavelengths of 335 and 440 nm, respectively. Fumonisin B1 quantification was performed by peak area measurements and compared with a reference standard solution. The standard solution was obtained by dissolving pure fumonisin FB1 (Sigma-Aldrich) in acetonitrile:water (1:1 v/v) at a concentration of 100 µg/mL.
Statistical analysis. A completely randomized 3 factor design (plant, concentration, and time and their interactions) for the radial and germination percent inhibition was carried out. Samples were taken at random from the incubator in triplicate. Experiments on radial growth were done in triplicate while germination assays were done in duplicate. For the fumonisin B1 production assay, 2 plants at two concentrations were applied in triplicate. The program JMP 2004 (JMP Inc., USA) computed the analysis of variance and the means were compared with the Tukey multiple range test (p < 0.05).
RESULTS
Spore germination. The spore germination percentage ofF. verticillioides was calculated for each methanolic extract. The results showed that spores inoculated on PDA only germinated completely within 28 h of incubation, while spores placed on the control with methanol germinated after 100 h of incubation (Table 1). However, there was a significant difference (p < 0.05) in the percentage of spore germination inhibition between methanolic extract and the control with methanol (Table 2). Results showed that extracts from Baccharis glutinosa and Larrea tridentata at all concentrations, were the most effective in controlling spore germination of F. verticillioides. In the same way, in all concentrations tested, these extracts reduced the maximal percentage of germinated spores, Smax (< 12.4%). Extracts from Azadirachta indica and Ambrosia confertiflora significantly inhibited spore germination, only at two of the concentrations tested (11.1 and 16.7%, respectively). According to the adjusted logistic parameters, there was not an observed pattern that relates the spore germination rate (k) to the vegetal extract or concentration (Table 2).

Radial growth. The control with methanol showed an inhibitory effect on F. verticillioides since plates were completely covered with the mycelium after 336 h of incubation (Table 3), while control plates with PDA only, F. verticillioides completely covered the plate after 192 h. Due to its inhibitory effect, methanol was used as control to determine the radial inhibition percentage of extracts (Table 4). Extracts from B. glutinosa and L. tridentata were the most effective to control fungal colony growth at all concentrations. Extracts from A. indica and A. confertiflora, however, were not able to control effectively the colony growth at any concentration, even though fungi grew faster than the control with methanol. Compared with control, all extracts at all concentrations tested, significantly affected the radial extension rate, U (µm/h), of F. verticillioides. Extracts from B. glutinosa and L. tridentata presented the lowest radial extension rates (< 0.008 µm/h). The minimum inhibitory concentration that inhibited 50% of radial growth, CI50, was determined to the extracts that showed the best inhibitory effects (B. glutinosa and L. tridentata). The CI50 for B. glutinosa and L. tridentata extracts were 7.4% and 4.0% (v/ v), respectively.

Biomass production. Table 5 shows that extracts from B. glutinosa and L. tridentata were the most effective in inhibiting biomass production of F. verticillioides at all concentrations. These results were also inconsistent, because F. verticillioides was inhibited by more than 50% with the A. confertiflora extract at a 11.1% concentration, whereas the inhibition was lower at higher concentrations. A. indica was not effective in controlling biomass production at any concentration tested. For all treatments, a pattern which relates maximal biomass production (Xmax) to the specific growth rate (µmax) of the fungi in the presence of the methanolic extract was not observed.

Fumonisin production. The presence of fumonisin B1 was detected in all treatments by HPLC analysis (Table 6). There were not significant differences (p > 0.05) among the treatments. Results showed that in the corn grain treated with either B. glutinosa and L. tridentata extracts at 7.4 and 4.0% concentration, respectively, there was no inhibition of fumonisin B1 production. On the contrary, the fumonisin B1 production was increased with the treatment compared with the control. Analysis determined that there was no difference (p > 0.05) between the control, and each of the treatments.

DISCUSSION
Baccharis glutinosa, Larrea tridentata, and Azadirachta indica extracts were effective in inhibiting spore germination and radial growth of F. verticillioides. The inhibitory effect of extracts did not increase when concentration increased. Indeed, these results were inconsistent, and in some cases the highest concentrations had no inhibition in some of the treatment, as observed with A. indica and A. confertiflora extracts. This agrees with the findings of Juglal et al. (2002), who also reported that neem oil did not affect mycelial growth of Fusarium moniliforme Sheld and Aspergillus parasiticus ; however, fumonisin B1 production increased as in our study. The methanolic extracts with the highest fungistatic potential were B. glutinosa and L. tridentata, since they inhibited the radial and mycelial growth of F. verticillioides. This means that in order to inhibit 50% of F. verticillioides radial growth, 7.4 (v/v) of the B. glutinosa and 4.0% (v/v) of the L. tridentata extracts are required in the media. However, the same concentrations of these extracts were not effective in inhibiting the production of fumonisin B1 in corn grain, whereas the extracts that can inhibit growth on synthetic media, are not effective on corn at the same concentrations. Nevertheless, further studies are necessary to evaluate the effectiveness of the extracts at other concentrations to inhibit fumonisin B1 production by F. verticillioides. Similar results were reported by Vellunti et al. (2003), who found that essential oils from plants have an inhibitory effect on fumonisin B1 production by Fusarium proliferatum (Matsushima) Nirenberg, as well as in its growth. Bullerman (1974) and Bullerman et al. (1977) found that aflatoxin production was inhibited without affecting fungal growth. In contrast, López et al. (2004) reported that Aloysia triphylla (L' Hér.) Britton and Origanum vulgare oils inhibited the mycelia growth ofF. verticillioides, however, fumonisin B1 production was inhibited by O. vulgare while A. triphylla increased it. Cespedes et al. (2006) reported that Tagetes lucida Cav. methanolic extracts inhibited F. moniliforme growth, however, fumonisin production was not evaluated. Also, Cárdenas-Ortega et al. (2005) reported that essential oil of Chrysactinia mexicana Gray caused inhibition of A. flavus growth in liquid culture media. The A. confertiflora extract at a 11.1% concentration showed a similar effect (p > 0.05) to that observed when either B. glutinosa, or L. tridentata extract was used. The A. indica extract showed less than 50% inhibition, which is contrary to that reported in a previous study in which an ethanolic extract from A. indica inhibited the incidence of Colletotrichum glocosporioides (Penz.) Penz. and Sacc. in mango (Mangifera indica L.) (Bommarito et al., 1998). This might suggest that the extract effectiveness may depend on both the target organism and the substrate. The inhibitory activity of L. tridentata extract on growth of F. verticillioides found in the present study agrees with its effect against the growth of A. flavus and A. parasiticus reported previously by Vargas-Arispuro et al. (2005). They analyzed the composition of the ethanolic extract of L. tridentata leaves, finding norhydroguaiaretic acid (NDGA) and methyl-norhydroguaiaretic (methyl-NDGA) as the main components responsible for the antifungal effect. Fumonisin production was not inhibited with the two extracts at the tested concentrations, even though when they reduced the radial growth of F. verticillioides. Although when radial growth was restringed by the plant extracts, we observed atypical apical growth and the aerial mycelium reached the cover of the Petri dishes. These could be due to the stressful environment generated by the plant extracts in the Petri dishes and physiological responses to overcome these conditions may result in over expression of mycotoxin production. The use of these plant extracts alone does not appear feasible to control fumonisin production by F. verticillioides. Our findings evidence that the use of methanolic crude plant extracts of Baccharis glutinosa and Larrea tridentata inhibited fungal growth, but they did not affect fumonisin production.
ACKNOWLEDGEMENTS
The study was funded by the National Council of Science and Technology (CONACyT) through the project 38926-N at the University of Sonora. Authors acknowledge M.A. Jesús Borboa-Flores for the field plant sampling.
LITERATURE CITED
Abbas, H.K., Cartwright, R.D., Shier, W.T., Abouzied, M.M., Bird, C.B., Rice, L.G., Ross, P.F., Sciumbato, G.L., and Meredith, F.I. 1998. Natural occurrence of fumonisins in rice with Fusarium sheath rot disease. Plant Disease 82:22-25. [ Links ]
Acuña, A., Lozano, M.C., de García, M.C., and Díaz, G.J. 2005. Prevalence of Fusarium species of the Liseola section on selected Colombian animal feedstuffs and their ability to produce fumonisins. Mycopathologia 160:63-66. [ Links ]
Bacon, C.W., and Nelson, P.E. 1994. Fumonisin production in corn by toxigenic strains of Fusarium moniliforme and Fusarium proliferatum. Journal of Food Protection 57:514-521. [ Links ]
Blackwell, B.A., Edwards, O.E., Fruchier, A., Apsimon, J.W., and Miller, J.D. 1996. NMR Structural studies of fumonisin B1 and related compound from Fusarium moniliforme. pp. 75-91. In: L.S. Jackson, J.W. Devries, and L.B. Bullerman (eds.). Fumonisins in Food. Plenum Press. New York, USA. 412 p. [ Links ]
Bommarito, S.G., Greennough, R.D., and Tudela, A.F. 1998. Inhibition of mango anthracnose (Colleotrichum gloeosporioides) and stemend rot (Phomposis magniferae) by ethanolic leaf extracts of neem (Azadirachta indica Juss) in the Northern Mariana Islands. Phytopathology 88:S9. [ Links ]
Bullerman, L.B. 1974. Inhibition of aflatoxin production by cinnamon. Journal of Food Science 39:1163-1165. [ Links ]
Bullerman, L.B., Lieu, F. Y., and Seier, S.A. 1977. Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol. Journal of Food Science 42:1107-1109. [ Links ]
Bullerman, L.B., Ryu, D., and Jackson, L.S. 2002. Stability of fumonisins in food processing. Advances in Experimental Medicine and Biology 504:195-204. [ Links ]
Cárdenas-Ortega, N.C., Zavala-Sánchez, M.A., Aguirre-Rivera, J.R., Pérez-Gónzalez, C., and Pérez-Gutiérrez, S. 2005. Chemical composition and antifungal activity of essential oil of Chrysactinia mexicana Gray. Journal of Agricultural and Food Chemistry 53: 4347-4349. [ Links ]
Castellá, G., Bragulat, M.R., and Cabañes, F. J. 1999. Fumonisin production by Fusarium species isolated from cereals and feeds in Spain. Journal of Food Protection 62:811-813. [ Links ]
Castelo, M.M., Sumner, S.S., and Bullerman, L.B. 1998. Stability of fumonisins in thermally processed corn products. Journal of Food Protection 61:1030-1033. [ Links ]
Castoria, R., Lima, G., Ferracane, R., and Ritieni, A. 2005. Occurrence of mycotoxin in Farro samples from Southern Italy. Journal of Food Protection 68:416-420. [ Links ]
Cespedes, C.L., Avila, J.G., Martínez, A., Serrato, B., Calderón-Mugica, J.C., and Salgado-Garciglia, R. 2006. Antifungal and antibacterial activities of Mexican Tarragon (Tagetes lucida). Journal of Agricultural and Food Chemistry 54:3521-3527. [ Links ]
Etcheverry, M., Torres, A., Ramírez, M.L., Chulze, S., and Magan, N. 2002. In vitro control of growth and fumonisin production by Fusarium verticillioides and F. proliferatum using antioxidants under different water availability and temperature regimes. Journal of Applied Microbiology 92:624-632. [ Links ]
Finney, D.J. 1952. Probit Analysis. Cambridge University Press. 2th edition. London, England. 318 p. [ Links ]
Gallardo-Reyes, E.D., Ibarra-Moreno, G.M., Sánchez-Mariñez, R.I., Cuamea-Cruz, G., Molina Gil, D., Parra-Vergara, N.V., Rosas-Burgos, E.C. y Cortez-Rocha, M.O. 2006. Micobiota de maíz (Zea mays L.) recién cosechado y producción de fumonisina B1 por cepas de Fusarium verticillioides (Sacc.) Nirenb. Revista Mexicana de Fitopatología 24:27-34. [ Links ]
García-Camarillo, E.A., Quezada-Viay, M.Y., Moreno-Lara, J., Sánchez-Hernández, G., Moreno-Martínez, E. y Pérez-Reyes, M.C.J. 2006. Actividad antifúngica de aceites esenciales de canela (Cinnamomum zeylanicum Blume) y orégano (Origanum vulgare L.) y su efecto sobre la producción de aflatoxinas en nuez pecanera [Carya illinoensis (F.A. Wangenh) K. Koch]. Revista Mexicana de Fitopatología 24:8-12. [ Links ]
Hendrich, S., Miller, K.A., Wilson, T.M., and Murphy, P.A. 1993. Toxicity of fumonisins in nixtamalized corn-based diets fed to rats: effect of nutritional status. Journal of Agricultural and Food Chemistry 41:1649-1654. [ Links ]
Holmes, G.J., and Eckert, J.W. 1999. Sensitivity of Penicillium digitatum and P. italicum to postharvest citrus fungicides in California. Phytopathology 89: 716-721. [ Links ]
Infante, G.S. y Calderón, A.L. 1994. Manual de Análisis Probit. Centro de Estadística y Cálculo, Colegio de Posgraduados. Montecillo, Edo. de México. 107 p. [ Links ]
Juglal, S., Goviden, R., and Odhav, B. 2002. Spice oils for the control of co-occurring mycotoxin-producing fungi. Journal of Food Protection 65:683-687. [ Links ]
Larralde, C.C., López, I.F., and Viniegra, G.G. 1997. Morphometric evaluation of the specific growth rate of Aspergillus niger grown in agar plates at high glucose levels. Biotechnology and Bioengineering 56:287-294. [ Links ]
Leslie, J.F., Pearson, C.A.S., Nelson, P.E., and Toussoun, T.A. 1990. Fusarium spp. from corn, sorghum, and soybean fields in the Central and Eastern United States. Phytophatology 80:343-350. [ Links ]
López, F., Larralde, P., and Viniegra, G. 1997. Mass transfer and growth kinetics in filamentous fungi. Chemical Engineering Science 52:2629-2639. [ Links ]
López, A.G., Theumer, M.G., Zygadlo, J.A., and Rubinstein, H.R. 2004. Aromatic plant essential oils activity on Fusarium verticillioides Fumonisin B1 production in corn grain. Mycophatologia 158:343-349. [ Links ]
López-Benítez, A., López-Betancourt, S.R., Vázquez-Badillo, M.E., Rodríguez-Herrera, S.A., Mendoza-Elos, M. y Padrón-Corral, E. 2005. Inhibición del crecimiento micelial de Fusarium oxysporum Schlechtend. f. sp. lycopersici (Sacc.) Snyder y Hansen, Rhizoctonia solani Kühn y Verticilllium dahliae Kleb. mediante extractos vegetales acuosos. Revista Mexicana de Fitopatología 23:183-190. [ Links ]
Marasas, W.F.O., Miller, J.D., Riley, R.T., and Visconti, A. 2000. Environmental health criteria for fumonisin B1. World Health Organization. Geneva, Switzerland. 150 p. [ Links ]
Marasas, W.F.O. 1996. Fumonisins: history, world-wide occurrence and impact. Chap. 1, pp.1-16. In: L.S. Jackson, J.W. Devries, and L.B. Bullerman (eds.). Fumonisins in Food. Plenum Press. New York, USA. 412 p. [ Links ]
Marasas, W.F.O., Nelson, P.E., and Toussoun, T.A. 1984. Toxigenic Fusarium species: identity and mycotoxicology. The Pennsylvania State University Press. University Park, Pennsylvania, USA. 328 p. [ Links ]
Marín, S., Magan, N., Abellana, M., Canela, R., Ramos, A.J., and Sanchis, V. 2000. Selective effect of propionates and water activity on maize mycoflora and impact of fumonisin B1 accumulation. Journal of Stored Product Research 36:203-214. [ Links ]
Marín, S., Homedes, V., Sanchis, V., Ramos, A.J., and Magan, N. 1999a. Impact of Fusarium moniliforme and F. proliferatum colonization of maize on calorific losses and fumonisin production under different environmental conditions. Journal of Stored Products Research 35:15-26. [ Links ]
Marín, S., Sanchis, V., Sanz, D., Castel, I., Ramos, A.J., Canela, R., and Magan, N. 1999b. Control of growth and fumonisin B1 production by Fusarium verticillioides and F. proliferatum isolates in moist maize with propionate preservatives. Food Additives and Contaminants 12:555-563. [ Links ]
Moreno, M.E. 1988. Manual para la Identificación de Hongos en Granos y sus Derivados. Editorial UNAM. Primera edición. México, D.F. 109 p. [ Links ]
Moreno, M.E. and Ramírez, G.J. 1985. Protective effect of fungicides on corn seed stored with low and high moisture content. Seed Science and Technology 13:285-290. [ Links ]
Nelson, P., Desjardins, A., and Plattner, R. 1993. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry and significance. Annual Review of Phytopathology 31:233-252. [ Links ]
Nelson, P.E. 1992. Taxonomy and biology of Fusarium moniliforme. Mycopathologia 117:29-36. [ Links ]
Paul, G.C., Kent, C.A., and Thomas, C.R. 1993. Viability testing and characterization of germination of fungal spores by automatic image analysis. Biotechnology and Bioengineering 42:11-23. [ Links ]
Pitt, J.I., and Hocking, A.D. 1999. Fungi and Food Spoilage. 2nd ed. Aspen Publishers, Inc. Gaithersburg, MD, USA. 593 p. [ Links ]
Plascencia-Jatomea, M., Viniegra, G., Olayo, R., Castillo-Ortega, M.M., and Shirai, K. 2003. Effect of chitosan and temperature on spore germination of Aspergillus niger. Macromolecular Bioscience 3:582-586. [ Links ]
Rabea, E.I., Badawy, M.E.T., Stevens, C.V., Smagghe, G., and Steubaurt, W. 2003. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules 4:1457-1465. [ Links ]
Sánchez, E., Heredia, N., and García, S. 2005. Inhibition of growth and mycotoxin production of Aspergillus flavus and Aspergillus parasiticus by extracts of Agave species. International Journal of Food Microbiology 98:271-279. [ Links ]
Scott, P.M., and Lawrence, G.A. 1995. Analysis of beer for fumonisins. Journal of Food Protection 58:1379-1382. [ Links ]
Shephard, G.S., Sydenham, E.W., Thiel, P.G., and Gelderblom, W.C.A. 1990. Quantitative determination of fumonisin B1 and B2 by high performance liquid chromatography with fluorescence detection. Journal of Liquid Chromatographie 13:2077-2087. [ Links ]
Shephard, G.S., Van der Westhuizen, L., Gatyeni, G.M., Katerere, D.R., and Marasas, W.F.O. 2005. Do fumonisin mycotoxins occur in wheat?. Journal of Agricultural and Food Chemistry 53:9293-9296. [ Links ]
Smilanick, J.L., Harstell, P.I., Henson, D., Fouse, D.C., Assemi, M., and Harris, C.M. 1990. Inhibitory activity of suphur dioxide on the germination of spores of Botrytis cinerea. Phytopathology 80:217-220. [ Links ]
Tequida-Meneses, M., Cortez-Rocha, M.O., Rosas-Burgos, E.C., López-Sandoval, S., and Corrales-Maldonado, C. 2002. Effect of alcoholic extracts of wild plants on the inhibition of growth of Aspergillus flavus, Aspergillus niger, Penicillium chrysogenum, Penicillium expansum, Fusarium moniliforme, and Fusarium poae moulds. Revista Iberoamericana de Micología 19:84-88. [ Links ]
Vargas-Arispuro, I., Reyes-Báez, R., Rivera-Castañeda, G., Martínez-Téllez, M.A., and Rivero-Espejel, I. 2005. Antifungal lignans from the creosote bush (Larrea tridentata). Industrial Crops and Products 22:101-107. [ Links ]
Vellunti, A., Sanchis, V., Ramos, A.J., Egido, J., and Marin, S. 2003. Inhibitory effect of cinnamon, clove, lemongrass, oregano and palmarose essential oils on growth and fumonisin B1 production by Fusarium proliferatum in maize grain. International Journal of Food Microbiology 89:145-154. [ Links ]
Verastegui, M.A., Sánchez, C.A., Heredia, N.L., and García-Alvarado, J.S. 1996. Antimicrobial activity of extracts of three major plants from the Chihuahuan desert. Journal of Ethnopharmacology 552:175-177. [ Links ]
Voss, K.A., Bacon, C.W., Meredith, F.I., and Norred, W.P. 1996. Comparative subchronic toxicity studies of nixtamalized and water-extracted Fusarium moniliforme culture material. Food Chemistry and Toxicology 34:623-632. [ Links ]





![Susceptibility of three apple [Malus sylvestris (L.) Mill. var. domestica (Borkh.) Mansf.] cultivars and management of scab [Venturia inaequalis (Cooke) Wint.] in production systems in the state of Hidalgo, Mexico](/img/en/next.gif)








