Introduction
Coffee is an understory plant, native to Ethiopia and introduced in the nineteenth century to Mexico (Moguel & Toledo, 1999). Coffee cultivation is a major agroecosystem due to its social, economic, and environmental importance (Jha et al., 2014; Flores, 2015). In Mexico, coffee agroecosystems are found in mountainous and flat areas in the Neotropical part of this country and play an important role in the conservation of biodiversity, as it is usually planted under the shade of different tree species, making it a relatively structured and complex agroecosystem (Lin & Perfecto, 2012; Jha et al., 2014).
Some 3,000 species of animals associated with coffee have been recorded in the world, of which 850 are known to feed on the plants and about 30 species are considered pests, the coffee berry borer Hypothenemus hampei (Ferrari, 1867) being the most important economically (Johnson et al., 2020). Methods used to fight pests include cultural control (Bustillo-Pardey, 2006; Aristizábal et al., 2016), synthetic insecticides (Bustillo-Pardey, 2006), pathogenic fungus (Escobar-Ramírez et al., 2019) and parasitoids (Yousuf et al., 2021); however, the role of predators, including spiders, has been little explored.
Spiders are a megadiverse group found in all habitats except polar regions and are known for their sensitivity to changes in habitat conditions, therefore they are useful for studying environmental quality (Ibarra-Núñez, 2014). They are abundant in natural and cultivated environments. Since they are generalist predators, they are considered to have high potential as natural enemies of insect pests (Pekár & Kocourek, 2004; Armendano & González, 2011).
In Mexico, studies have been conducted on the abundance and richness of spider species in coffee agroecosystems (Ibarra-Núñez, 1990; Ibarra-Núñez & García-Ballinas, 1998; Méndez-Castro & Rao, 2014), as well as ecological studies on the types of prey captured by these spiders (Ibarra-Núñez et al., 2001; Henaut et al., 2001). In addition, changes in the composition and abundance of species have been studied as farming techniques intensify (Pinkus et al., 2006; Marín & Perfecto, 2013), while Hajian-Forooshani et al. (2014) and Marín et al. (2016) analyzed the influence of local and landscape factors on arboreal and soil spiders respectively. Most of these studies were made in coffee orchards from a tropical humid area of Chiapas (where previously there were tropical rain forests), but almost nothing is known about other Mexican states that have coffee orchards with other environmental conditions. In the south of the state of Oaxaca, coffee orchards are growing in sub-humid tropical areas, surrounded by tropical deciduous forests. In this area, there are different coffee orchard systems, some corresponding to traditional polyculture shadow, and others to monoculture shadow (Moguel & Toledo, 1999).
Our hypotheses are that uncultivated areas (Tropical Forest) have higher spider diversity than coffee plantations, while coffee systems with Polyculture shade management have higher spider diversity than coffee systems with Monoculture shade management. The objective of this work was to analyze the impact of agronomic management and seasons on spider assemblage composition (abundance, species richness and diversity), in two coffee plantations with different management systems and a portion of tropical forest in two adjacent municipalities of the La Costa region of the state of Oaxaca, Mexico.
Materials and methods
The samplings were carried out in four sites: two coffee agroecosystems with different agronomic management and two sites with tropical forests corresponding to native vegetation. These sites are in La Costa region of Oaxaca, with a subhumid-warm climate (mean annual temperature 23 °C, mean annual rainfall 2,250 mm) (CONANP, 2003). The coffee plantations are 18 km apart and differ in agronomic management. The farm “Loma de Perico” (8 hectares) is in the municipality of San Mateo Piñas (96° 19' 34.2" N, 15° 59' 16.8" W, 829 m.a.s.l.) and has a traditional polyculture system (after Moguel & Toledo, 1999). The shade cover is provided by diverse tree species: Cecropia obtusifolia Bertol. (1840), Bursera simaruba (L.) Sarg. (1890), Inga spp., Ficus tecolutensis (Liebm.) Miq., Anonona muricata L. (1753), Musa spp., Citrus sinensis (L.) Osbeck, Theobroma cacao L.) and Manguifera sp. The second site is in the farm “La Aurora” (50 hectares), in the municipality of Santa María Huatulco (96° 17' 00.1" N, 15° 55' 26.4" W, 1,050 m.a.s.l.), and is a shaded monoculture system (after Moguel & Toledo, 1999). Leguminous shade trees (Inga spp.) provide shade for coffee plants and occasionally Ceiba pentandra (L.) Gaertn. (1791). Both sites have adjacent areas with deciduous tropical forest (after Rzedowski, 1978), 200 m away from the traditional polyculture site, and 350 m away from the shaded monoculture site (Fig. 1). The study area has two well-defined seasons: the dry season, from November to April and the rainy season, from May to October (CONANP, 2003).

Figure 1 Location of the sampling sites in two municipalities in the La Costa region of Oaxaca, Mexico.
Field work. The spider samplings were carried out in two periods, the first period from January 2014 to January 2015, the second one from July 2016 to July 2017. A standardized protocol, concerning sampling methods and sampling effort was applied to every season, with six samplings in each one, in both dry and rainy seasons. For the coffee sites, each sampling lasted two days, while for the forest sites each sampling lasted one day. The forest sites were considered equivalent because they have a similar vegetation structure, so half of the sampling effort was made in each of them in order to collect data from both sites.
Three sampling methods were used to capture spiders with different ecological preferences, foraging strategies and from different strata of the study sites (Cardoso et al., 2011): manual collection, foliage beating and pitfall traps (Ibarra-Núñez et al., 2011). For each technique, in each sampling period, the sampled coffee plants were separated from each other by 2.5 meters and marked with yellow plastic tape to avoid repeated sampling. In the forest sites, we sampled shrubs of a similar size and architecture to the coffee plants (plants of genus Acalypha, Homalium and Saurauia).
For the manual collection and foliage beating methods, 20 plants were sampled in the coffee sites (10 plants per day), while in the tropical forest 10 plants were sampled at each site. Exhaustive visual search and extraction of spiders were performed on leaves, branches, and trunks, allocating 15 minutes for each plant. In the foliage beating method, a white cloth (1.20 m x 1.20 m) was put on the soil surrounding the coffee plant and shaken vigorously for about 30 seconds, then the fallen organisms were put in labeled plastic bags with 80% ethanol, for further separation later in the laboratory. The pitfall traps were plastic containers (diameter 11 cm, height 15 cm) filled to – of their capacity with a soap solution and buried flush with the ground. In each of the coffee sites 10 traps were placed, while in each of the tropical forest sites five traps were placed. In all sites, the spacing between traps was 2.5 meters. The traps were active for 48 h, after which the content was extracted, put on 80% ethanol, and transported to the laboratory for identification.
The spiders were identified at the genus level with the keys by Ubick et al. (2017) and at the species level with specialized literature available in the World Spider Catalog (2022). Juveniles were identified at generic or specific level, when possible, by comparing them with adults, or separated as morphospecies. Some too-young, immature specimens could not be identified below genus or specie level. These specimens were excluded from the diversity analyses, but not from the abundance analyses at family level. Part of the collected specimens were deposited at Colección de Artrópodos Benéficos, Centro Interdisciplinario de Investigación para el Desarrollo Integral Regional (CIIDIR), campus Oaxaca, Instituto Politécnico Nacional, and other specimens were deposited at Colección de Arácnidos del Sureste de México (ECOTAAR), El Colegio de la Frontera Sur, Tapachula, Chiapas, Mexico
Data analysis. Abundance. A previous analysis showed that data was not homoscedastic, therefore we used a two-ways Generalized Linear Model (McCullagh & Nelder, 1989) to determine significant differences in total abundance of spiders (include adults and all immature specimens) among vegetation (Monoculture, Polyculture, and Tropical Forest) and seasons (dry and rainy) for each collection method used. The Generalized Linear Model (GLM) with Poisson distribution error and Log-link function was chosen since it is recommended when working with counts or abundances (Cayuela, 2009).
Due to the environmental conditions during the rainy season, it was not possible to carry out the foliage beating and pitfall traps methods in all sites, therefore, these values were coded as null observation in the data matrix (9.5% of the data).
Diversity. For the diversity analyses only those individuals (adults or juveniles) to which a specific name was assigned or those considered as morphospecies were considered. The completeness of the inventories for each vegetation type was calculated by two methods, one method based on the ratio of observed species richness to estimated richness using the Chao 1 estimate (Cardoso et al., 2008) (observed richness / estimated total richness x 100), and the second method by determining the proportion of "singletons" (number of species with a single individual / total of observed species x 100). These estimators were chosen because they are the indicators commonly used to assess sampling completeness.
Total species richness was estimated by two non-parametric estimators (Chao 1 and ACE) using the EstimateS software, version 9.1 (Colwell, 2013). Samples were randomized 1000 times (Magurran, 2004; Colwell, 2013). We chose these estimators because they have been applied to different spider inventories (Sørensen et al., 2002; Scharff et al., 2003; Cardoso et al., 2008), which will allow a broader comparison of completeness statistics between inventories.
Differences in species richness were evaluated by Rarefaction curves (sample-size-based) with 95% of confidence intervals between sites and seasons (Chao et al., 2014). Effective species numbers were determined using Hill numbers (Jost, 2019), as it makes possible to do direct comparisons of the number of species among the different sampling sites (Jost & González-Oreja, 2012; Moreno et al., 2018). Additionally, the Hutcheson t-test was applied to calculates significance values for differences between pairs of Shannon Index (Magurran, 2004). Furthermore, rank abundance curves were elaborated to observe differences in evenness between sites. All analyzes were carried out with the R software (R Core Team, 2022) using the packages: Ggplot2 (Wickham, 2009), iNEXT (Hsieh et al., 2016), Lattice (Sarkar, 2008), ecolTest (Salinas & Ramírez-Delgado, 2021) and vegan (Oksanen et al., 2022). The map of the studied sites was created using QGIS 3.22.9 “Biatowieza”.
Results
We collected a total of 2,210 spiders, including adults (832) and juveniles (1,378) (Table 1): 686 individuals from the Polyculture site (dry season = 386, rain season = 300), 796 from the Monoculture site (dry season = 428, rain season = 368), and 728 from the Tropical Forest (dry season = 378, rain season = 350). We collected 1,018 spiders during the rainy season and 1,192 during the dry season. Manual collection was the method with the highest return with 1,123 spiders, followed by foliage beating with 1,003 and, finally, the pitfall traps with 84 spiders.
Table 1 List of species (with their respective abundance) collected at two coffee plantations (Monoculture shade and Polyculture shade) and Tropical Forest in the La Costa region of Oaxaca, Mexico. †New species records for Oaxaca, ⸹undescribed taxa, nd: not determined, M: Monoculture, P: Polyculture, TF: Tropical Forest.
| TAXA | M | P | TF |
|---|---|---|---|
| AGELENIDAE | |||
| ⸹Hoffmannilena sp.1 | 1 | 0 | 1 |
| ⸹Rualena sp.1 | 2 | 0 | 1 |
| nd | 13 | 0 | 37 |
| AMAUROBIIDAE | |||
| Amaurobiidae sp | 0 | 1 | 1 |
| ANYPHAENIDAE | |||
| Wulfila tantillus Chickering, 1940 | 22 | 9 | 38 |
| nd | 17 | 25 | 10 |
| ARANEIDAE | |||
| †Acacesia tenella (L. Koch, 1871) | 0 | 16 | 1 |
| Allocyclosa bifurca (McCook, 1887) | 2 | 0 | 1 |
| Araneus expletus (O. Pickard-Cambridge, 1889) | 1 | 0 | 0 |
| †Araneus lineatipes (O. Pickard-Cambridge, 1889) | 0 | 2 | 0 |
| †Araneus pegnia (Walckenaer, 1841) | 1 | 1 | 0 |
| Argiope argentata (Fabricius, 1775) | 0 | 0 | 4 |
| †Argiope blanda O. Pickard-Cambridge, 1898 | 0 | 2 | 0 |
| ⸹Carepalxis sp.1 | 6 | 14 | 3 |
| ⸹Carepalxis sp.2 | 0 | 1 | 0 |
| Cyclosa conigera F. O. Pickard-Cambridge, 1904 | 0 | 1 | 0 |
| †Cyclosa jalapa Levi, 1999 | 1 | 0 | 0 |
| Cyclosa walckenaeri (O. Pickard-Cambridge, 1889) | 1 | 0 | 0 |
| †Eriophora edax (Blackwall, 1863) | 3 | 9 | 5 |
| ⸹Eustala sp. | 0 | 2 | 2 |
| †Gasteracantha cancriformis (Linnaeus, 1758) | 0 | 2 | 0 |
| Larinia directa (Hentz, 1847) | 1 | 1 | 0 |
| Mangora picta O. Pickard-Cambridge, 1889 | 4 | 2 | 3 |
| ⸹Mangora sp. | 2 | 1 | 2 |
| †Micrathena funebris (Marx, 1898) | 0 | 0 | 2 |
| Micrathena mitrata (Hentz, 1850) | 9 | 11 | 9 |
| †Micrathena quadriserrata F. O. Pickard-Cambridge, 1904 | 2 | 2 | 5 |
| Trichonephila clavipes (Linnaeus, 1767) | 0 | 1 | 2 |
| ⸹Pozonia sp. | 0 | 1 | 0 |
| †Verrucosa arenata (Walckenaer, 1841) | 6 | 9 | 8 |
| †Witica crassicauda (Keyserling, 1865) | 16 | 5 | 6 |
| nd | 28 | 23 | 29 |
| BARYCHELIDAE | |||
| Barychelidae sp. | 1 | 0 | 0 |
| CLUBIONIDAE | |||
| Elaver aff. E. richardi | 1 | 0 | 1 |
| nd | 4 | 4 | 2 |
| CORINNIDAE | |||
| Castianeira sp. | 2 | 2 | 1 |
| Creugas aff. C. uncatus | 0 | 1 | 0 |
| Creugas sp.1 | 1 | 0 | 2 |
| †Myrmecotypus pilosus (O. Pickard-Cambridge, 1898) | 0 | 1 | 0 |
| nd | 3 | 4 | 6 |
| CTENIDAE | |||
| †Ctenus calcaratus F. O. Pickard-Cambridge, 1900 | 6 | 0 | 0 |
| ⸹Leptoctenus sp.1 | 3 | 1 | 2 |
| ⸹Leptoctenus sp.2 | 1 | 0 | 1 |
| nd | 21 | 11 | 10 |
| DYCTINIDAE | |||
| †Mallos hesperius (Chamberlin, 1916) | 6 | 5 | 1 |
| EUCTENIZIDAE | |||
| Eucteniza sp1 | 2 | 0 | 0 |
| GNAPHOSIDAE | |||
| Cesonia aff C. clasica | 1 | 2 | 3 |
| nd | 0 | 2 | 0 |
| HERSILIIDAE | |||
| †Neotama mexicana (O. Pickard-Cambridge, 1893) | 0 | 0 | 1 |
| LINYPHIIDAE | |||
| ⸹Pocobletus sp. | 5 | 5 | 4 |
| †Frontinella tibialis F. O. Pickard-Cambridge, 1902 | 14 | 9 | 6 |
| †Diplothyron trifalcatus (Banks, 1909) | 5 | 1 | 3 |
| ⸹Diplothyron sp. | 4 | 0 | 0 |
| Linyphiidae sp.1 | 31 | 3 | 1 |
| Linyphiidae sp.2 | 5 | 0 | 1 |
| †Selenyphantes longispinosus (O. Pickard-Cambridge, 1896) | 1 | 0 | 0 |
| Selenyphantes sp. | 0 | 1 | 2 |
| nd | 11 | 4 | 4 |
| LIOCRANIDAE | |||
| Liocranidae sp. | 14 | 3 | 1 |
| LYCOSIDAE | |||
| Hogna sp. | 0 | 0 | 2 |
| Pirata sp. | 1 | 0 | 18 |
| ⸹Sosippus sp. | 0 | 5 | 1 |
| nd | 2 | 3 | 11 |
| MIMETIDAE | |||
| ⸹Mimetus sp. | 14 | 0 | 1 |
| OONOPIDAE | |||
| †Orchestina chaparrita Izquierdo, 2017 | 7 | 2 | 2 |
| OXYOPIDAE | |||
| †Hamataliwa banksi (Mello-Leitão, 1928) | 0 | 4 | 1 |
| Peucetia longipalpis F. O. Pickard-Cambridge, 1902 | 0 | 1 | 0 |
| Peucetia viridans (Hentz, 1832) | 0 | 0 | 2 |
| nd | 2 | 0 | 0 |
| PHILODROMIDAE | |||
| Philodromidae sp1. | 27 | 40 | 26 |
| PHOLCIDAE | |||
| Modisimus sp. | 7 | 1 | 0 |
| Physocyclus globosus (Taczanowski, 1874) | 0 | 1 | 0 |
| ⸹Psilochorus sp. | 1 | 0 | 2 |
| ⸹Spermophora sp. | 4 | 15 | 14 |
| nd | 1 | 6 | 4 |
| SALTICIDAE | |||
| Colonus sylvanus (Hentz, 1846) | 30 | 10 | 9 |
| ⸹Corythalia sp | 1 | 0 | 1 |
| †Cotinusa distincta (G. W. Peckham & E. G. Peckham, 1888) | 9 | 0 | 6 |
| †Cylistella adjacens (O. Pickard-Cambridge, 1896) | 1 | 0 | 0 |
| †Lyssomanes jemineus G. W. Peckham, E. G. Peckham & Wheeler, 1889 | 8 | 15 | 5 |
| †Lyssomanes reductus Peckham & Peckham, 1896 | 1 | 0 | 0 |
| ⸹Mexigonus sp.1 | 36 | 22 | 34 |
| ⸹Mexigonus sp.2 | 4 | 2 | 1 |
| ⸹Mexigonus sp.3 | 3 | 1 | 0 |
| ⸹Synageles sp. | 2 | 1 | 1 |
| nd | 98 | 69 | 54 |
| SCYTODIDAE | |||
| Scytodes fusca Walckenaer, 1837 | 0 | 1 | 2 |
| Scytodes sp. | 0 | 0 | 12 |
| SEGESTRIDAE | |||
| Segestridae sp. | 0 | 0 | 1 |
| SELENOPIDAE | |||
| Selenops sp. | 0 | 0 | 1 |
| SPARASSIDAE | |||
| †Curicaberis minax (O. Pickard-Cambridge, 1896) | 0 | 1 | 0 |
| ⸹Curicaberis sp.1 | 0 | 3 | 1 |
| ⸹Curicaberis sp.2 | 0 | 0 | 1 |
| nd | 3 | 4 | 2 |
| TETRAGNATHIDAE | |||
| †Azilia affinis O. Pickard-Cambridge, 1893 | 1 | 0 | 7 |
| †Chrysometa alboguttata (O. Pickard-Cambridge, 1889) | 0 | 0 | 2 |
| †Chrysometa palenque Levi, 1986 | 0 | 0 | 4 |
| Chrysometa aff. C. yungas | 0 | 0 | 1 |
| ⸹Chrysometa sp. | 3 | 1 | 3 |
| ⸹Dolichognatha sp. | 3 | 0 | 5 |
| †Leucauge argyrobapta (White, 1841) | 0 | 1 | 0 |
| ⸹Leucauge sp. | 15 | 7 | 10 |
| ⸹Tetragnatha sp. | 5 | 6 | 19 |
| nd | 21 | 2 | 32 |
| THERAPHOSIDAE | |||
| Tliltocatl schroederi (Rudloff, 2003) | 0 | 2 | 0 |
| THERIDIIDAE | |||
| Anelosimus baeza Agnarsson, 2006 | 5 | 2 | 3 |
| Anelosimus elegans Agnarsson, 2006 | 1 | 1 | 0 |
| Chrosiothes goodnightorum (Levi, 1954) | 0 | 1 | 0 |
| Chrysso albomaculata O. Pickard-Cambridge, 1882 | 1 | 0 | 0 |
| †Chrysso cambridgei (Petrunkevitch, 1911) | 0 | 4 | 2 |
| ⸹Chrysso sp1 | 7 | 0 | 0 |
| Coleosoma acutiventer (Keyserling, 1884) | 0 | 7 | 2 |
| †Dipoena nigra (Emerton, 1882) | 0 | 1 | 0 |
| Dipoena aff. D. boquete | 1 | 0 | 4 |
| ⸹Dipoena sp. | 0 | 5 | 0 |
| †Euryopis lineatipes O. Pickard-Cambridge, 1893 | 0 | 8 | 0 |
| Faiditus dracus (Chamberlin & Ivie, 1936) | 2 | 6 | 3 |
| †Faiditus godmani (Exline & Levi, 1962) | 5 | 3 | 1 |
| †Faiditus subdolus (O. Pickard-Cambridge, 1898) | 5 | 2 | 6 |
| Faiditus aff. F. chickering | 0 | 2 | 4 |
| †Hentziectypus florens (O. Pickard-Cambridge, 1896) | 15 | 10 | 22 |
| Neopisinus cognatus (O. Pickard-Cambridge, 1893) | 8 | 34 | 9 |
| †Nesticodes rufipes (Lucas, 1846) | 0 | 2 | 0 |
| †Nihonhimea tesselata (Keyserling, 1884) | 0 | 0 | 4 |
| †Phycosoma lineatipes (Bryant, 1933) | 0 | 0 | 1 |
| †Phycosoma altum (Keyserling, 1886) | 0 | 0 | 1 |
| Rhomphaea projiciens O. Pickard-Cambridge, 1896 | 2 | 1 | 2 |
| Spintharus flavidus Hentz, 1850 | 12 | 6 | 10 |
| Theridion adjacens (O. Pickard-Cambridge, 1896) | 1 | 0 | 0 |
| †Theridion evexum Keyserling, 1884 | 19 | 6 | 9 |
| Theridion hispidum O. Pickard-Cambridge, 1898 | 0 | 3 | 0 |
| †Theridion positivum Chamberlin, 1924 | 2 | 3 | 0 |
| ⸹Theridion sp. 1 | 1 | 0 | 0 |
| ⸹Theridion sp. 2 | 1 | 0 | 0 |
| †Thymoites illudens (Gertsch & Mulaik, 1936) | 0 | 0 | 2 |
| †Thymoites verus (Levi, 1959) | 0 | 0 | 1 |
| †Tidarren mixtum (O. Pickard-Cambridge, 1896) | 2 | 1 | 0 |
| Tidarren sisyphoides (Walckenaer, 1841) | 0 | 4 | 1 |
| †Wamba congener O. Pickard-Cambridge, 1896 | 1 | 0 | 1 |
| Wamba crispulus (Simon, 1895) | 3 | 5 | 1 |
| nd | 65 | 62 | 66 |
| THERIDIOSOMATIDAE | |||
| †Theridiosoma davisi Archer, 1953 | 1 | 2 | 2 |
| nd | 2 | 0 | 1 |
| THOMISIDAE | |||
| ⸹Bucranium sp. | 0 | 9 | 0 |
| ⸹Misumenoides sp. | 0 | 0 | 2 |
| ⸹Misunema sp. | 0 | 0 | 1 |
| ⸹Misumenops sp. | 0 | 1 | 0 |
| ⸹Modysticus sp. | 0 | 0 | 1 |
| ⸹Synema sp. | 1 | 0 | 1 |
| ⸹Tmarus sp. | 9 | 31 | 14 |
| ⸹Xysticus sp. | 0 | 2 | 2 |
| nd | 7 | 8 | 6 |
| TITANOECIDAE | |||
| Titanoecidae sp. | 0 | 4 | 0 |
| TRACHELLIDAE | |||
| †Trachelas ductonuda Rivera-Quiroz & Álvarez-Padilla, 2015 | 2 | 0 | 0 |
| ⸹Trachelas sp. | 3 | 2 | 0 |
| TRECHALEIDAE | |||
| Cupiennius spp. | 9 | 11 | 9 |
| ULOBORIDAE | |||
| ⸹Miagrammopes sp. | 0 | 0 | 1 |
| Philoponella semiplumosa (Simon, 1893) | 0 | 4 | 0 |
| Philoponella sp. | 0 | 0 | 1 |
| †Uloborus campestratus (Simon, 1893) | 1 | 1 | 0 |
| †Uloborus segregatus Gertsch, 1936 | 1 | 1 | 1 |
| †Uloborus trilineatus Keyserling, 1883 | 0 | 1 | 0 |
| nd | 2 | 1 | 3 |
| ZODARIIDAE | |||
| †Ishania simplex Jocqué & Baert, 2002 | 9 | 3 | 8 |
| Total | 796 | 686 | 728 |
The spiders collected represents 35 families, 100 genera (considering as a genus the unique morphospecies of a family), and 146 species. The list includes 81 previously described species (55.5%), 40 species (27.4%) that have been considered as undescribed taxa, six (4.1%) that were determined as related to species already described and 19 (13%) morphospecies (Table 1).
Theridiidae (35 species), Araneidae (25), Salticidae (10), Tetragnathidae (9), Linyphiidae and Thomisidae (8 each) were the families with the highest species richness for the data set. Theridiidae (27 species), Araneidae (20), Salticidae (9) and Thomisidae, Linyphiidae, and Tetragnathidae (7 species each) were the families with the highest species richness in the dry season. Theridiidae (28 species), Araneidae (17), Salticidae (9), Tetragnathidae (8), and Linyphiidae (6) were the families with the highest species richness in the rainy season. Theridiidae (20 species), Araneidae (14), Salticidae (10), Linyphiidae (7), and Tetragnathidae (5) were the families with the highest species richness in the Monoculture (Fig. 2a). Theridiidae (23 species), Araneidae (19), Salticidae (6), and Linyphiidae (5) were the families with the highest species richness in the Polyculture (Fig. 2b). Theridiidae (21 species), Araneidae (14), Tetragnathidae (8), Linyphiidae, and Salticidae (6 species each) were the families with the highest species richness in the Tropical Forest (Fig. 2c).

Figure 2 Richness of genus and species for each spider family in a) Monoculture coffee plantation, b) Polyculture coffee plantation, and c) Tropical Forest.
The Chao 1 estimator yielded 116 species for the Polyculture site, 126 species for the Monoculture site, and 127 species for the Tropical Forest. ACE estimator yielded 123 species for Polyculture, 115 species for Monoculture, and 137 species for the Tropical Forest. Completeness values of the inventories range from 68.25% (Monoculture with Chao 1) to 78.44% (Polyculture with Chao 1). Singletons proportions, all above 30%, were highest in the Tropical Forest and lowest in the Monoculture site (Table 2).
Table 2 Estimated species richness for two coffee plantations and Tropical Forest in the La Costa region of Oaxaca, Mexico obtained with the Chao 1 and ACE estimators, with their corresponding completeness of the inventories and percentage of singletons.
| Site | Observed species richness | Chao 1 | Completeness | ACE | Completeness | Proportions of singletons |
|---|---|---|---|---|---|---|
| Estimated species richness | Estimated species richness | |||||
| Monoculture | 86 | 126 | 68.25% | 115 | 74.78% | 33.72% |
| Polyculture | 91 | 116 | 78.44% | 123 | 73.98% | 34.06% |
| Tropical Forest | 97 | 127 | 76.37% | 137 | 70.80% | 38.14% |
Generalized Linear Model showed that the spiders’ abundance was significantly lower in the rainy season with the manual collection method (Fig. 3a), similarly, the Tropical Forest, the Polyculture site, and the rainy season showed a lower abundance of spiders with the foliage beating method (Fig. 3b); on the contrary, the abundance of spiders was higher in the rainy season but lowest for the Polyculture with the pitfall traps (Fig. 3c; Table 3).

Figure 3 Number of spider specimens collected in two coffee agroecosystems and tropical forest, and contrasting seasons in Oaxaca, Mexico with a) manual collection method, b) foliage beating method, and c) pitfall traps. The intervals show the standard errors.
Table 3 Abundance analysis of the spider specimens from two coffee plantations and Tropical Forest in the La Costa region of Oaxaca, Mexico. Values obtained with a two-ways Generalized Linear Model with Poisson distribution for each collection method. Bold letters show significant differences.
| MANUAL COLLECTION | Estimate (exp) | Std. Error | Z value | P (95%) |
| Site Polyculture | 0.014252 | 0.097477 | 0.146 | 0.884 |
| Site Tropical Forest | -0.019324 | 0.098299 | -0.197 | 0.844 |
| Season rain | -0.23076 | 0.06008 | -3.841 | 0.0001 |
| Site Polyculture: Season rain | -0.219302 | 0.148379 | -1.478 | 0.139 |
| Site Tropical Forest: Season rain | -0.003404 | 0.14501 | -0.023 | 0.981 |
| df | 35 | |||
| FOLIAGE SHAKING | Estimate (exp) | Std. Error | Z value | P (95%) |
| Site Polyculture | -0.19587 | 0.10323 | -1.898 | 0.0578 |
| Site Tropical Forest | -0.23159 | 0.10425 | -2.221 | 0.0263 |
| Season rain | -0.21954 | 0.1039 | -2.113 | 0.0346 |
| Site Polyculture: Season rain | 0.03371 | 0.1539 | 0.219 | 0.8266 |
| Site Tropical Forest: Season rain | 0.12423 | 0.15337 | 0.81 | 0.4179 |
| df | 35 | |||
| PITFALL TRAPS | Estimate (exp) | Std. Error | Z value | P (95%) |
| Site Polyculture | -1.2993 | 0.6513 | -1.995 | 0.046 |
| Site Tropical Forest | -0.3185 | 0.4647 | -0.685 | 0.4931 |
| Season rain | 0.7376 | 0.3666 | 2.012 | 0.0442 |
| Site Polyculture: Season rain | 0.7287 | 0.7379 | 0.988 | 0.3234 |
| Site Tropical Forest: Season rain | 0.4411 | 0.5457 | 0.808 | 0.419 |
| df | 35 |
Rarefaction curves of observed species richness (order q0) did not show differences between the sites and seasons (Fig. 4). Effective numbers of species for the order q1, indicate that the Tropical Forest had the greatest diversity with 51.9 effective species, followed by the Polyculture with 49.9 effective species, and finally Monoculture with 48.4 effective species (Fig. 5a). Tropical Forest had the highest diversity of species in the rainy season with 51.9 effective species (Fig. 5b), followed by Polyculture with 45.3 and the Monoculture with 40.6 effective species. Monoculture was the site with the highest diversity in the dry season with 38.8 effective species (Fig. 5c), followed by the Polyculture with 36.9 and, finally, the Tropical Forest with 32.5. Unlike the analyses with effective species, the Hutcheson test does detect a significant difference in diversity, i.e., spider diversity was significantly higher in the Tropical Forest than Monoculture in the rainy season (Table 4).
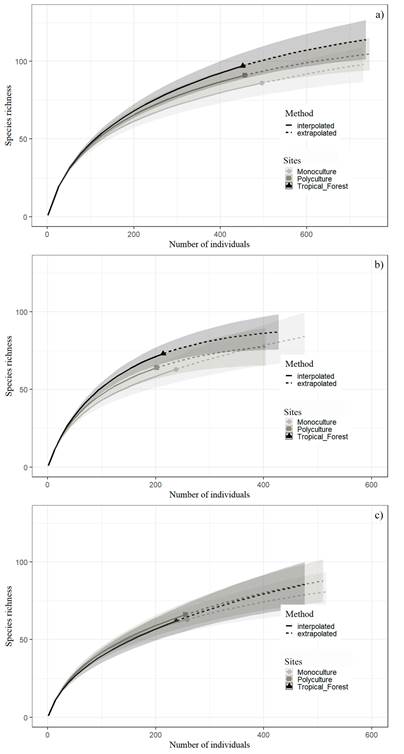
Figure 4 Spider species richness comparison between two agroecosystems with different management and a portion of tropical forest of the La Costa region of Oaxaca, México: a) total richness, b) rainy season, c) dry season. Comparisons based on Chao et al. (2014) methodology using sample-size-based. Rarefaction (solid lines) and extrapolation (dashed lines) curves, with 95% confidence intervals (gray-shaded regions).

Figure 5 Numbers of effective species (orders q1, q2) obtained for two coffee plantations and a portion of the Tropical Forest of Oaxaca. a) total diversity, b) rainy season, c) dry season. The intervals show the standard errors.
Table 4 Diversity analysis of the spider specimens from two coffee plantations and Tropical Forest in the La Costa region of Oaxaca, Mexico. Values obtained with the Shannon index and the Hutcheson t-test. Bold letters show significant differences.
| RAINY SEASON | Shannon index | Hutcheson t-statistic | Degrees of freedom | p-value |
| Polyculture vs Monoculture | 3.81 vs 3.70 | -1.3366 | 438.18 | 0.1821 |
| Polyculture vs Tropical Forest | 3.81 vs 3.94 | -1.6671 | 415.76 | 0.0963 |
| Monoculture vs Tropical Forest | 3.70 vs 3.94 | -2.9859 | 451.27 | 0.0030 |
| DRY SEASON | Shannon index | Hutcheson t-statistic | Degrees of freedom | p-value |
| Polyculture vs Monoculture | 3.60 vs 3.66 | 0.5791 | 503.13 | 0.5628 |
| Polyculture vs Tropical Forest | 3.60 vs 3.48 | 1.2812 | 485.13 | 0.2007 |
| Monoculture vs Tropical Forest | 3.66 vs 3.48 | 1.9042 | 463.51 | 0.0575 |
Diversity order q2 indicates that, the site with the highest number of effective species was the Monoculture site with 33.4 equally common species, followed by the Polyculture with 32.2 equally abundant species, and finally the Tropical Forest with 31.4 equally abundant species (Fig. 5a). Tropical Forest was the site with the highest number of effective species in the rainy season with 37.2 equally common species, followed by the Polyculture with 34 equally abundant species, and finally the Monoculture with 28.9 equally abundant species (Fig. 5b). Monoculture was the site with the highest number of effective species in the dry season with 27.2 equally common abundant species, followed by the Polyculture with 23.1 equally common species, and finally the Tropical Forest with 19.3 equally common species (Fig. 5c).
Rank abundance curves show differences in evenness among the sites. Mexigonus sp1, Linyphiidae sp1, Colonus sylvanus (Hentz, 1846) and Philodromidae sp1 (accounting 15.5% of abundance) were the most abundant species in the Monoculture (Fig. 6a). Philodromidae sp1, Neopisinus cognatus (O. Pickard-Cambridge, 1893), Tmarus sp1 and Mexigonus sp1 (accounting 18.5% of abundance) were the most abundant species in the Polyculture (Fig. 6b). Wulfila tantillus, Mexigonus sp1, Philodromidae sp1 and Hentziectypus florens (O. Pickard-Cambridge, 1896) were the most abundant species (accounting 16.4% of total abundance) in the Tropical Forest (Fig. 6c). In this way, the site with the highest evenness was the Monoculture, and the site with the lower evenness was the Polyculture (Fig. 6). For the data set, the most abundant species were Philodromidae sp, Mexigonus sp1, Colonus sylvanus, and Wulfila tantillus.
Discussion
The spider species recorded in these sites represents 50.51% of the number of species previously recorded for the state of Oaxaca (Nieto et al., 2022). We found a large percentage of species considered new (undescribed, 27.4%). These data expose the few studies conducted in the state of Oaxaca, since there are very few formal studies available (Martínez-Martínez et al., 2016; Santiago-Pacheco et al., 2017). For the state of Oaxaca, 51 species are new records (Nieto et al., 2022) (Table 1).
Completeness level (after Cardoso, 2009) of this study (70-80%) is lower than those of Lucio-Palacio and Ibarra-Núñez (2015) in cacao plantations (90%) and those of Maya-Morales et al. (2012) in a tropical cloud mountain forest (77-90%). Another indicator of the inventory completeness is the percentage of species recorded with a single individual “singleton”. Our study report 33-38% of singletons, that range of values is close to the average values estimated in several spider studies in the tropical regions (average 32%-33%, Coddington et al., 2009; Malumbres-Olarte et al., 2017). Lower percentages of singletons indicate more complete surveys while higher percentages of singletons are explained as undersampling or by a reduced sampling area (Coddington et al., 2009).
We found a significant seasonal change in the spider abundance. This change may be because most spiders are more active in one season as well as the high rainfall levels in this area (mean annual precipitation 2250 mm per year) (CONANP, 2003). Despite this, the change in spider abundance between seasons is a pattern that has already been widely recorded in other studies (Weeks & Holtzer, 2000; Jiménez-Valverde & Lobo, 2006; Cardoso et al., 2007; Lucio-Palacio & Ibarra-Núñez, 2015; Rodríguez-Rodríguez et al., 2015). It is also possible that the difference in spider abundance between seasons was due to the reduction of sampling during the rainy season. Other explanations for low spider abundance in the rainy season is the potential effect of environmental changes as well as changes in individual species from season to season (Maya-Morales et al., 2012).
Concerning the richness of species in the coffee plantations, the results showed that in these agroecosystems the spider species richness was high compared to Ibarra-Núñez (1990), who studied the arthropods associated with coffee trees and recorded 26 families and 65 spider species. Species richness was not affected by the intensity of crop management since the rarefaction curves did not show significant differences. This is consistent with other previous spiders’ inventories in coffee plantations (Pinkus et al., 2006; Marín et al., 2016) but it differs from a previous study that report an increase in species richness as the intensity of cultivation decreases (Perfecto et al., 1996). We found that the Tropical Forest was the site with the highest observed species richness, followed by the Polyculture shade system and, finally, the Monoculture shade system. This is different from Pinkus et al. (2006), who analyzed the composition of spiders in two coffee plantations with different management (differences in shade trees) and in a control site with native vegetation corresponding to a Tropical Forest. They recorded that the conventional coffee plantation was the site with the highest species richness observed (64 species), followed by the control site (56), and the organic coffee plantation (47) (unpublished data provided by G. Ibarra-Núñez, one of the coauthors of this study). Furthermore, there is another difference in the species richness observed in the dry season, since Pinkus et al. (2006) reported the highest species richness in the conventional coffee site (51 species), followed by the tropical forest (47) and finally the organic coffee (32), while in this study we found highest species richness for the dry season in the Polyculture (66), followed by the Monoculture (63) and finally the Tropical Forest (62). Pinkus et al. (2006) reported the conventional coffee plantations as the site with the highest species richness observed in the rainy season (45 species), followed by organic coffee (36) and the tropical forest (30), while in this study, we found that the Tropical Forest (73 species) had the highest species richness, followed by Polyculture (64) and Monoculture (63). However, in Pinkus’s study only one collecting method was used to collect spiders. Marín and Perfecto (2013) explored the influence of agricultural intensification of coffee plantations and aggressive ants in Chiapas, Mexico. They recorded 91 spider species in two coffee sites, and they concluded that there is not a negative effect of coffee intensification on spider diversity, since they recorded the highest species richness in the monoshade system and attributed this result partially to differences in tree cover. Although the effect of crop intensification on species diversity and abundance has been documented, we consider that the structures of spider assemblages are also influenced by other factors such as differences in microclimates, availability of refuges and hunting sites, shade cover and leaf litter depth, among others, however, more complete studies are needed to understand the complex interactions in these agroecosystems.
Effective numbers of species are a measure of diversity that can be computed from the values obtained with the Shannon-Wiener and Simpson indexes, allowing then to be compared with different studies that also provide the effective or equivalent number of species (Jost & González-Oreja, 2012; Jost, 2019). Pinkus et al. (2006) work is the most comparable to our study in the sense of studying two sites with different coffee crop management and an area of Tropical Forest. To do an appropriate comparison with our study, the raw data of Pinkus et al. (2006) (unpublished data provided by G. Ibarra-Núñez, one of the coauthors of this study) were used to calculate the Shannon-Wiener and Simpson indexes in view to determine the q1 order of the Hill numbers. In this sense, the site with the highest effective species number (q1) obtained for the data of Pinkus et al. (2006) was the conventional coffee (19.7) followed by the tropical forest (19) and the organic coffee (7.8), whereas in our study, the Tropical Forest had the highest effective species number (q1) (51.9) and the Monoculture (48.4) the lowest. For the data of Pinkus et al. (2006) in the rainy season, the conventional coffee (15.6 effective species) was the site with a higher spider diversity than the organic coffee (13.3 effective species). This is contrary to our data where the Polyculture (45.3 effective species) was the site with a higher spider diversity than the Monoculture (40.7 effective species). For the data of Pinkus et al. (2006) in the dry season, the conventional coffee (17.7 effective species) was the site with a higher spider diversity than the organic coffee (4.6 effective species). Similarly, in our study, for the dry season the Monoculture (38.8 effective species) was the site with a higher spider diversity than the Polyculture (36.9 effective species). Overall, spider diversity was significantly higher in the Tropical Forest than in Monoculture management in the rainy season, similar to that registered by Pinkus et al. (2006), where the spider diversity was significantly higher in the control site than in organic management in the same season. This may be due to the methods used, since in the study by Pinkus et al. (2006) only direct (visual) collection was used, while we used three methods, which may influence the results.
The Theridiidae and Araneidae families have greater species richness and abundance of individuals in Mexican coffee plantations (Ibarra-Núñez, 1990; Ibarra-Núñez & Garcia-Ballinas, 1998) and Mexican cocoa plantations which are very similar in terms of structure to coffee plantations (Ibarra-Núñez et al., 2004; Lucio-Palacio & Ibarra-Núñez, 2015). This could be explained because the tree cover of these agroecosystems provides suitable microhabitats for the establishment of weaving spiders. Similarly, in a Mexican fragment of Tropical Forest, Rivera-Quiroz et al. (2016) reported Theridiidae, Araneidae, and Salticidae as the richest families, representing 48.1% of the total species richness, while in a Tropical Forest in Borneo, Theridiidae, Salticidae and Araneidae were the richest families (Floren & Deeleman-Reinhold, 2005). On the other hand, in Mexican tropical mountain cloud forests, the families with high species richness were Theridiidae, Linyphiidae and Anyphaenidae (Ibarra-Núñez et al., 2011; Campuzano et al., 2019), while in a Mexican Deciduous Dry Forest Thomisidae, Oxyopidae, Araneidae, Salticidae, and Theridiidae were reported as the families with high species richness (Corcuera & Jiménez, 2009). In the present study, both coffee plantations had the same family richness: Theridiidae, Araneidae, Salticidae, and Linyphiidae. Tropical Forest was different in having Tetragnathidae more species rich than Salticidae and, in the order of family richness (Theridiidae, Araneidae, Tetragnathidae, and Salticidae).
Rank abundance-curves showed differences in evenness among sites, the Monoculture site had a more horizontal initial slope compared to Polyculture and Tropical Forest whose initial slope was more vertical. In this way, the percentage of the four most dominant species in Monoculture is lower compared to the four most dominant species in Polyculture and Tropical Forest. In addition, different species were dominant in the three sites. Philodromidae sp1 was the most dominant species in Polyculture. Species of this family have been reported as dominant in other crops such as pear and apple (Horton et al., 2001; Pekar & Kocourek, 2004), medicinal and aromatic gardens (Amal et al., 2019), and olive grove (Benhadi-Marin et al., 2020). Mexigonus sp1 was the dominant species in the Monoculture. A species belonging to this genus was reported as the second most dominant in a site with high perturbation in forest ecosystems (Reta-Heredia et al., 2018). Likewise, Durán-Barrón et al. (2009) and Desales-Lara et al. (2013) reported this genus as synanthropic in Mexico City and Estado de Mexico respectively, however, not all species of this genus are synanthropic, as other studies (Ibarra-Núñez et al., 2011; Sosa-Romero et al., 2016; Campuzano et al., 2019) reported that species of Mexigonus are abundant in sites with natural vegetation or low perturbation levels. Wulfila tantillus was the dominant species in the Tropical Forest. Jiménez and Tejas (1996) reported one species of this genus, Wulfila immaculellus (Gertsch, 1933), as the third dominant in fruit crops in Baja California, Mexico, while Llinas-Gutiérrez and Jiménez (2004) reported Wulfila tantillus with low abundance in wetlands of Baja California. We think that the high dominance of Wulfila tantillus could be attributed to the heterogeny of the Tropical Forests because these ecosystems have high richness and abundance of plants species which generates more refuges and hunting sites for these spiders. Proportions of the four most abundant species in the three studied sites show that the spiders community structure is similar among sites. We believe this may be so because there is not much difference in vegetation structure among the sites.
This is the first study on the diversity of spiders in coffee plantations in the state of Oaxaca, Mexico. The percentage of undescribed species (21.7%) indicates the lack of studies in the state. The shade management system in coffee plantations (either Polyculture or Monoculture) plays an important role in spider community structures and seems to allow spider communities to have a structure similar to that of the Tropical Forest. In addition, abundance, spider diversity and species composition among sites changed across seasons. As we thought, the observed spider richness was higher in the site with natural vegetation (Tropical Forest) than the sites with coffee plantations, however, we found no significant differences between the two crop management systems and, differences in species richness were not so pronounced to be significant. The observed richness among the Tropical Forest and the Monoculture were different, while the observed richness in the Polyculture presents an intermediate number of species. This suggests that this type of agroecosystem provides more adequate microhabitats for the establishment of spider species than the Monoculture site. We suggest promoting and maintaining shade coffee agroecosystems as they are sites with a high spider species richness in southeastern Mexico.











 nova página do texto(beta)
nova página do texto(beta)




