Introduction
The Michoacán state has a high diversity and endemism of vascular plants. The main vegetation types are tropical deciduous forest, coniferous forest, and Quercus forest (Villaseñor, 2016). Michoacán is the main avocado producer in Mexico (SIAP, 2019), the increase in exports to European and Asian markets, has implied increasing deforestation of pine and oak forests, for the establishment of new cultivation areas (Barsimantov & Navia, 2008).
Oribatid mites are one of the main groups of microarthropods present in soils (Norton & Behan-Pellelier, 2009). For Mexico, 104 families with 250 genera and about 440 species have been reported; only eleven species have been reported for the State of Michoacán (Palacios-Vargas & Iglesias, 2004; Pérez et al., 2014; Revelo-Tobar et al., 2021).
Galumnidae family has a great diversity of species; to date, 28 genera and 603 species have been described worldwide. In this family, the genera with the highest species richness are Galumna von Heyden, 1826 (203 spp.), Pergalumna Grandjean, 1936 (173 spp.), and Allogalumna Grandjean, 1936 (54 spp.) (Subias, 2004, updated 2021). In Mexico, 11 genera and 30 species have been reported. Of the genus Allogalumna the following species have been reported: Allogalumna (Allogalumna) borhidiiBalogh & Mahunka, 1979, Allogalumna (Allogalumna) cubanaBalogh & Mahunka, 1979 and Allogalumna (Acrogalumna) monttensis (Hammer, 1962) (Bernal et al., 2009; Damián-Chávez et al., 2019; Ojeda & Gasca-Pineda, 2019; Páez et al., 2019; Palacios-Vargas et al., 1998; 2011; Palacios-Vargas & Iglesias, 2004; Vázquez et al., 2016; Villagómez & Palacios-Vargas, 2017).
Adults of this group are mainly characterized by the presence of a pair of mobile and auriculate pteromorphs, octotaxic system conformed by porous areas arranged longitudinally on the notogaster, 10-15 pairs of notogastral setae or alveoli, serrated chelicerae, presence of cheliceral setae chb, cup-shaped bothridium, six pairs of genital setae, one aggenital pair, two pairs of anal setae, three adanal, mono, bi or tridactyl legs (Ermilov & Klimov, 2017; Norton & Behan-Pellelier, 2009).
Despite the considerable taxonomic information available on these organisms, information on the immature stages, their biology, ecology, and behavior are still very scarce. Of the family Galumnidae, the ontogenetic development of only 23 species has been studied, eight of them do not have information on all their developmental stages; in addition, only seven species have been reared in laboratory conditions and have information on their developmental time, feeding and behavior (Corpuz-Raros & Ermilov, 2019).
Allogalumna (Acrogalumna) longipluma is a widely distributed species, described by Berlese (1904). Its ontogenetic development has been studied by multiple researchers (Grandjean, 1935); Oudemans (1914) made descriptions and illustrations of the body and legs. The work of Seniczak et al. (2012) describes in greater detail the body morphology, discusses the quetotaxia of legs, and provides illustrations of the larva, protonymph and tritonymph. Haq and Adolph (1981), Sengbusch (1958), Sengbusch and Sengbusch (1970), Sengbusch (1954) have maintained this species in laboratory conditions and have generated information about its developmental time and feeding. However, no details are provided for each stage of development, adult lifetime, pre-oviposition period, details of quiescence, and molt.
In the present work we provide more information about the biology, photographs of the different stages of development, body diagrams of the proto- and deutonymph, and we make the first record of A. longipluma for Mexico.
Materials and methods
Two sampling sites were established. Intervened primary forest: constituted principally by Pinus devoniana Lindley 1839, Quercus rugosa Nee (1801), and dispersed undergrowth, located in the municipality of Nuevo San Juan Parangaricutiro (19° 23' 27.8" N, 102° 10' 30.8" W) at 2160 m a.s.l. Agricultural zone: conventionally managed avocado (Persea americana Var. Hass) farm, and located in the municipality of Uruapan (19° 23' 47.2" N, 102° 05' 58.1" W) at 1725 m a.s.l. Both municipalities belong to the state of Michoacán, Mexico.
Five soil samples were collected in each ecosystem, at a single depth level (0-15 cm). For the extraction of the specimens, Berlese-Tullgren funnels were used for two weeks, the first one only at ambient temperature and the second one with a light and heat source (40 watt bulbs). The specimens were collected in plastic containers containing a fragment of moistened paper towel to collect the live mites and place them in the rearing medium (Estrada-Venegas, 2020). These containers were checked every two days to avoid the death of specimens due to desiccation, inanition, or predation. The different stages of development of A. longipluma were transferred to rearing media made with calcium sulfate (gypsum), ground charcoal, and decomposed organic matter. The individuals were maintained with a diet based principally on dog croquettes (Biscuit, Pedigree®), yeast flakes, decomposed and crushed plant tissue; the food was provided ad libitum, but care was taken to avoid fungal exerted. Every two days, maintenance tasks were carried out: removal of food remains or dead individuals covered with fungi, elimination of contaminating mites (generally of the Sancassania genus), and moistening of the medium; eventually, springtails were used to control fungi in the rearing medium (Estrada-Venegas et al., 1999; Estrada-Venegas, 2020); however, this practice was discouraged because adult Collembola females have a high reproductive capacity and can affect the establishment of the study species.
With established breeding stock, eggs, larvae, or ovigerous females were isolated in observation units (breeding medium of 1.5 cm diameter). Each observation unit contained three to five individuals of the same age to facilitate observation. They were checked every 12 hours for extended periods and recorded: oviposition strategies, eggs care, eggs development, hatching, time of all developmental stages, behavior before quiescence, quiescence time, molt type, reproduction, and feeding preferences. Individuals were maintained at 22.8 °C and 63 % humidity.
Photographs of live specimens were taken with the aid of a Canon Rebel T6® camera, adapted to an AmScope® SM-2TZZ stereo microscope. The slides were studied under a Carl Zeiss® Axioskop 2 plus phase-contrast microscope. Rendering was performed with the Helicon Focus 7® program, using method B (depth map). Final editing with GIMP version 2.10.20. All measurements are reported in microns (µm).
Results
A total of 487 specimens were collected and 81 live adults were extracted as brood stock for biological, behavioral, and morphological studies.
Allogalumna (Acrogalumna) longipluma (Berlese, 1904) (Oribata elimata l.).
Galumna decipiens Mihelčič, 1956
Galumna filata Oudemans, 1914
Galumna latipluma Mihelčič, 1952
Galumna longiporus Mihelčič, 1952
Oribata setiformis Hall, 1911
World distribution: wide distribution: Holarctic, Ethiopic, Oriental (India: Kerala and southeastern China), and New Zealand (Subias, 2004, updated 2021).
Material examined: 48 specimens (28 ♀ and 20 ♂) - MEXICO (new country record), Michoacán: El Rosario, Municipality of San Juan Nuevo, 19° 23' 27.8" N, 102° 10' 30.8" W, 2160 masl. 2 specimens (1 ♀ and 1 ♂) - Tanaxuri, Municipality of Uruapan, Mi Ranchito 1 farm, 19° 23' 47.2" N, 102° 05' 58.1"W, 1683 masl.
Habitat: litter and forest floor of Pinus devoniana, Quercus rugosa and avocado (Persea americana Var. Hass).
Biological cycle
Oviposition and fertility. The female has a telescopic ovipositor (Fig. 11), which allows her to deposit eggs in narrow hollows to protect them (Estrada-Venegas, 2008; 2012). Under laboratory conditions, females oviposited one or several eggs in openings in the environment, remains of organic matter, exuviae from other developmental stages, or among the pteromorphs of dead adults (Figs. 1-4). Females may oviposit four to five eggs, which is consistent with previous reports by Jacot (1934). Several females can use the same area for oviposition; up to 14 eggs were counted in an exuvia or four to six eggs in the same brood hole; in many cases, the use of the same site for oviposition can complicate the exit of the larvae after hatching, since they can become trapped and after two or three days they die of inanition. Oviposition began 50 days after adult emergence (Table 1) and lasted about 20 days.

Figures 1-11 Biology of Allogalumna (Acrogalumna) longipluma: 1) Newly oviposited eggs. 2-3) Oviposition strategies. 4) Egg close to hatching. 5) Larva. 6) Protonymph. 7) Deutonymph. 8) Tritonymph. 9) Adult. 10) Quiescence and molt. 11) Female with ovipositor extended. Scale = 100 µm.
Table 1 Biological cycle of Allogalumna (Acrogalumna) longipluma.
| n | Average (days) ± DE | |
|---|---|---|
| Egg | 14 | 10.1 ± 1.8 |
| Larva | 26 | 7.7 ± 1.9 |
| Quiescence I | 37 | 4.3 ± 0.7 |
| Protonymph | 36 | 7.1 ± 1.3 |
| Quiescence II | 34 | 4.1 ± 0.5 |
| Deutonymph | 25 | 8.3 ± 2.2 |
| Quiescence III | 23 | 4.3 ± 0.7 |
| Tritonymph | 13 | 9.4 ± 2.6 |
| Quiescence IV | 13 | 7 ± 1.2 |
| Pre-ovi | 3 | 50 |
| Adult | 13 | 155 ± 57 |
| Total | 217 ± 69.9 |
Egg. The incubation period of the egg was 10.1 ± 1.8 days (n = 14). This value is close to the incubation period of 11.8 and 10.8 days reported by Sengbusch (1954) and Sengbusch (1958); respectively, in these works the author reports that the brood was maintained at 25° C and 82% relative humidity.
Larva. At the beginning of emergence, the egg opens at the anterior pole towards the middle of the egg and allows the larva to emerge forward. Upon emergence, the larva remains attached to the chorion for 12 to 24 hours; during this time, several individuals were observed feeding on the remains of the chorion (Fig. 5). The larval period had a duration of 12 ± 2.6 days (7.7 ± 1.9 days active and 4.3 ± 0.7 quiescence), the result differs by +1 day with Sengbusch (1954) and -1 day with Sengbusch (1958).
Nymphs. The development period for protonymph was 11.2 ± 1.8 days (7.1 ± 1.3 days active and 4.1 ± 0.5 quiescence), for deutonymph was 12.6 ± 2.9 days (8.3 ± 2.2.2 days active and 4.3 ± 0.7 quiescence) and for tritonymph was 16.4 ± 3.8 days (9.4 ± 2.6 days active and 7 ± 1.3 quiescence). The results for protonymph and deutonymph resemble those obtained by Sengbusch (1954) and differ with Sengbusch (1958) by -1 day, the tritonymph time difference is +1 day with the first work and -1 with the second. As ontogenetic development proceeds, the nymphs feed more voraciously, and their locomotion becomes more agile.
Adult. The life span of adults was 155 ± 57 days, the variance is considerably high, principally because some individuals were infected by fungi that were developed in the rearing medium, inhibited their feeding until they were killed; these individuals lived between 104 to 107 days, however, two individuals lived 226 and 247 days respectively. None of the authors cited above report the lifetime of adults. During this stage, feeding is more voracious and constant than in the nymphal stages, while locomotion becomes less agile. Estrada-Venegas et al. (1999) mention that this is because adults are bigger than nymphs, which makes locomotion more difficult.
Reproduction. During the observation period, the deposition of spermatophores on the substrate was not evidenced, however, populations increased exponentially, which allows us to assume that this species reproduces by parthenogenesis. Pauly (1956) and Sengbusch (1958) report sperm transfer by spermatophores and also parthenogenesis. According to Norton (1994), about 10% of oribatid species can reproduce asexually in a facultative way, as a response to habitat alteration and allows them to double their reproductive rate.
Quiescence and molt. Individuals move away from food two to three days before entering quiescence, during this time they look for cracks, hollows, and exuviae of deutonymphs and tritonymphs to go through this process, these spaces can be occupied by one of the dozens of individuals, which, if they converge at the beginning of quiescence, can return to the same site in the four processes of quiescence and molting. The growth of the organisms causes the idiosome to expand, between the circumgastric suture and the dorsosejugal suture, this condition is an obvious sign that the individual is close to the quiescence period. The day before molting, the body becomes darker in color and the old cuticle becomes iridescent as it separates due to the formation of the new cuticle; the old cuticle breaks from the posterior region of the circumgastric suture, is directed anteriorly around the gastral line, and allows the individual to emerge posteriorly (hysterodehiscent) (Norton & Behan-Pellelier, 2009) (Fig. 10). Quiescence takes 4 to 4.3 days in the larva, protonymph, and deutonymph, while in the tritonymph it can take as long as nine to ten days (Table 1).
Feeding habits. This species showed a strong preference for dog croquettes (Biscuit, Pedigree®) and yeast flakes, occasionally they were observed consuming decomposed plant tissue, dead galumnids covered with fungus remains of springtails, and dead diplurans. However, they rejected pine pollen, algae, and lichens. Under laboratory conditions, adults showed greater voracity than nymphs and larvae. Sengbusch (1954) fed his galumnids with the bark of trunks covered with green alga Protococcus sp., also tried to feed them with apple peel, potato, germinated wheat seeds, rice, oats, yeast, sugar water, and malt; however, he obtained little or no successful results.
Morphological description
Egg (Figs. 1-4). Length 66.4 (62.7-68.7), width 46.1 (44.5-47.7). Eggs are oval, milky white iridescent, darkening as embryonic and pre-larvae development progresses to a slightly yellowish white coloration. The shape of the egg also changes as the days go by the anterior pole tends to narrow to a typical ovoid shape. In advanced stages of development (one or two days before emergence) the three pairs of legs directed towards the posterior pole of the egg and the sclerotized gnathosoma can be observed (Fig. 4).
Larva (Fig. 5). Body ovoid, notogaster rounded, anterior part of prodorso rounded, narrower than posterior. Body length 313.95 (313-314.9), width 223.9 (219.7-229.1); body sparsely sclerotized, color light beige on notogaster, while prodorso varies between dark beige and light brown. Prodorsal setae black; rostral (ro) 44.2 (44-45.2), lamellar (le) 45.9 (40.1-50.2) and interlamellar (in) 33.2 (32.3-34.6) setiform and barbed. Lamellar and rostral setae projected anteriorly, the latter shorter, thicker, and with its anterior ends slightly curved and convergent. Sensillum black, elongate 73.6 (64.1-79.0), fusiform, with wide capitulum 4.6 (4.3-5.0) and barbulate (Table 2); bothridium cup-shaped, the ends of it not converging appearing to form a coil, the basal part of the sensillum arranged wavy on the bothridium; exobotridial setae (ex) 22.8 (22.1-23.2) straight, barbulate and located posterolateral to the bothridium. Legs more sclerotized than rest of body, total length (trochanter-tarsus) leg I:158.6 ± 5.8 µm, leg II: 159.3 ± 6.6 µm and leg III: 163.6 ± 15.6 µm; tarsus on leg I slightly larger than on legs II and III (Table 3). Tarsal formula of legs (solenidia in parentheses) I: 0-2-2-2(1)-3(1)-3(1)-16(1); II: 0-2-2-2(1)-2(1)-13(1); III: 0-2-1(1)-2(1)-13 (Table 4). Tarsus I-III monodactyl. Notogaster with 11 pairs of minute setae, three pairs of porose areas (Aa, A1, and A2) rounded, faint grayish almost imperceptible and arranged laterally on notogaster; setae c3 larger than setae c2. Epimeral region with chaetotaxial formula 3-2-1. Anal aperture 85.4 (64.5-86.3); setae h2 positioned contiguous to the anal plate and h1 slightly posterolateral to the former; opisthonotal glands nearly aligned with the anterior part of the anal plate and arranged on the flanks of the anogenital region.
Table 2 Measurements of some morphological characters of the immature and adult stages of Allogalumna (Acrogalumna) longipluma.
| Morphological characters | Larva | Protonymph | Deutonymph | Tritonymph | Adult | |
|---|---|---|---|---|---|---|
| Body length | 313.95 ± 1.4 | 379.3 ± 8.7 | 471.9 ± 13.1 | 658.5 ± 12.1 | 623.3 ± 18.6 | |
| Body width | 223.9 ± 4.8 | 288.5 ± 3.1 | 375.7 ± 14.3 | 531.2 ± 13.4 | 597.5 ± 23.4 | |
| Length of: | seta in | 33.2 ± 0.9 | 40 ± 1.8 | 49.2 ± 7.5 | 52.3 ± 3.7 | 123.3 ± 4.8 |
| seta le | 45.9 ± 4 | 64.6 ± 0.1 | 68.4 ± 6.3 | 87.2 ± 3 | 111.1 ± 2.5 | |
| seta ro | 44.2 ± 0.7 | 53.8 ± 1.1 | 61.3 ± 1.2 | 66.8 ± 1.1 | 70.5 ± 6.9 | |
| seta ex | 22.8 ± 0.5 | 30.5 ± 2.1 | 32.1 ± 1.8 | 45.5 ± 3.2 | - | |
| seta c2 | 13.2 ± 1.2 | 14 ± 0.1 | 22.9 ± 1.2 | 27.8 ± 4 | - | |
| seta c3 | 28.4 ± 2.5 | 40.2 ± 0.3 | 49.4 ± 2.5 | 65.1 ± 4.6 | - | |
| seta ss | 73.6 ± 5.1 | 78.3 ± 3 | 99.2 ± 2.6 | 117.9 ± 7.5 | 206.5 ± 13.7 | |
| Head width | seta ss | 4.6 ± 0.3 | 3.7 ± 0.2 | 4.0 ± 0.3 | 4.1 ± 0.5 | 2.6 ± 0.3 |
| Genital opening | - | 33.6 ± 0.8 | 46.9 ± 1.4 | 73 ± 1.9 | 95.6 ± 6 | |
| Anal opening | 85.4 ± 1.2 | 103.4 ± 5.2 | 120.0 ± 8.6 | 162.4 ± 4.6 | 149 ± 9.2 | |
Table 3 The leg length of immature and adult stages of Allogalumna (Acrogalumna) longipluma.
| Leg | Trochanter | Femur | Genu | Tibia | Tarsus | Total | |
|---|---|---|---|---|---|---|---|
| I | L | 14.3 ± 2.9 | 47.2 ± 0.4 | 18.8 ± 0.7 | 25.9 ± 1.2 | 52.5 ± 0.7 | 158.6 ± 5.8 |
| PN | 16.8 ± 0.8 | 56.8 ± 2.8 | 18.9 ± 0.5 | 31.9 ± 0.8 | 58 ± 1 | 182.4 ± 5.9 | |
| DN | 17.1 ± 0.6 | 64.3 ± 1.9 | 23.3 ± 0.8 | 34.2 ± 1.8 | 63.2 ± 0.7 | 202.2 ± 5.7 | |
| TN | 21.1 ± 0.7 | 79.8 ± 0.9 | 28.1 ± 0.4 | 50.4 ± 1.1 | 87.5 ± 0.5 | 266.9 ± 3.6 | |
| AD | 75.8 ± 2.9 | 118.2 ± 1.4 | 62.2 ± 0.3 | 74 ± 1.3 | 130.1 ± 2.6 | 460.3 ± 8.4 | |
| II | L | 23.1 ± 1.4 | 51.3 ± 2.9 | 17.9 ± 1.1 | 21.7 ± 0.8 | 45.4 ± 0.4 | 159.3 ± 6.6 |
| PN | 13.2 ± 0.7 | 48.5 ± 3.9 | 17.7 ± 0.2 | 30.6 ± 2.6 | 48.3 ± 0.8 | 158.4 ± 8.2 | |
| DN | 28.2 ± 1.2 | 64.5 ± 1.3 | 20.4 ± 2.2 | 35.1 ± 2.3 | 62.5 ± 0.9 | 210.7 ± 8 | |
| TN | 44.8 ± 2.3 | 66.7 ± 1.2 | 26.7 ± 2.9 | 44 ± 0.2 | 62.9 ± 1.9 | 245 ± 8.4 | |
| AD | - | 132.2 ± 7.2 | 49.1 ± 1.5 | 76.1 ± 1.8 | 101.6 ± 1.9 | 359 ± 12.4 | |
| III | L | 24.4 ± 2.6 | 47.4 ± 0.9 | 19.0 ± 0.9 | 30.3 ± 1.5 | 42.5 ± 9.6 | 163.6 ± 15.6 |
| PN | 37.9 ± 1.3 | 45.3 ± 1.3 | 19.8 ± 0.6 | 33.7 ± 0 | 53.7 ± 0.7 | 190.3 ± 3.9 | |
| DN | 23.1 ± 0.5 | 54.8 ± 0.8 | 20.9 ± 0.4 | 43.5 ± 0.5 | 60.8 ± 0.2 | 203 ± 2.4 | |
| TN | 42.3 ± 1.5 | 69.4 ± 0.4 | 24.6 ± 2.4 | 48.1 ± 10.1 | 84.6 ± 0.6 | 268.9 ± 15 | |
| AD | 49.9 ± 3.7 | 91.2 ± 3 | 31.8 ± 0.8 | 69.8 ± 1.1 | 117.7 ± 0.5 | 360.4 ± 9.1 | |
| IV | PN | 40.8 ± 1 | 47.3 ± 1.8 | 23.2 ± 0.9 | 35.9 ± 0.5 | 63.2 ± 0.4 | 210.4 ± 4.7 |
| DN | 27 ± 0.3 | 61.6 ± 1.3 | 27.8 ± 1.4 | 54.8 ± 0.4 | 80.2 ± 0.7 | 251.5 ± 4.1 | |
| TN | 33 ± 1.3 | 77.6 ± 1.5 | 31.0 ± 4.4 | 69.5 ± 1.3 | 95.7 ± 0.3 | 306.9 ± 8.8 | |
| AD | 69.4 ± 0.9 | 84.7 ± 1.8 | 61.7 ± 0.2 | 106.4 ± 0.8 | 143.2 ± 0.9 | 465.4 ± 4.5 | |
Table 4 Chaetotaxy of the legs ontogeny (solenidia in Greek letters) of Allogalumna (Acrogalumna) longipluma.
| Leg | Trochanter | Femur | Genu | Tibia | Tarsus | |
|---|---|---|---|---|---|---|
| I | L | - | d bv' | (l) σ | (l) v' φ1 | (ft) (tc) (p) (u) (a) s (pv) (pl) e ω1 |
| PN | - | - | - | - | ω2 | |
| DN | - | (l) | - | φ2 | - | |
| TN | v' | - | v' | v'' | (it) | |
| AD | - | - | - | - | v´ l'' | |
| II | L | - | d bv' | (l) σ | (l) φ | (ft) (tc) (p) (u) (a) s (pv) ω1 |
| PN | - | - | - | - | - | |
| DN | - | (l) | - | v' | ω2 | |
| TN | v' | - | v' | - | (it) | |
| AD | - | - | - | v'' | - | |
| III | L | - | d ev' | l' σ | (v) φ | (ft) (tc) (p) (u) (a) s (pv) |
| PN | - | - | - | - | - | |
| DN | - | - | - | - | - | |
| TN | v' | - | - | l' | (it) | |
| AD | - | - | - | - | - | |
| IV | PN | - | - | - | - | (p) ft'' (u) (pv) |
| DN | - | d, ev' | d, l' | v', φ | (tc) (a) s | |
| TN | v' | - | - | l' | - | |
| AD | - | - | l'' | - | ||
Protonymph (Figs. 6, 12). body length 379.3 (371.5-391.3), width 288.5 (285.2-291.6). Length of setae ro, 53.8 (52.5-53.9), le 64.6 (64.4-64.7), in 40 (38.5-42.4), ex 30.5 (27.5-32.5), ss 78.3 (75.9-82.3); sensillar capitulum dilatation shows a slight reduction 3.7 (3.5-4. 0) compared to the larva; the exobotridial setae are larger than the larva, the same situation occurs with the lamellar and rostral setae, they show the similar thickness and are arranged completely parallel on the prodorsum. The setae c3 almost doubles its length compared to the previous stage 40.2 (40.0-40.4), while c2 shows only a slight increase of 14 (13.9-14.0) (Table 2). Legs (Figs. 13-16) much more sclerotized than the larva, total length (trochanter-tarsus) leg I: 182.4 ± 5.9 µm, leg II: 158.4 ± 8.2 µm, leg III: 190.3 ± 3.9 µm and leg IV: 210.4 ± 4.7 µm; leg I and III larger than the larva, leg II shows no difference in size. The segments of leg IV are larger than in the other legs, except the femur which is longer in leg I (Table 3). Chaetotaxy formula of legs (solenidia in parentheses) I: 0-4-2(1)-3(2)-16(2); II: 0-4-2(1)-3(1)-13(2); III: 0-2-1(1)-2(1)-13; IV: 0-2-2-2-1(1) -12 (Table 4). The solenidion ω2 appears on tarsus I and the setae (p), ft'', (u), (pv) on tarsus IV. Notogaster with 15 pairs of minute setae how in the larva and four porose areas (Aa, A1, A2, A3), all circular, the first three of dark grayish color quite evident, the coloration of A3 fainter and less evident; this pattern is also repeated in the deutonymph and tritonymph. Epimeral region with chaetotaxy formula 3-1-2-1. Anal aperture 103.4 (94.8-107.4), genital plate primordium 33.6 (32.4-34.4), with a pair of genital setae and acetabulum. Opisthonotal glands are larger and conspicuous.
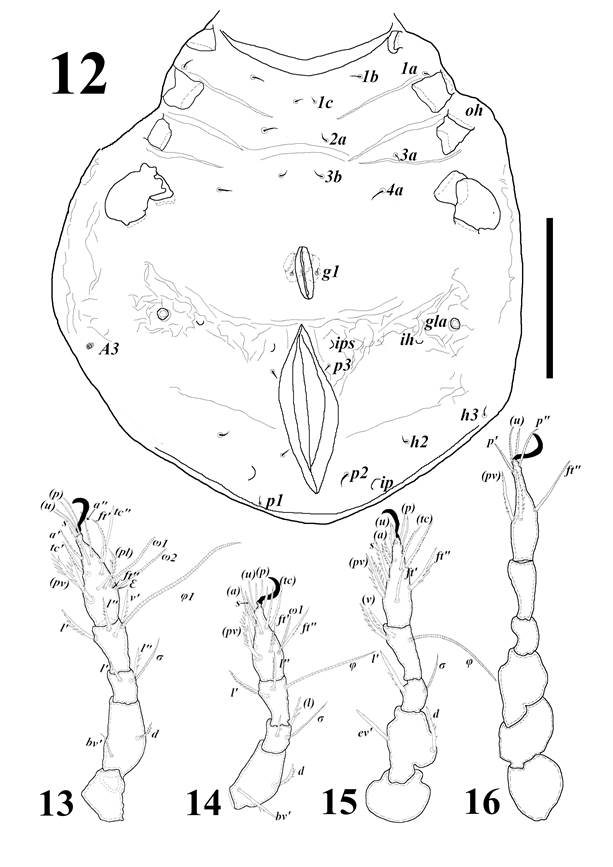
Figures 12-16 Allogalumna (Acrogalumna) longipluma, protonymph: 12) Entire body, ventral view. 13) Leg I. 14) Leg II. 15) Leg III. 16) Leg IV. Scale = 100 µm.
Deutonymph (Figs. 7, 17). Body length 471.9 (453.1-482.3), width 375.7 (359.4-391.7). Prodorsum triangular, length of setae ro, le, in, ex and sensillum increases in size relative to protonymph; setae in and le are more barbulate than in protonymph. The sensillum shows considerable changes in its length 99.2 (97.1-102.9), but not in its capitulum width, and remains with the same dimensions until tritonymph. The notogastral setae are smaller than in the protonymph, becoming almost imperceptible; porose areas increase in size. The setae c3 shows a developmental rate similar to that of the previous nymphal stage (9-11 µm), in contrast, c2 shows a higher elongation rate of 22.9 (22.0-24.2) (Table 2). The length of legs I and III show an increase of 20 and 13 µm respectively concerning the protonymph, in legs II and IV the growth is greater (62 and 41 µm) (Figs. 18-21). The length of legs I and III show an increase of 20 and 13 µm respectively concerning the protonymph, in legs II and IV the growth is greater (62 and 41 µm) (Figs. 18-21). Chaetotaxy increases, the most evident is found in leg IV, where setae d, ev' (femur), d, l' (genua), v', φ (tibia) and setae (tc), (a), s (tarsus) are added, chaetotaxy formula of legs (solenidia in parentheses): I: 0-4-2-2(1)-3(2)-16(2); II: 0-4-2(1)-3(1)-13(2); III: 0-2-1(1)-2(1)-13; IV: 0-2-2-2-1(1)-12 (Table 4). In epimere IV, seta 4b is added, epimeral region formula 3-1-2-2-2, maintained until tritonymph and adult. Anal aperture 120.0 (113.3-132.0), two pairs of anal setae, three adanal, lyrifissure iad located anterior to ad3 setae; genital plate primordium 46.9 (45.2-47.9) with three pairs of genital setae.

Figures 17-21 Allogalumna (Acrogalumna) longipluma, deutonymph: 17) Complete body, ventral view. 18) Leg I. 19) Leg II. 20) Leg III. 21) Leg IV. Scale = 100 µm.
Tritonymph (Fig. 8). Body growth is considerably greater than in the previous stage of development, length 658.5 (647.9-675.0), width 531.2 (511.8-542.0); further sclerosing of the body causes it to take on a brown, slightly reddish coloration; prodorsal setae increase in length relative to the deutonymph (Table 2). Similarly, the development and size of the humeral organ (oh) is greater. Porose areas Aa presents an anteromedial orientation in notogaster; the growth rate of legs (Table 3) is greater than in deutonymph; setae v' are added on trochanters I-IV and genua I-II, seta v'' on tibia I, seta l' on tibiae III-IV and seta (it) on tarsus I-III; chaetotaxy formula of legs (solenidia in parentheses): I: 1-4-3(1)-4(2)-18(2); II: 1-4-3(1)-3(1)-15(2); III: 1-2-1(1)-3(1)-15; IV: 1-2-2-2(1)-12 (Table 4). Anal opening 162.4 (158.6-170.1); genital plate primordium 73 (69.5-74.4), five pairs of genital setae, and three acetabulum also present in the adult stage.
Adult (Figs. 9, 11): Body length 623.3 (590.3-634.4), width 597.5 (568.1-628.5); body strongly sclerotized, light brown post molt and progressively darkening in later days to dark brown coloration. Prodorsal setae rather long, seta in 123.3 (119.4-131.2) larger, setae le 111.1 (108.2-114.4) larger than setae ro 70.5 (64.3-83.0); the latter with a curvature in the anterior middle portion that converges at the ends. Sejugal porose areas Ad elongate widened medially and located posterior to setae in. Sensillum elongate, setiform, and barbulate from middle onwards 206.5 (191.1-217.3); length up to double compared to tritonymph, width almost reduced by half 2.6 (2.3-3.0). L-line absent. Notogaster with five pairs of porose areas; Aa'' large, subtriangular, arranged next to pteromorph joint, Aa' two to three times smaller, circular or oval, located between lm and la setae, A1 circular, between lp and h3, A2 oval, elongate, located posterolateral to the anterior, A3 more elongate than last and accommodated slightly transverse to notogaster, between h1, p1, and p2. Pteromorphs auriculate, with multiple descending branching lines; a stria crosses transversely the median region, between alveolus c2 and lirifisura ia, terminating as a sclerotized canal near the pteromorph joint. Setae c2 and c3 are absent. Median pore absents in female, male with 44 (40-54) pores between porose areas A3. Legs considerably larger than tritonymph, total length trochanter tarsus (I: 460.3 ± 8.4 µm, II: 359 ± 12.4 µm, III: 360.4 ± 9.1 µm and 465.4 ± 4.5 µm). Setae v', l'' are added on tarsus I, v''', l'' on tibia II and IV respectively; chaetotaxy formula of legs (solenidia in parentheses): I: 1-4-3(1)-4(2)-20(2); II: 1-4-3(1)-4(1)-15(2); III: 1-2-1(1)-3(1)-15; IV: 1-2-2-3(1)-12. Anal aperture reduced compared to tritonymph 149 (134.7-157.6), anal and adanal setae minute, lirifisura iad paraanal; genital aperture 95.6 (90.0-104.7), six pairs of genital setae, g1-4 arranged medially on plate, g5-6 on upper edge. Opisthonotal glands are positioned on notogaster next to porose areas A1.
Discussion
The measurements of morphological characters show some similarity with those reported by Seniczak et al. (2012), the protonymph, deutonymph, and adult are slightly smaller in size. Allogalumna longipluma differs from A. monttensis, A. borhidii and A. cubana (species reported for Mexico) by the length and width of the body; this can be up to twice as large. They possess only four pairs of porose areas; Aa porose areas elongate and crescent-shaped in A. monttensis, surrounded by rings in the case of A. borhidii and of smaller size in A. cubana. Prodorsal setae of A. longipluma considerably longer and barbulate; interlamellar setae in A. cubana is represented by only one alveolus, in A. monttensis almost imperceptible. Sensillar capitulum: fusiform and large, observed only in juveniles of A. longipluma. Well defined lamellar (L) and sub lamellar (S) lines and presence of postanal porose areas in A. monttensis (Balogh & Mahunka, 1979; Hammer, 1962).
The chaetotaxy of A. longipluma legs resembles that of Galumna obvia (Ermilov et al., 2013), with subtle variations in the order of occurrence of setae l', v'' in tibiae II-III and absence of setae l'' in tibiae IV.
This work is part of the project: mites associated with avocado cultivation in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)



