Introduction
The tropical deciduous forest (TDF), also known as tropical dry forest (Becerra, 2005) is an ecosystem existing at the transition from humid to semiarid climates of Central and South America, southeastern Africa, southern and southeastern Asia, Australia, and certain oceanic island groups. It is typically composed of deciduous trees and shrubs that drop their leaves during the coldest and driest season (autumn /winter), after bearing abundant foliage during a hot and rainy season (spring /summer). Simultaneously, numerous species of annual plants finish their life cycle during the dry season and germinate from seeds during the rainy season. Thus, the vegetation switches annually from relatively open and mostly dormant into a dense verdant community, and back again (Halffter et al., 2001; Martínez-Yrízar & Sarukhán, 1990).
In the state of Guerrero of Mexico, TDF occupies about half of the total forest surface (Miranda & Hernández-X, 1963) and forms part of a distinct Mexican floristic group, one of 12 major subdivisions recognizable within the neotropical TDF biome (Banda-R. et al., 2016). Guerrero’s TDF persists mostly on landforms with less easily farmed topology, often including closely spaced ridge/gully systems. Intense torrential rainfall and runoff during the wet season result in mosaics of removal versus accumulation of organic matter. This creates a great variety of conditions for the development of microbiological communities of bacteria and fungi, edaphic microflora, and microscopic soil animals, including nematodes (Montaño et al., 2010; Barbosa et al., 2016; Ballina Gómez, et al., 2012). High levels of soil heterogeneity and soil biodiversity correlate with high rates of endemism in tropical dry forest flora and fauna, including some endemic taxa of Nematoda (Mundo-Ocampo et al., 2002; Franco-Navarro et al., 2019).
Deciduous plant communities of coastal Mexico have served the needs of human societies for centuries before the Spanish Conquista. In the Costa Chica of eastern coastal Guerrero, where soil sampling for this study was conducted, archeological evidence indicates extensive ancient human population centers. These include (among others) a major urban site that flourished over a millennium ago, covering some 90 ha at Piedras Labradas, Ometepec (Reyes Álvarez, 2017). Since the arrival of European colonists, increasingly intensive types of agriculture and animal husbandry are practiced over large parts of the Costa Chica’s landscapes, but subsistence farming nevertheless remains predominant. Traditional practices continue to be applied on many small family farms, including episodic crop cultivation alternating with multi-year or multi-decade periods of fallowing and TDF regrowth.
Nematodes are ubiquitous in all habitats with available organic carbon sources, playing key roles as primary and intermediate consumers in soil food webs as the planet’s most abundant multicellular animals (Bongers & Ferris, 1999). They also serve as indicators of soil health, including, for example, to detect toxic effects of metals and other pollutants resulting from anthropogenic activities (Azpilicueta et al., 2015). The mixture of geomorphological, hydrological, meteorological, biological, and anthropogenic factors underlying the present distribution of TDF has created a patchwork of soil properties and past use histories. Against this background, the goal for this study is to present a first faunistic assessment of TDF soil nematode diversity on a local scale, as a first step in developing broader-scale strategies to assess both the recent impacts on TDF nematodes from ongoing activities, as well as potentially revealing patterns that might arise from legacy effects from past human land uses.
Materials and methods
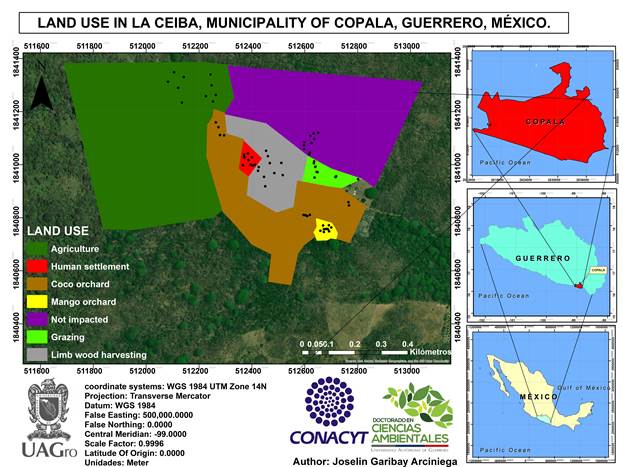
Site selection and soil sampling. The selected area is located at the coordinates 16° 39’ 5.8” N 98° 52’ 59.1” W 40 m asl in the municipality of Copala, within the Costa Chica region of the state of Guerrero, Mexico.
The local inhabitants selected an area of 102.91 hectares where seven land uses were established, as depicted in Figure 1: 1) a coconut orchard (13.47 hectares); 2) a mango orchard (0.55 hectares); 3) deciduous non-impacted forest (22.69 hectares) without known prior anthropogenic activities; 4) a human settlement with two wattle and daub houses (0.61 hectares); 5) an agricultural area (31.34 hectares), in which corn, beans, hibiscus and sesame were mainly cultivated; 6) a grazing area (1.05 hectares) for goats and cattle, with dry clay soils and organic residues from manure deposited by the grazing animals; 7) a wattle-enclosed area (5.73 hectares) where the long-repeated manual process of harvesting wood limbs, fence-posts and firewood left high content of organic deposits. All clearing and management were performed with hand tools; mechanized equipment or slash-and-burn were not used on any of these seven sites.

Figure 2 Field images of land uses: 1) Coconut orchard; 2) Mango orchard; 3) Deciduous Non Impacted Forest; 4) Human settlement; 5) Grazing; 6) Agriculture; 7) Limb wood harvesting.
Ten soil samples were randomly collected from each land use site during three weeks at the start of the 2018 wet season, minimizing temporal heterogeneity while the vegetation was producing new foliage, but had not yet closed in enough to hinder access. Three subsamples at each sampling point were collected, with a separation of approximately 50 cm around a tree or shrub plant at a depth of 25-30 cm, and these subsamples were combined to comprise each of the 70 samples of approximately 1.5 kg of soil. Nematodes were extracted by the combined sieving and Baermann funnel method (De Ley et al., 1998). A volume of 300 ml was taken from each composite sample, the soil was mixed with filtered water in a graduated container and filtered through two soil sieves with mesh numbers 35 (427 microns pore size) and 400 (37 microns pore size). Sediment obtained with the 400-mesh sieve was placed on filter paper on top of a wire mesh support in a glass Baermann funnel. The funnel, sealed at the stem with rubber tubing and a clamp, was filled with water and allowed to stand for 48 hours. Next, water was collected from each funnel and concentrated in a 10 ml vial. To estimate total nematode abundance, an aliquot of 1 ml from each sample was placed in a counting dish and the nematodes were counted under an Olympus dissecting microscope. The quantified totals in 1 ml were extrapolated to 10 ml, to estimate the total population per sample. Each counted aliquot was returned to the rest of the 10 ml vial and then preserved in a 5.0% formaldehyde solution heated to 45-50 °C (Baldwin & Mundo-Ocampo, 1995; De Ley et al., 1998).
Nematode identification and estimation of population numbers. After 24 hours the samples were washed with running water through a 400-mesh sieve, to remove all fixative. The nematodes were then transferred to Petri dishes with solution A (70 ml of 95% ethanol, 5 ml of glycerin and 25 ml of distilled water) and placed in a desiccator until most of solution A had evaporated and the specimens were partially infiltrated with glycerin. Solution B (90 ml of 95% ethanol and 10 ml of glycerin) was added, and the samples were then placed in an oven at ± 40 °C until all water and ethanol evaporated such that the nematodes became embedded in pure glycerin (De Ley et al., 1998). One hundred nematode specimens were randomly picked from each site, mounted in absolute glycerin on 10 paraffin-ringed microscopic slides with 10 specimens each; they were covered and sealed with coverslips and paraffin (De Ley et al., 1998). The permanent mounts were observed under a Nikon E 600 differential interference microscope for identification of genera and families, using keys and monographs as well as by comparison with type and reference specimens from the University of California-Riverside nematode collection (Mundo-Ocampo & Baldwin, 2010; De Ley et al., 1998). In addition, abundance was appraised for each of five trophic groups, following Parmelee et al. (1995). Colonizer-persister (c-p) classes or values for each genus were assigned, following the classification proposed by Bongers (1990) and Bongers et al. (1995). This approach organizes terrestrial and freshwater nematode genera and species in the following five functional groups according to their life history characters and resilience to disturbances: Class c-p1 contains nematodes with very short generation time, capable of rapidly colonizing soil after major disturbances, or rebuilding numbers after moderately severe impacts; these nematodes are typically bacterial feeders with highest metabolic activity and often employ arthropods as phoretic vectors for their dispersal. Class c-p2 are short generation time nematodes with a relatively high reproduction rate, with less specialized dispersal/colonization abilities but often having high tolerance for extreme temperatures, soil desiccation and/or freezing, and situations of minimal bacterial/fungal biomass availability; this class mainly includes bacterial feeders, fungal feeders and selected predators. Class c-p3 nematodes have moderately long generation times and are moderately sensitive to disturbances, including not only bacterial feeders, fungal feeders but also plant parasites, omnivores and certain predators. Class c-p4 includes nematodes with typically long generation time and higher dependence on stable soil conditions, often with larger adult sizes and mainly feeding by omnivory or predation. Class c-p5 finally includes nematodes with the lowest reproduction rates and largest body sizes. This group mainly includes large Dorylaimida and Mononchida feeding as omnivores, known to be most sensitive to drastic changes in soil chemistry including addition of toxic pollutants.
To determine the Alpha and Beta diversity, including the Simpson and Shannon indices, various statistical methods were applied (Halffter et al., 2005; Moreno et al., 2011; Parmelee et al., 1995; Pla, 2006) as implemented in Biodiversity Calculator (Zaiontz, 2020). In addition, the Margalef biodiversity index was calculated (Gamito, 2010; Maclaurin & Sterelny, 2008; Halffter et al., 2005; Ferriol & Merle, 2002). Genera were assigned to trophic groups following Yeates (1971). To calculate significant values for differences in trophic group abundance per land use, Two Factor ANOVA without Replication was applied via the Real Statistics Using Excel platform, Milan Italy, (Zaiontz, 2020). Another method that does not require replicated treatments or factors is the Hutcheson t test, which calculates significance values for differences between pairs of Shannon Index values (Hutcheson, 1970). Genera were assigned colonizer-persister values following Bongers, (1990); Bongers et al. (1995) and the Maturity Index for each land use was computed from abundance data of the c-p classes following Bongers and Ferris (1999).
Results
Total abundance and percentage values for the identified genera in all land use sites are displayed in alphabetical order in Table 1. Genus-level diversity ranged from 12 to 18 taxa for each of the sampled land uses, or 27 total genera for all samples combined. In terms of families, Cephalobidae was the most abundant (26.7%) including, in descending order of abundance, genera Cephalobus, Pseudacrobeles, Acrobeles, Eucephalobus and Acrobeloides (Table 1) followed by Dorylaimidae (13.0%) with genera Eudorylaimus, Dorylaimus and Xiphinema. The genera listed in Table 1 represent a total of 18 families.
Table 1 Total abundance of Genera by land use, percentages for each genus in total sampling, genus trophic type (Ba= bacterivore, Fu= fungivore, Om= omnivore, Pl= herbivore, Pr= predator), colonizer-persister (c-p) classes or values and Maturity Index per land use of Nematoda in seven land use systems: MO= Mango orchard, A= Agriculture, CO= Coconut orchard, G= Grazing, HS= Human settlement, LW= Limb wood harvesting and DNiF= Deciduous Non Impacted Forest (henceforward, abbreviations will be used in tables). Plant parasites groups were excluded from MI calculations. Estimated for fresh soil volume of 300 ml. Abbrev. TG cp V= Trophic Group / c-p value.
| Genera abundance by land use | MO | A | CO | G | HS | LW | NI | Totals | % | TG cp V |
|---|---|---|---|---|---|---|---|---|---|---|
| Acrobeles | 0 | 46 | 0 | 0 | 122 | 0 | 120 | 287 | 1.1 | Ba/2 |
| Acrobeloides | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 96 | 0.3 | Ba/2 |
| Aphelenchoides | 35 | 116 | 73 | 114 | 0 | 289 | 359 | 985 | 3.9 | Fu/2 |
| Aphelenchus | 29 | 231 | 656 | 419 | 689 | 385 | 0 | 2408 | 9.5 | Fu/2 |
| Cephalobus | 121 | 323 | 1021 | 837 | 486 | 1300 | 1017 | 5105 | 20.2 | Ba/2 |
| Chromadorids comb. | 0 | 0 | 0 | 76 | 0 | 0 | 0 | 76 | 0.3 | Ba/3 |
| Criconemoides | 12 | 46 | 0 | 0 | 0 | 0 | 120 | 177 | 0.7 | Pl/3 |
| Cuticularia | 29 | 69 | 0 | 951 | 203 | 482 | 0 | 1733 | 6.8 | Ba/1 |
| Dolichodorus | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 96 | 0.3 | Pl/3 |
| Dorylaimus | 29 | 208 | 255 | 114 | 324 | 241 | 239 | 1410 | 5.6 | Om/4 |
| Eucephalobus | 40 | 46 | 0 | 0 | 0 | 0 | 120 | 206 | 0.8 | Ba/2 |
| Eudorylaimus | 29 | 208 | 255 | 152 | 324 | 241 | 299 | 1508 | 5.9 | Om/4 |
| Helicotylenchus | 17 | 139 | 365 | 381 | 405 | 193 | 419 | 1917 | 7.6 | Pl/3 |
| Meloidogyne | 0 | 46 | 0 | 190 | 122 | 289 | 479 | 1126 | 4.4 | Pl/3 |
| Mesorhabditis | 0 | 0 | 0 | 0 | 0 | 96 | 0 | 96 | 0.3 | Ba/1 |
| Monhysterids comb. | 0 | 92 | 109 | 0 | 0 | 193 | 120 | 514 | 4.0 | Ba/2 |
| Mononchus | 0 | 92 | 73 | 0 | 0 | 96 | 0 | 262 | 1.0 | Pr/4 |
| Paratrichodorus | 0 | 0 | 0 | 0 | 122 | 0 | 0 | 122 | 0.4 | Pl/4 |
| Paratylenchus | 12 | 0 | 0 | 0 | 0 | 96 | 0 | 108 | 0.4 | Pl/3 |
| Plectus | 29 | 46 | 0 | 0 | 0 | 0 | 0 | 75 | 0.3 | Ba/2 |
| Pratylenchus | 0 | 0 | 73 | 114 | 122 | 289 | 0 | 597 | 2.3 | Pl/3 |
| Pseudacrobeles | 12 | 92 | 255 | 304 | 0 | 193 | 180 | 1036 | 4.1 | Ba/2 |
| Rhabditis | 58 | 92 | 0 | 0 | 0 | 0 | 958 | 1108 | 4.4 | Ba/1 |
| Rhabditonema | 35 | 370 | 328 | 0 | 932 | 96 | 838 | 2598 | 10.3 | Ba/1 |
| Trophurus | 17 | 0 | 0 | 0 | 122 | 0 | 0 | 139 | 0.5 | Pl/3 |
| Tylenchinae comb. | 75 | 0 | 109 | 152 | 81 | 144 | 479 | 1041 | 4.1 | Pl/2 |
| Xiphinema | 0 | 46 | 73 | 0 | 0 | 0 | 239 | 359 | 1.4 | Pl/5 |
| Total abundance: | 575 | 2310 | 3645 | 3805 | 4050 | 4815 | 5985 | 25185 | 100 | |
| % Abundance: | 2.2 | 9.1 | 14.4 | 15.1 | 16.0 | 19.1 | 23.7 | 100 | ||
| Total genera: | 16 | 18 | 13 | 12 | 13 | 18 | 15 | |||
| Maturity Index: | 1.9 | 2.2 | 2. 7 | 1.8 | 2.0 | 2.1 | 1.8 |
Maturity Index values were generally low and ranged from 1.8 to 2.2, with the Coconut Orchard being a notable outlier at 2.8.
The Deciduous Non-impacted Forest (DNiF) presented the highest abundance (5,985 individuals), accounting for 23.8% of the total number of extracted nematodes. No predatory nematodes were found at this site. Conversely, the mango orchard site registered the lowest abundance value (575), representing 2.3% of the total nematode numbers; however, it was the second highest with respect to the number of genera (16). The wood harvesting area ranked second in abundance, accounting for 19.1 % of the total number of nematodes, and with the highest number of genera (18). The site recovering from past grazing had a median number with respect to nematode abundance, but its number of genera (12) was the lowest among all the sites. The Coconut grove presented intermediate values of abundance and genera diversity, along with the sites recovering for a decade after multicropping or use as a small settlement.
Total number of nematodes per trophic group and per land use are given in Table 2 and in Figure 4; Simpson’s diversity index s is showed in Figure 3. The bacterivores were the most abundant trophic group overall while predators were the least abundant one, and herbivores alternated with fungal feeders for second or third highest numbers
Table 2 Total abundance of Trophic groups by land use. Estimated for fresh soil volume of 300 ml per sample.
| Trophic groups | MO | A | CO | G | HS | LW | DNiF | Totals |
|---|---|---|---|---|---|---|---|---|
| Bacterivores | 322 | 1,178 | 1,713 | 2,093 | 1,742 | 2,456 | 3352 | 12,855 |
| Fungivores | 63 | 347 | 729 | 533 | 689 | 674 | 359 | 3,393 |
| Omnivores | 58 | 416 | 510 | 266 | 648 | 482 | 539 | 2,918 |
| Predators | 0 | 92 | 73 | 76 | 0 | 96 | 0 | 338 |
| Herbivores | 132 | 277 | 620 | 837 | 972 | 1,107 | 1,736 | 5,681 |
| Totals: | 575 | 2,310 | 3,645 | 3,805 | 4,050 | 4,815 | 5,985 | 25,185 |
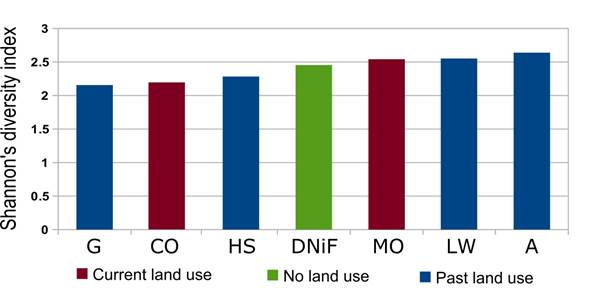
Figure 3 Shannon index values for genus richness in the studied land use systems. See Table 5 for differences among sites (Hutcheson t test and p values).
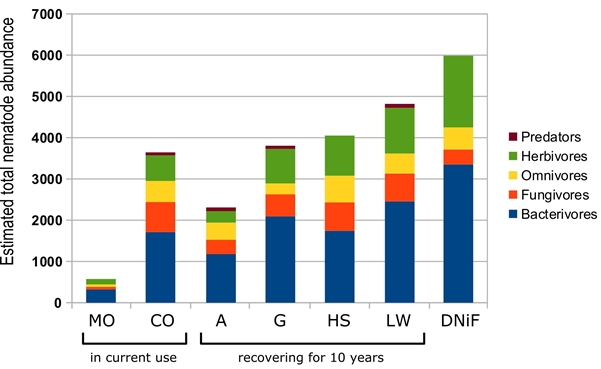
Figure 4 Total abundance (number of individuals) of nematodes by trophic group in each studied site.
Table 3 illustrates and compares Shannon’s diversity index, Simpson’s diversity index, Equitability and Margalef Richness diversity indices calculated from genus and trophic group abundances in all the land uses. The obtained Simpson Dominance Index values fluctuated between 0.85 for the "Grazing" land use and 0.91 for the "Agriculture" land use, showing that genus abundances were distributed evenly for all seven sites. The obtained Equitability index values were close to or identical with Simpson index values. The most abundant genus per site (see Table 1) was always a bacterial feeding genus, but its identity was not always the same: Cuticularia was most abundant in the “Grazing” site only, while Rhabditonema had the highest count in the “Agriculture” and “Human Settlement” land uses, with Cephalobus being the most abundant genus in the remaining four sites. In two sites the fungal-feeding genus Aphelenchus ranked with second highest abundance value (in the Coconut orchard and the Human settlement), while the plant parasitic Tylenchinae scored the second highest count in the Mango orchard. The following genera of bacterial feeders ranked second highest in the four remaining sites: Cephalobus in “Agriculture” and “Grazing”, Cuticularia in “Limb wood gathering” and Rhabditis in DNiF.
Table 3 Diversity indices calculated for genera and trophic groups (in brackets), for the different studied land uses.
| Land use / Diversity indices | MO | A | CO | G | HS | LW | DNiF |
|---|---|---|---|---|---|---|---|
| Simpson Dominance Index (D) | 0.9034 (0.6139) | 0.9131 (0.6693) | 0.8532 (0.6904) | 0.8520 (0.6243) | 0.8739 (0.7933) | 0.8870 (0.6571) | 0.8966 (0.5909) |
| Shannon Index | 2.541 (1.147) | 2.639 (1.320) | 2.19 (1.332) | 2.156 (1.201) | 2.283 (1.302) | 2.552 (1.265) | 2.454 (1.071) |
| Equitability Index | 0.9176 (0.7127) | 0.9131 (0.8199) | 0.8552 (0.8273) | 0.8675 (0.7465) | 0.8900 (0.8089) | 0.8831 (0.7860) | 0.9064 (0.6652) |
| Margalef Richness Index | 2.358 (0.6293) | 2.195 (0.5165) | 1.463 (0.4877) | 1.334 (0.4841) | 1.444 (0.4815) | 2.005 (0.4717) | 1.610 (0.4599) |
The values obtained for genus diversity with the Margalef index ranged between 1.334 and 2.358, representing weakly to moderately balanced richness and equity. However, Gamito (2010) cautioned that the Margalef index should be considered carefully, mainly when it is analyzed in combination with the Simpson and Shannon diversity indices because the Margalef index is more sensitive to sample size.
Results of two-factor ANOVA without replication (Table 4) show significant differences among all sites with respect to nematode trophic group abundances. Conversely, differences among all trophic groups were also highly significant with respect to abundance per site. To potentially gain further insight into the main factors contributing to these differences, we applied two-factor ANOVA on several reduced datasets in which, for example, the most (bacterivores) and least (predators) abundant trophic groups were excluded (data are shown in Table 4 for some but not all of these additional comparisons).
Table 4 Two-factor ANOVA without replication results: p values for differences between trophic group abundances. Values in bold font, indicate significant differences at p<0.05.
| Trophic groups included | Sites included | Differences among trophic groups | Differences among sites |
|---|---|---|---|
| All five Trophic groups | All seven | 0.000000416 | 0.01298 |
| Fungivores, Omnivores & Herbivores | All seven | 0.05033 | 0.05821 |
| Fungivores, Omnivores | All seven | 0.31751 | 0.02161 |
| Fungivores, Herbivores | All seven | 0.13783 | 0.25663 |
| Omnivores, Herbivores | All seven | 0.05841 | 0.16655 |
| All five Trophic groups | Coco, Settlement, Agriculture, Limb wood, Grazing | 0.000000032 | 0.04525 |
| All five Trophic groups | Settlement, Agriculture, Limb wood, Grazing | 0.000005066 | 0.05469 |
The seven sites were significantly different when comparing only the abundances of fungivores and omnivores. Comparing all five trophic groups after excluding the two sites with respectively highest and lowest abundance of nematodes (DNiF and the Mango orchard) resulted in highly significant differences among trophic groups, versus moderately significant differences between the five remaining sites. Repeating the analysis with only those four sites which were formerly impacted and then abandoned ten years ago, produced a p value just above the significance threshold for differences among sites, while differences among all trophic groups were highly significant.
Pairwise comparisons of the Shannon Index using Hutcheson's t test also revealed a mosaic of significant differences between 11 pairs of sites (Table 5; Fig. 3). In seven of these, differences were highly significant (p<0.01), including 4 pairs where both sites were abandoned a decade earlier. No straightforward pattern appeared among these highly significantly different pairs, but some land uses stood out in two different respects. Firstly, the coconut orchard differed with high significance from three land uses (mango orchard, former agriculture and former limb wood gathering), the greatest number of highly significant differences for any one land use. Secondly, the comparison of the former grazing land with the former agriculture area yielded the lowest p value of all land use pairs.
Table 5 Hutcheson t test results: p values for all pairwise comparisons of Shannon Index values calculated from genus richness and abundances. Values in bold font indicate significant differences. *) p<0.05; **) p<0.01.
| MO | 0.0024** | |||||
| NI | 0.0209* | 0.4015 | ||||
| HS | 0.4403 | 0.0152* | 0.1012 | |||
| A | 0.0001** | 0.3440 | 0.4000 | 0.0009** | ||
| G | 0.7477 | 0.0006** | 0.0066* | 0.2569 | 1.7E-5** | |
| LW | 0.0047** | 0.9210 | 0.0738 | 0.0240* | 0.4588 | 0.0014** |
| CO | MO | DNiF | HS | A | G | |
Another 4 pairwise comparisons scored as significantly different (p<0.05); two of these included the DNiF site as compared respectively to the coconut orchard and to the formerly grazed site, while the two others compared the site formerly used for a settlement to respectively the mango orchard and the former site for gathering limb wood.
Discussion
The absence of predators at DNiF is surprising because nematodes that are primarily predatory are usually considered to function as “persisters”, becoming established only when soil conditions remain relatively stable over longer periods of time (Bongers, 1999). In addition to nematodes scored as predators in Table 1, it should also be kept in mind that facultative predation is probably one of the various feeding mechanisms of some nematodes classified as omnivores, including the genera Dorylaimus and Eudorylaimus, encountered in this study, which also are considered to function more as persisters (Bongers & Ferris, 1999). Moreover, Kardol et al. (2010) suggest that extensive interactions driven by climate change effects might cause local changes in plant community composition. Perhaps such changes have been affecting soil nematode diversity at our study sites, for example through recent pulses of increased OM deposition into soils after limbs or entire plants die from shifting temperature extremes, or through increased deposition of limiting nutrients with falling ash from the widespread Central and South American wildfires of recent years. In a meta-analysis of 87 studies, Jiang et al. (2022) found that a statistically significant increase in soil NH4+ content follows burning of TDF, but not burning of rainforest or savanna vegetation. Also, comparisons of N addition studies worldwide led Xing et al. (2022) to conclude that N deposition in dry and warm regions lowers nematode diversity while stimulating their total abundance, with NH4+ increases particularly reducing omnivores and predators.
Alternatively, a more challenging scenario for future research could instead be that our site designated as DNiF continues to be affected by undocumented high -or low- impact land uses by previous generations of smallholder farmers. If so, then it was not a valid representative of undisturbed conditions but would have to be characterized instead as a site recovering for multiple decades from unknown past anthropogenic impacts.
Current agricultural practices at the mango orchard include assiduous manual removal of understory shrub seedlings as well as weeds, resulting in a distinctly bare soil surface compared to the coconut orchard or the fallowed sites (Fig. 2). This may explain the lowest nematode abundance found here, compared to the other sites, due to organic matter removal and/or soil compaction by human foot traffic. Trampling and compaction decrease habitable pore space (Bouwman & Arts, 2000) and are known to alter nematode diversity and abundance in other ecosystems, for example by decreasing numbers of bacterivores and omnivores/predators under turf grass. The high nematode abundances in the limb wood cutting site contrast greatly with the mango orchard.
As observed in Figure 2A, the coconut grove was not weeded manually but instead was used intermittently for grazing by the farm’s domesticated ruminants. Infrequent occurrences of trampling and grazing by hoofed herbivores may have caused the soil in the coconut orchard to suffer moderate levels of compression, compared to the past grazing, multi-cropping, and settlement sites, and especially contrasting with the mango orchard. For all three past use sites, soil bioturbation activities by larger soil animals during their ten fallow years could have counteracted some of the preceding soil compaction, without yet returning soil porosity and aeration to levels that match the DNiF site and the former limb wood gathering site.
The low Maturity Index values of 1.8-2.2 for all land uses except the Coconut Orchard are quite striking, compared for example to values between 2.6 and 2.8 recorded by Xiao et al. (2014) from rainforest and rubber plantations in southern China, or values of 3.0 to 4.1 calculated by Bloemers et al. (1997) from rainforest with different human impacts in Cameroon. Maturity Index values are calculated as the sum by weighted rankings of nematode abundances, on a scale differentiating easily dispersed fast-reproducing colonizer species from slowly reproducing persister species (Bongers, 1999). They can reflect successional states of soil nematode communities as well as degrees of disturbance from agricultural practices, inorganic pollutants, or soil acidification (Bongers & Ferris, 1999; Sánchez-Moreno & Talavera, 2013). However, this approach is only rarely applied to ecosystems in which all or most nematodes presumably undergo anhydrobiosis seasonally or repeatedly each year, or in tropical areas where mean annual soil temperatures exceed 22 °C and nematode reproduction is presumably accelerated in diverse groups. It remains to be investigated whether it is appropriate for tropical biomes to follow the underlying premises and the standardized colonizer-persister rankings employed in MI calculations. In our own data, it is for example puzzling that the DNiF site produced the lowest MI of all seven land uses.
The Hutcheson t test is not usually applied to nematode community surveys, but examples of its use in recent studies for other types of organisms include Lacerda-Júnior et al. (2019) and Rivera-Rivera and Cuevas (2020). Our results obtained with this method (Table 5) are largely consistent with the interpretations discussed above, including the possibility that the DNiF site was in fact disturbed by unknown uses in the distant past, and/or that 10 years of recovery at the four fallowed sites was insufficient to return nematode communities to an undisturbed-like state. Pairwise differences with significant p values for the Hutcheson t test apply particularly for pairs that include the currently used site with the most intense ongoing impacts (the mango orchard with its weed-free soil surface), or the two fallowed sites with greatest prior surface impacts (the former settlement and former vegetable growing site). The latter two experienced more frequent human ingress, presence, and maintenance than the formerly grazed or limb wood gathering sites, suggesting again that soil compaction may be a significant contributor to heterogeneity, and that belowground recovery from compaction may take well over a decade.
Our work is the first published nematode study of local land use heterogeneity within a TDF mosaic landscape. Nevertheless, recent papers have reported on tropical or subtropical nematode communities from multiple forest types and/or from other seasonally arid ecosystems with human impacts. Mejía-Madrid, (2018) analyzed soil nematodes from 11 sites sampled between 2013 and 2015 at elevations of 100 to 2400 m asl. in four, focusing mainly on TDF, tropical rainforest and temperate conifer vegetation zones, at locations either with low disturbance or significant impacts. This author did not report on genus richness but found 22 different families across his entire survey, compared to 18 families in ours. The three forest zones yielded total abundances averaging 350 to 11020 nematodes per 100 ml fresh soil, with the upper value obtained at 2071 m asl in grassland converted from TDF. These values far exceed our estimates: converted totals from our sites range between 19 and 200 individuals per 100 ml fresh soil. Multiple factors could explain such discrepancy, but the main one could be that his samples collecting spanned two years including both dry and wet seasons, sites were located up to 500 km apart, altitude differences ranged over 2000 m, and the samples included combinations of eleven different soil types. Our strategy on the other hand deliberately limited the sampling window to under one month in a total area smaller than 1 km2.
Seasonality of nematode abundances within TDF soils has not yet been reported on, but a related ecosystem was investigated by Mejía-Madrid and Sánchez-Moreno (2022), who sampled soils below two deep-rooting legume species (Prosopis laevigata and Cercidium praecox) during wet and dry seasons of 2018 in the semiarid Zapotitlán Valley in Puebla, Mexico. While C. praecox and several other woody perennials do shed all leaves during the dry season, local stands of P. laevigata and some shrubs maintain green foliage and produce new leaves all year (Pavón & Briones, 2001). Mejía-Madrid and Sánchez-Moreno (2022) sampled soils below surfaces with or without biological soil crusts, within a protected reserve, suggesting that these soils were likely relatively undisturbed. By converting their nematode counts to comparable units, we calculate dry season mean abundances equaling 126 to 863 nemas per 100 ml fresh soil, versus wet season means of 667 to 2078 nemas per 100 ml fresh soil. Across all samples, they reported 87 genera, with genus richness varying from 18 to 47 between the different season/legume/surface combinations. These values mostly exceed ours, perhaps due to soil compaction on our sites, caused by recent or decade-old trampling. It probably also reflects sampling depth differences: Mejía-Madrid and Sánchez-Moreno (2022) collected the top 10-12 cm of soil, while our samples reached a depth of 25-30 cm. Total abundance of soil nematodes is often higher in the upper 15-20 cm than at depths below, causing a roughly inverse relationship between nematode counts and whole soil core depths (see for example Čermák et al., 2011; Arieira et al., 2016).
Faunistically, our results yield some similarities with the wet season data in the supplementary table of Mejía-Madrid and Sánchez-Moreno (2022), but also revealed some interesting differences. While they encountered a much greater diversity of 19 dorylaim genera (versus only 3 in our sites), the two most abundant among these were Dorylaimus and Eudorylaimus in both studies. In three of the four wet season sample sets of Mejía-Madrid and Sánchez-Moreno (2022), fungal feeder Aphelenchus was the most abundant genus, while moss-associated bacterial feeder Wilsonema had the highest count in their fourth sample set (crust-covered soil under P. laevigata). The bacterial feeding genus Cephalobus was most abundant in 4 of our 7 sites, versus never ranking highly in their data from Zapotitlán Valley, not even within the bacterial feeding trophic group only (of which they reported 24 genera, versus 11 in our study). These notable differences in composition could be attributed to a range of differences between the Zapotitlán site and our Costa Chica site, not only in primary productivity and decomposition at both locations, but also in soil properties, soil surface features and spatiotemporal aspects of microhabitat heterogeneity. For example, well-developed biological soil crusts occurred in half of all sample points analyzed by Mejía-Madrid and Sánchez-Moreno (2022), but not in any of our seven land uses. The algae, fungi and mosses forming such crusts would potentially present a more varied trophic environment for greater diversity of omnivorous nematodes in the Zapotitlán Valley study, particularly within Dorylaimida. Also, pebbles and small rocks were abundant on the surfaces in Zapotitlán Valley, where human foot traffic was restricted to well-marked trails away from the sample points. Rocky material was largely absent from surfaces in Costa Chica, but soil compaction from past or ongoing human and animal activity was probably widespread across several of the areas (i.e., those in use as orchards, the area formerly grazed by animals and the site of the former human habitation). Differences in surface roughness could result in leaf litter and other organic matter remaining in situ between the rocky material at the Zapotitlán site, possibly sustaining greater fungal biomass and higher numbers of fungal feeders like Aphelenchus, versus reduced soil porosity and smoother surfaces at the Costa Chica site, potentially channeling more water runoff and litter transport away from the site.
Although nematode surveys from other continents have not yet included TDF ecosystems, a few studies of paleotropical rainforests do address human impacts from various land uses. Bloemers et al. (1997) compared soil nematode diversity indices and abundances from transects within Mbalmayo Forest Reserve in Cameroon that preserved relatively intact primary forest, old secondary forest, or which were subject to eight different combinations of human impacts. They sampled soils to depths between 15-30 cm and identified a total of 194 genera, with genus richness per treatment varying from a minimum of 25 genera in active slash and burn tracts, to a maximum of 52 genera in completely manually cleared tracts with replanting. Their converted total counts ranged between 84 and 694 nemas per 100 ml of fresh soil, respectively from completely mechanically cleared tracts without replanting versus from old secondary forest (with near-primary forest following very closely behind). Their higher counts are not attributable to any differences in sampling depth but might instead be due to higher levels of primary production, water availability and/or belowground nutrient levels in rainforest compared to TDF.
They reported no abundance data for the genera encountered in their samples, but did specify how many tracts each genus was found in. The only genera occurring in all 24 tracts were Cephalobus and the bacterial feeder Prismatolaimus (the latter is generally common in mesic or saturated soils).
Last but not least, several studies from southeast Asia compare nematode composition and abundance between relatively intact rainforest and rubber plantations. Unlike our results or those of other research cited here, Krashevska et al. (2019) found no significant differences in total nematode counts from soil or plant litter between Sumatran lowland rainforest and sites converted to rubber plantations or oil palm orchards. Converting their abundance values from dry soil weights, the three systems yielded on average 224, 114 and 257 nematodes per 100 ml fresh soil sample. Standard deviations were very large, however, equaling or exceeding the corresponding averages. It thus seems likely that undocumented biotic or abiotic factors modulated nematode abundances at their sites and is worth noting that the average water content of their soil samples reached 43-45% also with very high SD’s. Moreover, they reported nematode abundances from the litter layer that were 60x to 80x higher than in their corresponding soil samples, again with very high-water content and SD values close to or exceeding the averages.
Xiao et al. (2014) compared soil nematode communities of tropical rainforest in Yunnan, China with two types of rubber plantations and one mixed rubber/tea cultivation system. They listed no water content values of their samples, unfortunately precluding conversion of their nematode counts to fresh soil volume units. Nevertheless, they did find significant differences between the four land uses in nematode abundances, genus richness, Shannon index and Maturity Index. Their SD values are substantially lower than the corresponding averages, suggesting that the elevated standard deviations in Krashevska et al. (2019) relate to local factors rather than peculiarities of rubber cultivation in general. Most recently, Panklang et al. (2022) reported soil nematode community comparisons from southern Thailand of rainforest and smallholder rubber plantations up to 75 years old, with up to 2 replanting cycles. Conversion of their abundance data is again precluded, but they found significant differences for nematode counts and diversity in roughly declining order from rainforest to the oldest rubber plantations. Panklang et al. (2022) interpret these declines as driven by observed and statistically significant reductions in pH and in availability of several measured soil nutrients. Soil density and possible levels of soil compaction could potentially be other contributing factors but were not measured. Their study stands out as the only report we know of investigating nematode composition of a tropical forest-like cultivation system maintained/replanted for over seven decades.
The preceding example studies illustrate continuing challenges of methodological standardization and comparative analyses, due among others to differences in available or suitable equipment, land use selections, intensities of cultivation systems, spatial scales, and field conditions. Nevertheless, we believe they also clearly show the importance of expanding scientific research efforts into the faunistic and ecology of soil biodiversity in different types of tropical and subtropical forest ecosystems, especially in landscapes with mosaics of human impacts and intact native plant communities.
Natural recovery or resilience of soils disturbed by anthropogenic activities are particularly challenging to estimate for Tropical Deciduous Forest conditions. This is not only due to the mosaic-like biotic and abiotic properties of TDF, but also because human activities primarily occur within and around subsistence-type farms and small settlements. In a study of three fertilizer treatments on 175 smallholders’ bean fields in Burundi, Sanabria and Wendt (2019) discuss the challenges of experimental design and analysis of data obtained from heterogeneous small acreage subsistence farms. They report that a classical ANOVA analysis, through assigned replicates via a randomized complete block design, violated assumptions of independence between replicates. ANOVA without replication but in combination with Generalized Linear Mixed Models produced measurably higher fitness and power, allowing for more informative testing of hypotheses about genuine soil/site/treatment interactions. We believe TDF ecosystems mirror some of the scientific particulars of the African smallholder farms studied by Sanabria and Wendt (2019). Our sampling effort and analysis as presented above was necessarily much more limited because our aim was to obtain first information on soil nematode diversity of TDF soils with varying degrees of current human use or past known uses.
Conclusion
Overall, our observational study suggests that nematode communities from formerly impacted soils with recovering TDF do not necessarily reach a similar state ten years after human land use. Moreover, they have not become indistinguishable in nematode composition from a directly adjacent site that was not subject to prior land use for well over a decade. Evidently, we must keep in mind that this first effort is only the beginning and that the analytical methods applied to our sampling design without replication have only limited interpretative power. Other factors could well explain all of the significant differences detected. That is, despite collecting 10 soil samples (each combined from 3 subsamples) per site, a wide range of other factors independent of legacy effects may underlie the differences reported here; these include present heterogeneity of soil properties, vegetation, surface slope, and microclimate.
The present pilot study may be utilized as the beginning toward establishing a methodological baseline for future studies. We can deduce that the different uses of soils in the studied portion of the TDF biome have influenced the diversity and abundances of soil-inhabiting nematode groups. This information will help develop detailed knowledge about how TDF biodiversity is impacted by a mosaic of different land uses. Such studies are needed in the development of management strategies to preserve or restore biological communities interwoven with sustenance farming, local traditions, and human livelihoods. Given our findings, we strongly recommend that (a) maximum-effort soil biodiversity surveys of TDF should aim to include and analyze as much information as can be obtained about the prior history of human impacts on the sites and soils to be sampled and (b) such histories should preferably span multiple decades rather than only the last 10-15 years.
From the pattern of nematode abundance observed in the present study, we specifically recommend that the following historical information should be included where possible in datasets for future multifactorial surveys of TDF nematodes: (1) estimates of past trampling levels from human and cattle traffic; (2) estimates of past amounts of plant litter left to decompose on site after clearing, harvesting or weeding and (3) estimates of past amounts of plant litter removed from a site after clearing, harvesting or weeding by being physically taken away, by burning near the site or by allowing domesticated animals access to defoliate the cuttings. Because reliable documentation and standardization of these factors is likely to be constrained by the realities of past and present subsistence farming, encoding these factors will no doubt require developing and experimentally testing a simple scoring scale with ordinal values (for example along similar lines as the Braun-Blanquet Cover Abundance Scale employed in vegetation studies).











 nueva página del texto (beta)
nueva página del texto (beta)