INTRODUCTION
In some vespertilionid and rhinolophid bats species of temperate zones, development of the testicles and spermatogenesis activity occur in spring-summer, but development of the accessory sex glands, courtship and breeding activity all take place in autumn-winter after the testes have regressed completely (León-Galván et al., 2005). This functional asynchrony causes unusually prolonged epididymal sperm storage that can extend for several months, during which the spermatogenesis remains completely inactive (Racey & Entwistle, 2000).
This prolonged epididymal sperm storage becomes even more interesting when we learn that at the time of their release from the testicles, the sperm have not yet acquired the ability to fertilize the oocyte. Rather, they undergo the modifications necessary for fertilization gradually as they progress from the caput down to the cauda region of the epididymis, in a process called epididymal sperm maturation (ESM) (Lewis & Aitken, 2001; Sullivan et al., 2007). This maturational process involves the following: DNA compaction, acrosome restructuring, change in the phospholipids/cholesterol ratio, and modifications of the plasma membrane (Baker et al., 2012; Cooper, 2011; Dacheux et al., 2012; Sullivan et al., 2007).
One of the main modifications of the sperm membrane during ESM is protein glycosylation (Chandra et al., 2008; Schroter et al., 1999; Toshimori, 1998; Tulsiani, 2006), which confers the ability to function as oocyte recognition molecules (Chandra et al., 2008; Toshimori, 1998; Toyonaga et al., 2011; Tulsiani, 2003) and so participate in the interaction between spermatozoa and oocyte (Fierro et al., 1996).
One method to determine the distribution of sugars in the cell membrane is by using lectins, because they bind to those molecules in a specific carbohydrate, making it possible to determine their quantity and location (Damjanov, 1987; Flesch et al., 1998; Vazquez et al., 1996). The use of lectins led to the detection of an increase in negatively charged carbohydrates on the surface of sperm during their journey through the epididymis (Arenas et al., 1996; Eddy et al., 1985; Fierro et al., 1996; Holt, 1980; Moore & Bedford, 1979). Kumar et al. (1990), meanwhile, observed large amounts of N-acetylglucosamine (NAc-Glu) and sialic acid in the acrosomal region of rodent sperm, which increased as they advanced from the caput to the cauda of the epididimys. They found the highest amount of sialic acid in sperm cells obtained from the corpus region of the epididymis, which can be attached to sperm cells. Also, it has been determined that residue of fucose participates significantly in the interaction between gametes (Fierro et al., 1996).
In most of the studied species of mammals, the finish of epididymal maturation process occurs at the end of the corpus region of the epididymis. However, the seasonal bat Corynorhinus mexicanus (G. M. Allen, 1916) which is characterized by a long period of epididymal sperm storage (León-Galván et al., 2005), the sperm maturation culminate in the cauda region, probably as a time-dependent adaptation of the prolonged phase of sperm storage (Cervantes et al., 2008; Rodríguez-Tobón et al., 2016), what makes this mammalian species, an interesting model to study the changes in the distribution of carbohydrates present in the membrane of sperm. Therefore, the objective of this study was to analyze changes in the distribution of N-acetylglucosamine and/or sialic acid (NacG-AcS), Fucose (Fuc) and Mannose (Man) carbohydrates in the different domains of the membrane of sperm cells as they advance from the caput to the cauda of the epididymis in C. mexicanus.
MATERIALS AND METHODS
Chemicals and animals. Unless stated otherwise, all chemicals were purchased from Sigma (St. Louis, MO, USA). Ringer solution was prepared as follows: NaCl 95mM, KCL 5mM, KH2PO4 1.1mM, CaCl2 1.7mM. Animal capture and handling were conducted in accordance with the Guidelines of the American Society of Mammalogists for the use of wild mammals in research (Sikes & Gannon, 2011). At present, the C. mexicanus bat is not included in any category of threatened animal species in the Mexican Official Standard, or in the NOM-059-SEMARNAT-2010 for the protection of wild native species in Mexico (SEMARNAT, 2010). The study protocol was approved by the Ethics Committee of the Biological and Health Sciences Division of the University (Universidad Autónoma Metropolitana-Iztapalapa, Mexico City). The specimens studied were from permit SGPA/DGVS/06624/13 for collecting wild fauna issued to Dr. Miguel Angel León Galván by the Dirección General de Vida Silvestre de México (an agency of SEMARNAT).
Three adult male C. mexicanus bats were captured fortnightly during the period of epididymal sperm maturation and storage (September 11 - October 30), which precedes the mating season, inside a tunnel in the state of Tlaxcala, central Mexico (19° 37´ 14´´ N, 98° 02´ 02´´ W; 3,220 m altitude). All captures were done before the animals left their roost (12:00 - 16:00 hours) using an expanded hand-net (Bioquip Tropic net). The adult category and the reproductive condition of the individuals included in this study were stablished in accordance with reports by León-Galván et al. (2005), Cervantes et al. (2008), and Rodríguez-Tobón et al. (2016), taking into account the following variables: body weight, recorded using an Ohaus® (Ohaus Corporation, Parsippany, NJ, USA) portable electronic balance (±0.01 g); forearm length, measured with a vernier caliper (±0.1 mm); and by the external aspects of the sexual organs. A total of 26 bats were included in the study.
Obtaining sperm from the epididymis. The bats were anesthetized with diethyl ether and euthanized by decapitation. Both epididymides were dissected immediately under a stereoscopic microscope, cleaned of fat and connective tissue, washed with Ringer solution, sectioned in its three main anatomical regions (caput, corpus and cauda), and every pair of it (left and right) stored in a gauze moistened with cold Ringer. To extract of the sperm, every couple of epididymal regions were placed in 500 µL of Ringer at 37.8°C, finely minced and kept under agitation for 5 min to allow the spermatozoa to break free from the epididymal tubules. Subsequently, the minced material was collected with a Pasteur pipette and filtered by passage through a 20-µm-diameter-weft Lycra mesh (98% nylon/polyamide, 2% Lycra/elastane), and the fluid was collected in a 2 mL Eppendorf tube (Arenas-Ríos et al., 2005; Rodríguez-Tobón et al., 2016). The sperm suspension was washed by centrifugation at 500 g for 5 min; then, the supernatant was removed, and the cell pellet was re-suspended in 1 mL of Ringer. Sperm concentration and viability were quantified under a transmitted light microscope using standard methods (World Health Organization, 2010). A Neubauer hemocytometer chamber was loaded with 15 µL of sperm suspension. At least 200 cells were counted in duplicate to calculate sperm concentration, while viability was determined using eosin-nigrosin staining. Briefly, 10 µL of sperm suspension were mixed with an equal volume of staining solution, then the sample was smeared on a glass slide and left to dry. Once dry, the number of dead (stained) and viable (unstained) cells were counted (World Health Organization, 2010). A total of 200 cells were assessed in each replicate (the viability only was used as parameter of quality control) (data not shown). The time that elapsed between sperm sampling and the above procedures never exceeded 30 min.
Membrane carbohydrates. To evaluate carbohydrate distribution according to the transit of the spermatozoa through the epididymal duct of C. mexicanus, lectins conjugated to fluorescein isothiocyanate (FITC) were used, as follows: WGA, ConA, and UEA, which bind to residues of NacG-AcS, Man and Fuc, respectively. A negative control for each sample was also prepared. The lectins were incubated for 30 min with their corresponding sugar at a concentration of 0.3 M before being place in contact with the sperm (Fierro et al., 1996).
Lectins were used at a dilution of 1:50 and incubated with sperm samples for 30 min. Then, two washes were performed with PBS before fixing with 1% paraformaldehyde in PBS for one hour at room temperature. Observations were done under an Olympus BX41 epifluorescence microscope to determine the carbohydrates distribution pattern of lectins over the sperm membrane. The fluorescence intensity of the lectins conjugated to fluorescein isothiocyanate (FITC) was quantified using a flow cytometer (FACScan, Becton-Dickinson, San Jose, CA, USA). CELLQuest software was used for data analysis. Data were normalized and expressed as the fluorescence index (FI) = (mean fluorescence intensity) (% input events) (De Maistre et al., 1996; Jimenez et al., 2006).
Statistical analysis. Each proof was replicated with sperm obtained from each one of the three epididymal regions of three bats captured by each date of the epididymal sperm storage period. The percentages data were transformed to arco-sin ((x)1/2). Data were expressed as mean ± SD, and analyzed using NCSS 07.1.21 (Statistical software 2007, Utah). Data were examined by ANOVA followed by the Tukey’s test. Homogeneity of variances was tested using Levene's test. All tests were performed at p ≤ 0.05.
RESULTS
Once the epididymal spermatozoids of C. mexicanus had been marked with lectins-FITC, and observed at the epifluorescence microscope, it was possible to distinguish different patterns of carbohydrates distribution on the membrane of spermatozoa obtained from the three anatomical regions of the epididymis (caput, corpus and cauda), and according to the different capture dates (September - October) of the animals. In the case of NacG-AcS, five distinct patterns were identified (Fig. 1): I) unmarked sperm; II) sperm with tenuous marking on the equatorial segment, and more intense marking on the annulus; III) sperm with intense marking from the sub-acrosomal region to the annulus; IV) sperm with intense marking in the acrosomal region, and tenuous marking on the mitochondrial sheath and flagellum; and V) sperm marked from the apical part of the head to the annulus with a band in the equatorial segment. The distribution of NacG-AcS among the different regions of the epididymis indicates that the percentage of pattern III presented the highest percentage at ~70% characteristic of a spermatozoa that has not completed its maturation process. Patterns II-IV among the different regions of the epididymis (caput vs. corpus vs. cauda), showed no differences over the first three capture dates, but in the sperm obtained from the cauda on October 22, ( pattern V ) was ~30% higher (p < 0.05) decreasing the amount of negative charges of the plasmatic membrane by the decrease of NacG-AcS, than the other patterns on the sperm obtained from the corpus (Fig. 2).
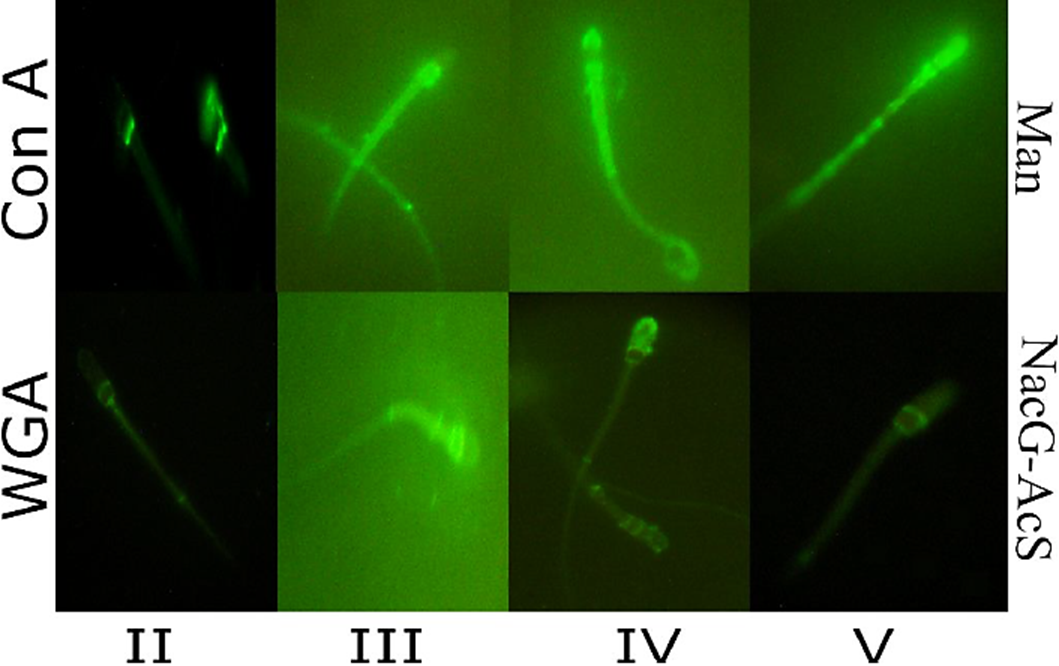
Figure 1 Lectin staining patterns of Corynorhinus mexicanus bat sperm recognized by epifluorescence microscopy. Carbohydrates distribution patterns (II-V) in the membrane of epididymal spermatozoa of bat, determined using two different lectins: Triticum vulgaris agglutinin (WGA), which recognizes N-acetiylglucosamine and/or sialic acid (NacG-AcS); Pattern I which correspond to cells without mark is not shown; II) sperm with tenuous marking on the equatorial segment, and more intense marking on the annulus; III) sperm with intense marking from the sub-acrosomal region to the annulus; IV) sperm with intense marking in the acrosomal region, and tenuous marking on the mitochondrial sheath and flagellum; and V) sperm marked from the apical part of the head to the annulus with a band in the equatorial segment; and Canavalia ensiformis agglutinin (Con A), specific to Mannose (Man); Patterns I) unmarked sperm; II) sperm with marking on the base of the head; III) sperm with intense marking on the sub-acrosomal region and towards the mitochondrial sheath; IV) sperm with intense marking on the mitochondrial sheath and the acrosomal cap; and V) sperm with intense marking beginning at the apical part of the head and continuing over the mitochondrial sheath up to the annulus. Magnification of Images: 1000X.
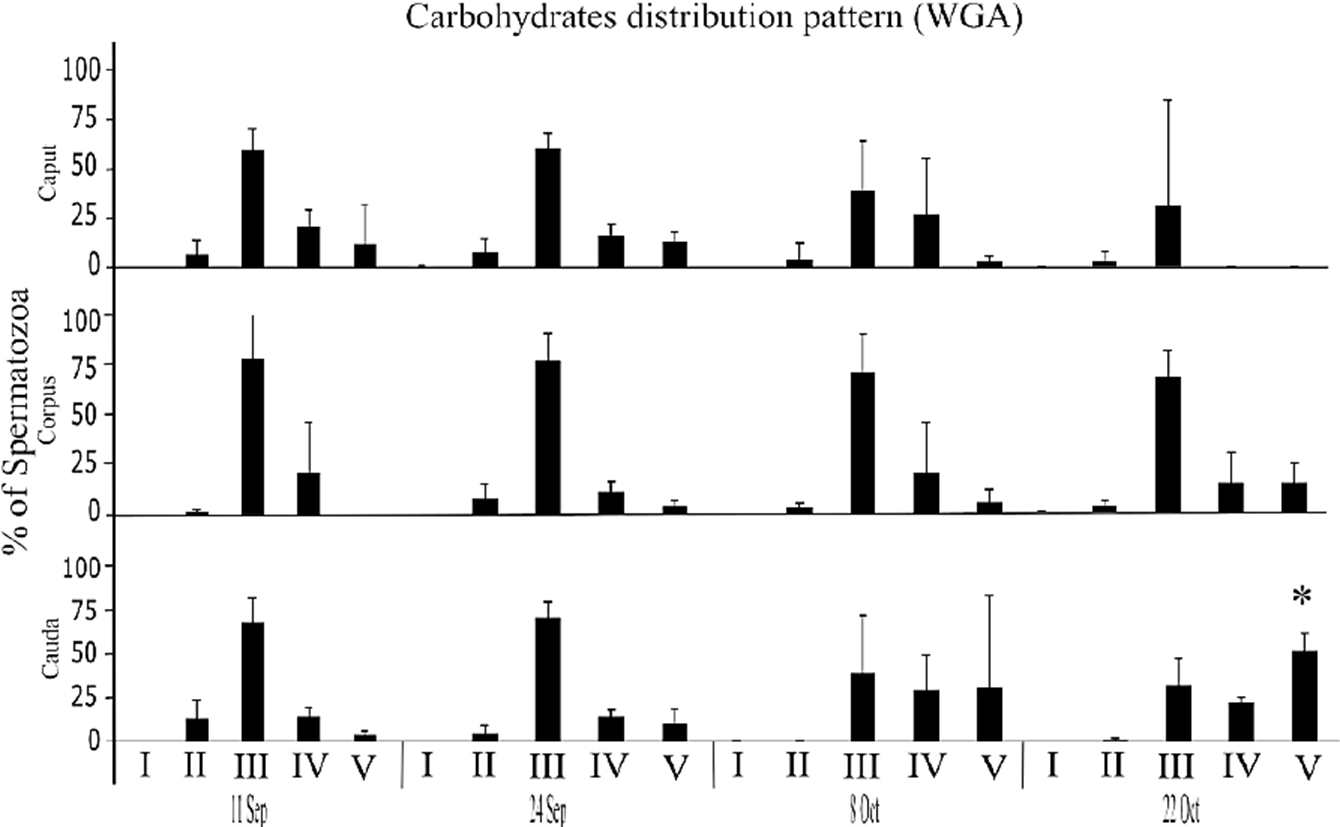
Figure 2 Percentage of bat sperm with pattern II-V of NacG-AcS carbohydrate distribution, recognized by WGA labeling. The average values (+ SD) of the data of each of the three epididymal regions of n = 3 bats are presented for each capture date throughout the epididymal sperm storage period. The comparisons were made by ANOVA, Tukey’s test, at p < 0.05. Asterisks indicate significant differences when compared the obtained values between the three regions on the same study date.
In the same way, the distribution of Man on the spermatozoa membrane presented five different patterns (Fig. 1): I) unmarked sperm; II) sperm with marking on the base of the head; III) sperm with intense marking on the sub-acrosomal region and towards the mitochondrial sheath; IV) sperm with intense marking on the mitochondrial sheath and the acrosomal cap; and V) sperm with intense marking beginning at the apical part of the head and continuing over the mitochondrial sheath up to the annulus. The distribution of Man among the different regions of the epididymis indicates that the percentage of patterns II, IV and V differed between September and October (p < 0.05). There was an increase of ~40% of the cells that presented pattern IV towards the month of October (the sperm prepares for its encounter with the oocyte) and a reduction of Man in the sperm that presented pattern V (~50%), on the later capture dates (Fig. 3). No spermatozoa were marked with UEA-FITC the lectin specific to Fuc; for that reason, not any fluorescence pattern for this carbohydrate distribution was observed.
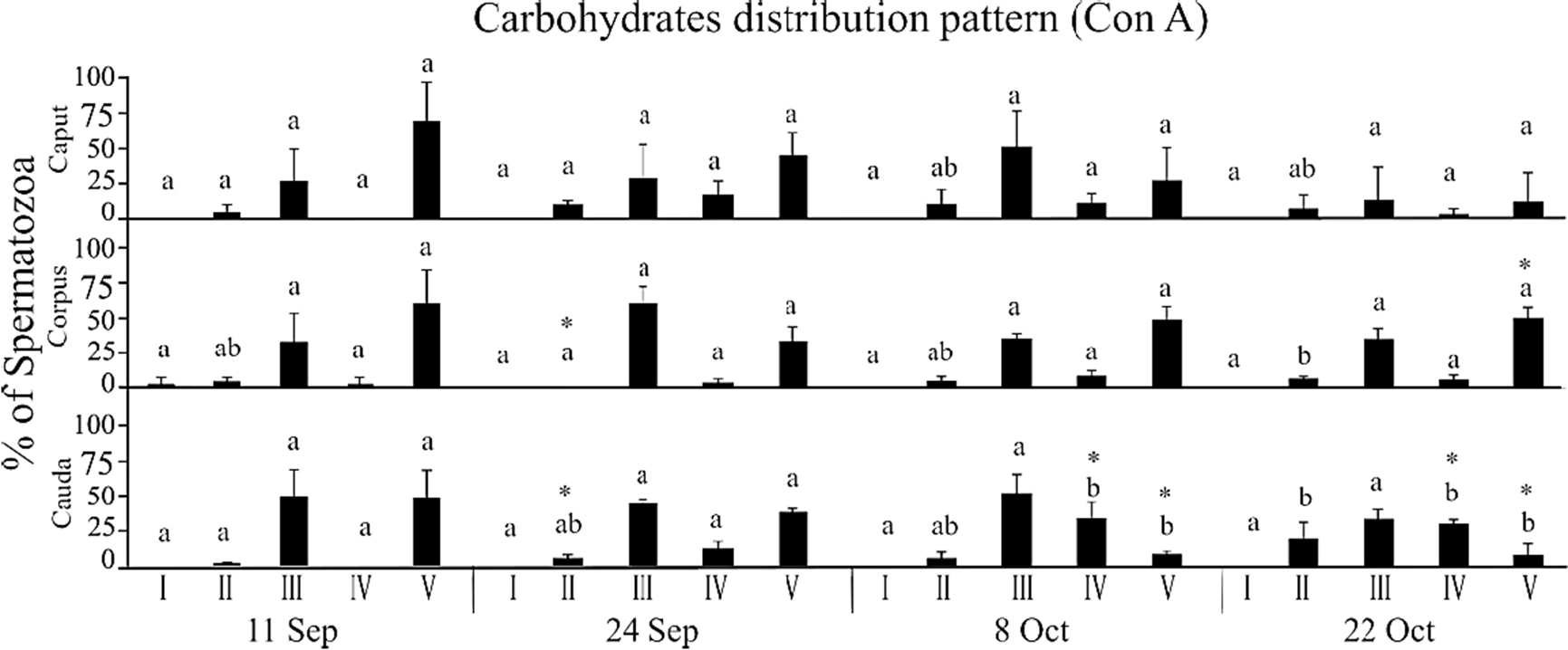
Figure 3 Percentage of bat sperm with pattern II-V of Man carbohydrate distribution, recognized by Con A labeling. The average values (+ SD) of the data of each of the three epididymal regions of n = 3 bats are presented for each capture date throughout the epididymal sperm storage period. The comparisons were made by ANOVA, Tukey’s test, at p < 0.05. Different letters indicate significant differences when comparing the values of the same region of the epididymis between the different study dates. Asterisks indicate significant differences when compared the obtained values between the three regions on the same study date.
The quantity of cells that fluoresced due to the presence of carbohydrates marked with lectins-FITC was ascertained using flow cytometry. In the case of NacG-AcS, no differences were found among the sperm samples obtained from different regions of the epididymis (Fig. 4). However, upon comparing the sperm obtained from the caudal region according to the different capture dates, we found an increase between September 11 and 24, as those two dates had higher values of fluorescence than the final two (October 8, 22), evidence that sperm require a storage time in the epididymis to reach maturity, since they enter the epididymis with a higher concentration of NacG-AcS, decreasing the amount of negative charges of the plasmatic membrane of the cells and inhibiting its motility.
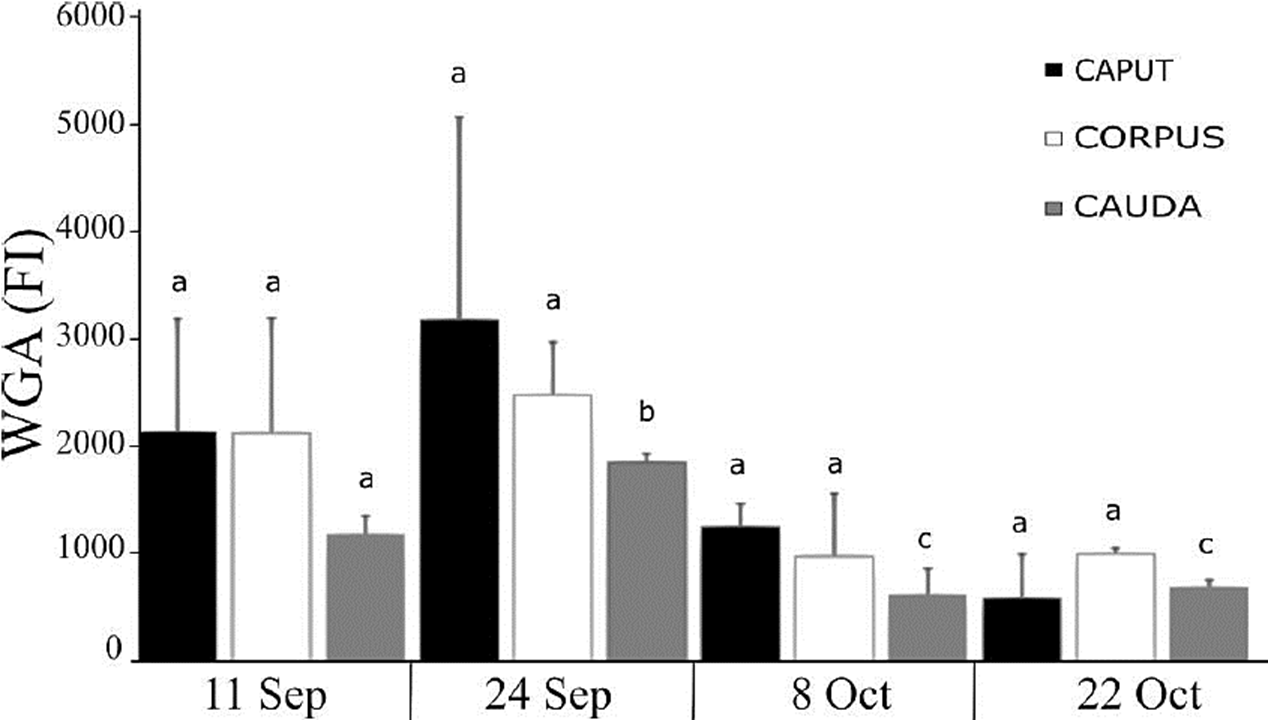
Figure 4 Presence of NacG-AcS in the sperm membrane of C. mexicanus bat, reported as the fluorescence index (FI) resulting from the fluorescein isothiocyanate WGA lectin binding to sperm obtained of the three epididymal regions of bats, as evaluated by flow cytometry. The average values (+ SD) of the data of each of the three epididymal regions of n = 3 bats are presented for each capture date throughout the epididymal sperm storage period. The comparisons were made by ANOVA, Tukey’s test, at p < 0.05. Different letters indicate significant differences when comparing the values of the same epididymal region between the different study dates. There are no significant differences when comparing the values between the three different regions of the epididymis on the same study date.
Fluorescence microscopy failed to detect the presence of Fuc, but flow cytometry allowed us to observe that it was indeed present in the sperm membrane, though in lower amounts than NacG-AcS and Man. We found a lower amount of Fuc in the sperm from the caput and cauda compared to those in the corpus for the dates of September 11 and 24, though this was not the case on subsequent dates. There were no differences in the comparison of each region of the epididymal tubule with the same region on different study dates (Fig. 5).
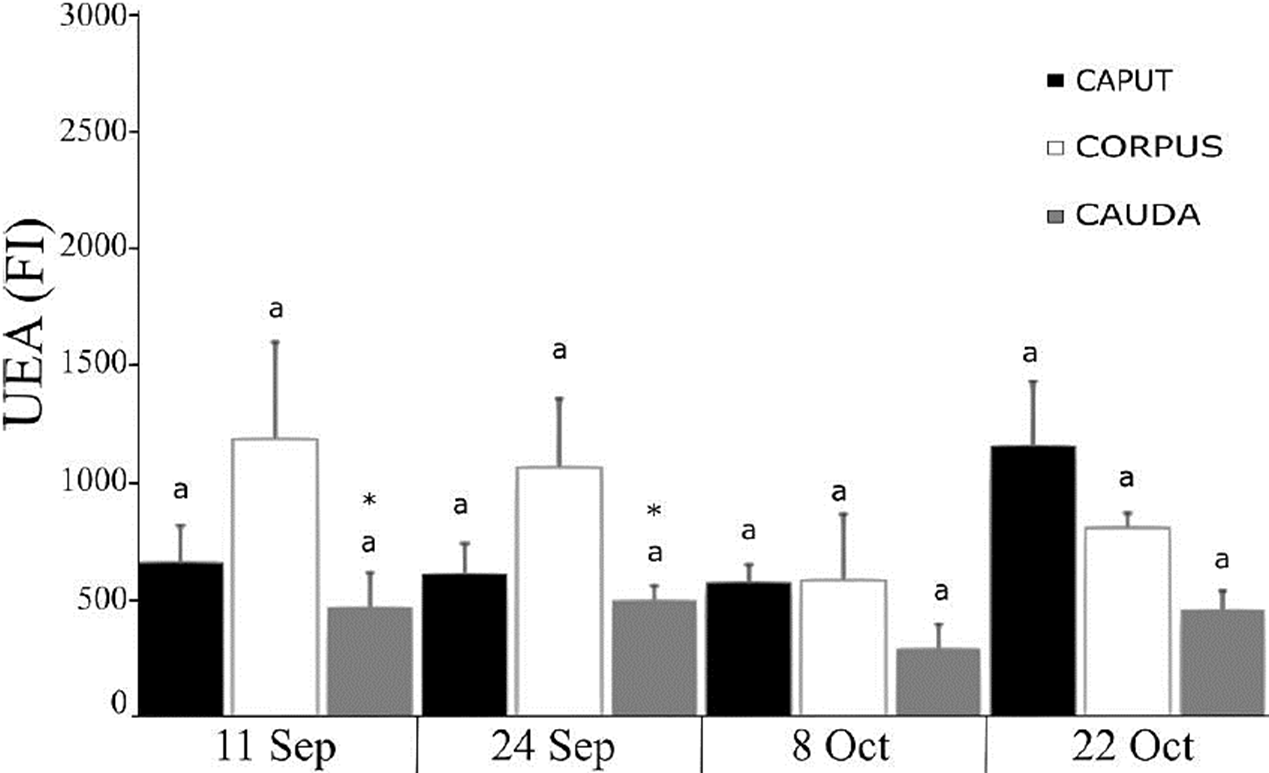
Figure 5 Presence of Fuc in the sperm membrane of C. mexicanus bat, reported as the fluorescence index (FI) resulting from the fluorescein isothiocyanate UEA lectin binding to sperm obtained of the three epididymal regions of bats, as evaluated by flow cytometry. The average values (+ SD) of the data of each of the three epididymal regions of n = 3 bats are presented for each capture date throughout the epididymal sperm storage period. The comparisons were made by ANOVA, Tukey’s test, at p < 0.05. Different letters indicate significant differences when comparing the values of the same epididymal region between the different study dates. There are no significant differences when comparing the values between the three different regions of the epididymis on the same study date.
In the case of Man, analysis determined that fluorescence intensity decreased in the corpus and cauda from September 24 to October 8 (Fig. 6) which could indicate the preparation of the sperm for its encounter with the oocyte, though no differences appeared on the latter date. There were no differences upon comparing the different regions of the epididymis (Fig. 6).
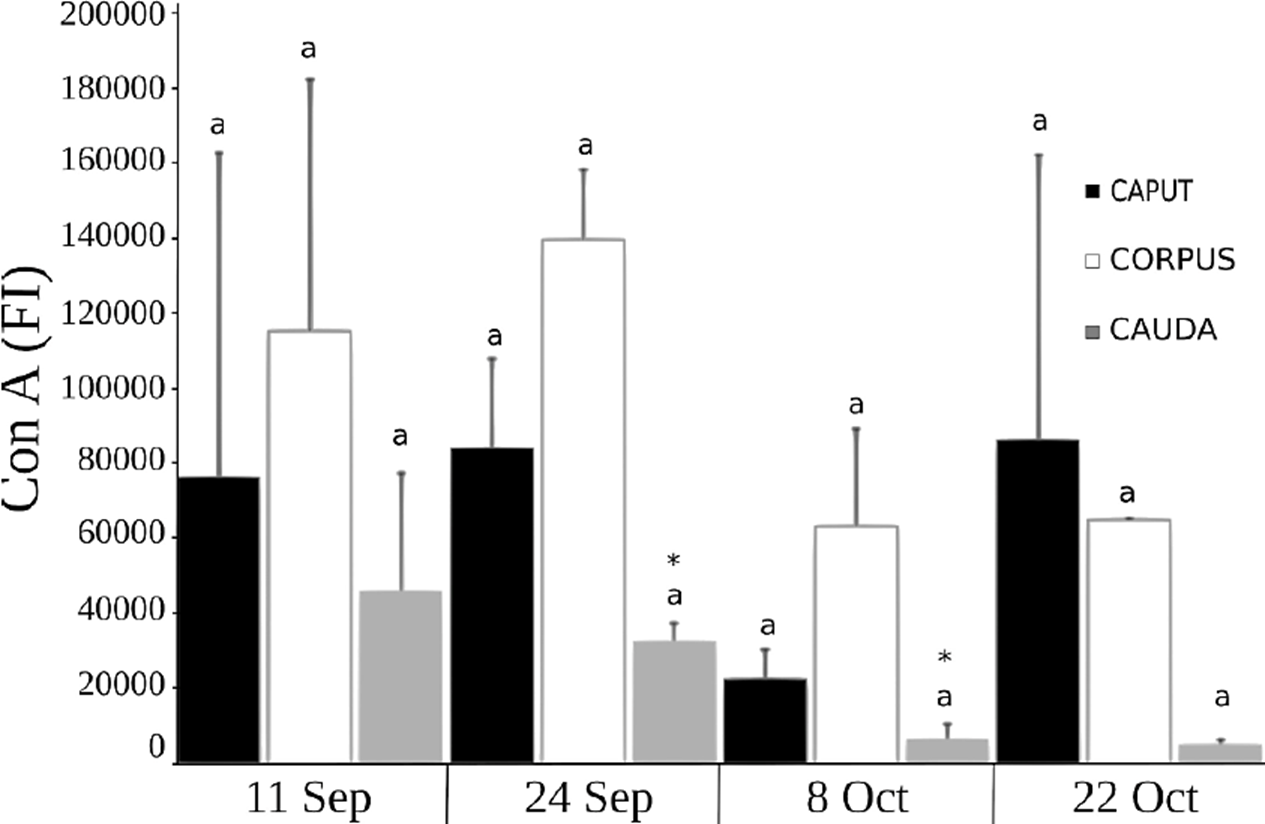
Figure 6 Presence of Man carbohydrates in the sperm membrane of C. mexicanus bat, reported as the fluorescence index (FI) resulting from the fluorescein isothiocyanate Con A lectin binding to sperm obtained of the three epididymal regions of bats, as evaluated by flow cytometry. The average values (+ SD) of the data of each of the three epididymal regions of n = 3 bats are presented for each capture date throughout the epididymal sperm storage period. The comparisons were made by ANOVA, Tukey’s test, at p < 0.05. Different letters indicate significant differences when comparing the values of the same epididymal region between the different study dates. There are no significant differences when comparing the values between the three different regions of the epididymis on the same study date.
DISCUSSION
The acrosome forms during spermiogenesis. In mammals it originates in the Golgi apparatus, which is an organelle rich in carbohydrates, especially sialic acid and sulfated carbohydrates (Kopecny & Flechon, 1981; Navaneetham et al., 1996). For this reason, we expected to find a greater presence of NacG-AcS at the level of the acrosomal cap of the sperm cells of C. mexicanus bat, as it has been described in other mammals (Clermont & Tang, 1985). Reports on rats, mice, rabbits, hamsters, goats and Rhesus monkeys (Macaca mulatta) indicate that the bonding site of NacG-AcS is in the acrosomal region (Kumar et al., 1990), and that after transiting through the entire epididymis it is distributed along the whole spermatozoa. In the C. mexicanus sperms, the presence of NacG-AcS was detected from the sub-acrosomal region and as it the spermatozoids pass through of the epididymis the fluorescence is located in the mitochondrial sheath towards the site where the annulus of the sperm is found; however, in contrast to species with a continuous reproductive pattern in which epididymal maturation ends before the sperm enter the caudal region of the epididymis, our study found a redistribution of NacG-AcS in the sperm when they arrived in the caudal region (September 11), but that after storage until October 22 the distribution of NacG-AcS changed. This modification of the distribution is dependent on the time during which the sperm remained stored. This phenomenon concurs with reports on this same species, which found according to the phosphorylation of tyrosine residues from the proteins and the sperm’s ability to become enabled that maturation ends in the caudal region and is dependent on storage time (Rodríguez-Tobón et al., 2016). This phenomenon has not been reported for other mammals.
In the aforementioned mammalian species (Kumar et al., 1990), the fluorescence intensity of the NacG-AcS on the sperm increases as they transit through the epididymis up to ejaculation. In C. mexicanus, in contrast, FI decreases as the sperm travel through the epididymal duct and as storage time in the cauda goes on (Fig. 4). This leads us to think that in C. mexicanus NacG-AcS could function as decapacitation factors (Harayama et al., 1998; Toyonaga et al., 2011), rather than oocyte-recognizing molecules (Chandra et al., 2008; Toshimori, 1998; Toyonaga et al., 2011; Tulsiani, 2003).
Fucuse residues participate in the interaction between ovule and sperm (Fierro et al., 1996); however, the present study failed to detect the presence of this sugar on the sperm membrane (Fig. 1), as has been reported by Jimenez et al. (2006) for ejaculated pig sperm. However, upon performing flow cytometry the presence of Fuc in C. mexicanus sperm was corroborated, though in lower quantities than those found for NacG-AcS and Man (Fig. 5). This suggests that the contribution of Fuc in the spermatozoa obtained from the caput and corpus regions of the epididymis is greater than those cells obtained from the cauda.
Navaneetham et al. (1996) mention that in Macaca fascicularis sperm obtained from the three regions of the epididymis, Mannose residue was found predominantly towards the acrosome; this is similar to descriptions of felines sperms (Toyonaga et al., 2011). However, as the graph for Man shows (Fig. 3), patterns III and V for the presence of Man in the sperm of C. mexicanus during their entry into, and transit through, the 3 regions of the epididymis, were found over the entire spermatozoa as well as in the post-acrosomal region, though at a lower percentage (September 11). Also, at the end of storage time in the caudal region, this sugar was distributed from the entire sperm to the head (i.e., sub-acrosomal and acrosomal regions) (Fig. 3).
Studies of rat sperm have succeeded in determining differences in the marking pattern of Man in the different regions of the epididymis, indicating that quantities are greater around the cephalic region than in the cauda of the epididymis (Olson & Danzo, 1981). Considering that Man levels were much higher than those of the other sugars measured (Fig. 6), it seems clear that a large contribution of glycoproteins from the epididymis takes place, and that these would be incorporated into the membrane of C. mexicanus sperm primarily at the level of the caput and corpus, but would decrease drastically towards the cauda (September 24-October 8) (Fig. 6), as has been described for rats.
CONCLUSIONS
This study allowed to recognize changes in the distribution of carbohydrates present in the plasma membrane of the sperm during its transit through the three large anatomical regions of the epididymis in the seasonal bat Corynorhinus mexicanus.
The amount of NacG-AcS increased in the sperm of the caudal region of between September 11 and 24, and again in October. A redistribution of NacG-AcS occurred when the sperm have been stored in the caudal region for at least one month, as these sugars were found from the apical part of the head to the annulus, with a band in the equatorial segment.
Man levels decreased when sperm are in the corpus and cauda after September 24 and up to October 8, though no differences existed on the latter date. A redistribution of Man took place between September and October, as the level of this sugar increased in the mitochondrial sheath and the acrosomal cap.
From September 11 to 24, a lower amount of Fuc was found in the sperm of the caput and caudal regions compared to those in the corpus.
This biochemical modification of the sperm membrane can be considered as part of the process of epididymal sperm maturation, and is probably associated with the phenomenon of prolonged sperm storage that is characteristic of the species, adaptation that allows the males to synchronize with the period of receptivity of the females, and then, carry out the mating in the months of November and December, when the activity of spermatogenesis is totally in recess.











 nueva página del texto (beta)
nueva página del texto (beta)


