Introduction
The jaguarundi (Herpailurus yagouaroundi) is a threatened species in Mexico (NOM-059; SEMARNAT, 2010) and is listed under Appendix I of CITES (Caso et al., 2015). While widely distributed along the Pacific and Gulf coasts (Leopold, 1959; de Oliviera, 1998; Aranda, 2005), much less is known of its distribution in interior Mexico including the state of San Luis Potosí (SLP), and it remains the least studied felid in Mexico. San Luis Potosí hosts a wealth of ecosystems and biodiversity due to high floristic diversity associated with its geographic location in the Nearctic-Neotropical zoogeographical transitional region, rugged topography, and climatic complexity (Escalante et al., 2005). Despite this diversity, historically there were only three visual records of jaguarundi from SLP (Dalquest, 1953; Leopold, 1959). However, recent observations of jaguarundi in SLP (Méndez-Salinas, 2009; Villordo-Galván et al., 2010; Hernández-SaintMartín et al., 2013; Benítez-Alemán, 2014) indicate presence in some areas, although the overall distribution of jaguarundi and its habitat associations in SLP remain uncertain.
Establishment of protected areas is commonly espoused as a practical method of conserving natural communities including habitats preferred by rare species (Primack, 1993; Ochoa-Ochoa et al., 2009). However, only 1.6% of SLP is protected at the Federal level and 6.6% at the State level (Chapa-Vargas & Monzalvo-Santos, 2012), well below recommendations of 12-18%. Additionally, many natural communities in SLP face continued alteration due to a variety of human-related activities, including logging, conversion to agriculture, growth of communities, and livestock grazing (INEGI, 2002; Chapa-Vargas & Monzalvo-Santos, 2012). Because of this, and despite being less impacted by human development than many other areas of Mexico, many areas of SLP lost 40% to nearly 100% of natural communities between 1966 and 2000 (Huber-Sannwald, 2002; INEGI, 2002; Chapa-Vargas & Monzalvo-Santos, 2012). Hence, protected areas alone are unlikely to provide a suitable long-term solution to conserving jaguarundi.
Thus, a key to the long-term viability of jaguarundi is their adaptability to human-induced changes in natural communities, particularly tolerance of human-related development. Despite this, presence of jaguarundi in human-altered environments, and attributes that may facilitate that presence in SLP, are unknown. Unlike many rare felids, however, jaguarundi are apparently adaptable to a variety of habitat types (Aranda, 2005; Charré-Medellín et al., 2012; Caso, 2013; Farías et al., 2015; Giordano, 2016), may be tolerant of habitat disturbance (de Oliviera, 1998; Carrillo et al., 1999; Caso et al., 2005; Giordano, 2016), and may prefer edge habitats (Cabrera & Yepes, 1960; Caso, 2013; Giordano, 2016). Thus, the highly diverse landscape of SLP potentially provides suitable habitat conditions, including areas with human-related development and fragmentation, such as rural communities, agricultural, and agroforestry environments.
Knowledge of the distribution and environmental attributes associated with the presence of species-of-concern is fundamental to the development of informed recovery plans. Consequently, because the overall distribution of jaguarundi, and the adaptability of jaguarundi for human-altered habitats in SLP is uncertain, our goal was to determine the current distribution of jaguarundi in SLP and to characterize both landscape-level and site-level environmental features that may facilitate its presence in light-moderately human-altered environments, such as rural communities, ejidos, ranches, and other agricultural landscapes.
Materials and methods
Study area. The state of SLP (Fig. 1) is located in east-central Mexico, between 21° 10’-24° 29’ N and 98° 20’-102° 18’ W (INEGI, 2002). San Luis Potosí has a great diversity of vegetation types, including piedmont scrub, scrubland, and grassland, as well as temperate (oak [Quercus spp.], pine [Pinus spp.]-oak, and cloud forests) and tropical-subtropical forests (Rzedowski, 1966). Four geographic regions are identified: Altiplano, Zona Media, Zona Centro and Huasteca Potosina (Fig. 1). Climates included dry and semi-dry (74% of the State), semi-warm (16%), warm (8%), and temperate (2%). Agriculture and cattle ranching are among the most important economic activities (INEGI, 2002).

Figure 1 Location of San Luis Potosí in Mexico and its four geographic regions: Altiplano, Zona Media, Zona Centro and Huasteca Potosina.
Jaguarundi occurrences. We obtained field records of jaguarundi occurrences following the methodology of de Oliveira (1994). Because we were primarily interested in presence in or near human-altered habitats, we conducted during October 2008-May 2009 interviews in and around 96 rural communities (mean population = 778 [range = 10-10,562]; excluding the six largest communities with >1,000 residents each, mean population = 296 [range = 10-975]), ejidos, and ranches within the four geographic regions of SLP. Around each area, we interviewed local residents who were familiar with the local fauna, including hunters, rural authorities, livestock producers, and farmers. For each interview we recorded the type of evidence indicating presence of jaguarundi (e.g., road kill, sighting, skins, skulls, etc.), approximate date of sighting, and place of sighting. We also evaluated the knowledge of the person interviewed regarding jaguarundi by showing a series of images of different felids and determining their ability to recognize jaguarundi (Rabinowitz, 1993).
We classed the combined information from the interview and the images by its reliability using the analytical criterion of Tewes and Everett (1986) following several other studies of cryptic felids (e.g., Shindle & Tewes, 1998; Grigione et al., 2007; Horne et al., 2009; Villordo-Galván et al., 2010; Martínez-Calderas et al., 2011). These criteria classes included: Class I, where observations were made by a reliable and experienced observer and supported by physical evidence; Class II, where a detailed description was provided by a reliable and experienced observer; and Class III, where details provided by the observer were vague and not specific (Tewes & Everett, 1986).
Environmental correlates. We located and geo-referenced each record of jaguarundi using a Global Positioning System receiver. We used or created digital maps (INEGI, 2002, 2013) in ArcMap 10.1 (ESRI; Redlands, California, USA) to determine environmental variables associated with jaguarundi occurrences. These variables included (1) vegetation cover type; (2) distance to the nearest water source; (3) distance to the nearest community; (4) distance to paved and unimproved roads; (5) elevation; and (6) human population density. We selected these variables because they have previously been shown to be important for habitat evaluations of other felid species (e.g., Carrol & Miquelle, 2006; Martínez-Calderas, 2009) and several specifically addressed associations with human-altered habitats, as well as characteristics of human-altered habitats that could be contrasted with the overall SLP landscape.
We also determined several patch and edge related statistics (Patch Analyst 5.2; Rempel et al., 2012), including total number of patches, patch density, total amount of edge, edge density, patch relative density, and relative patch richness, to assess if jaguarundi presence was associated with more or less fragmented habitats. We used a 2.5-km radius circular plot centered on jaguarundi locations and random points to calculate these statistics, which corresponded to male jaguarundi home range size in northeastern Mexico (Caso, 2013). We generated 10,000 random points across SLP and extracted these same variables for each random point. We then compared jaguarundi locations and random points for the continuous variables using MANOVA (Morrison, 1990) and for cover type using Fisher’s exact test (Zar, 1996) to contrast habitat correlates of jaguarundi from rural survey areas with the SLP landscape.
Landscape modeling. We constructed models of habitat correlates of jaguarundi presence using both logistic regression (Zar, 1996) and maximum entropy modeling (Maximum Entropy 3.1 [MaxEnt]; Phillips et al., 2006) to identify the environmental variables associated with jaguarundi presence in our surveyed areas (Trisurat & Bhumpakphan, 2018). We used the environmental variables noted above for distribution modeling. For logistical models, we modeled all possible additive candidate models, and used stepwise logistic regression to identify the environmental variables most associated with jaguarundi presence. We used stepwise regression because it is more conservative than information-theoretic model selection methods (Arnold, 2010).
For maximum entropy modeling, we modeled all possible candidate models using the complementary log‐log (clog-log) transformation. We compared models by testing whether the model with the highest area-under-curve (AUC; Swets, 1988) differed from more parsimonious models. We used the critical ratio test (Pearce & Ferrier, 2000 as modified by Baldwin & Bender, 2008) to compare the highest AUC model with other models to determine if the increase in explanatory value was significant at α = 0.05 following Baldwin & Bender (2008). If models did not differ, we selected the most parsimonious model. We calculated standard errors for AUC values using 30% of the locations as test data. We derived thresholds for probability of jaguarundi presence by maximizing sensitivity and minimizing specificity (Fielding & Bell, 1997; Phillips et al., 2006). We used these thresholds to convert probabilities to binary response (presence-absence) and used the equal test sensitivity and specificity threshold values to calculate successful classification percentages. Last, we compared the final model to 1,000 random null models following Raes & ter Steege (2007) and determined the probability that the fit (AUC) of the final model was greater than the AUC of the random models.
Because fine resolution cover type maps may poorly reflect interspersion or mosaics of cover types as compared to coarser resolution cover type maps that include mosaic habitats in cover type designations (e.g., 300-m resolution maps such as GlobCover [2008]), we also assessed whether cover types in preferred logistic and maximum entropy models were more indicative of uniform or mosaic patches. For this, we created a 300-m resolution window around jaguarundi occurrences and random points and extracted the fine resolution cover types (from the 30-m resolution raster used in modeling; INEGI, 2013) within this neighborhood. We then determined whether the fine resolution cover type at the sample point was the predominant cover type in the neighborhood and identified which other cover types, if any, were present in the neighborhood. We considered the neighborhood essentially a uniform cover type if ≥90% of the 30-m pixels were of the same cover type, and defined mosaics by the two predominant cover types if no single cover type comprised ≥90% of the pixels.
Last, we compared final logistical and maximum entropy models using AUC and successful classification outcomes (Trisurat & Bhumpakphan, 2018) but using parametric bootstrapping and contingency tables, respectively (Efron & Tibshirani, 1993; Zar, 1996). We included the latter because a model that poorly classifies the data it was built from is unlikely to have any true predictive ability (Hosmer & Lemeshow, 1989). We also compared similarity of model predictions (i.e., predicted likelihood of jaguarundi presence) using Warren’s I statistic (Warren et al., 2008) at N = 10,000 random locations.
Site characteristics. At each occurrence we recorded site-level descriptive variables including (1) actual vegetation cover type at the occurrence site (as opposed to mapped cover type); (2) predominant vegetation cover type seen in the surrounding area of the site of occurrence (i.e., aspect dominant cover type); (3) percent ambush cover; and (4) degree of habitat disturbance. We defined ambush cover as the estimated proportion of an individual jaguarundi detectible through the vegetation when recumbent (lying down) as if waiting for prey or hiding from predators. We estimated this variable using a 1.5 x 0.6 m screen placed at the location of the jaguarundi sighting and 0.5 m above the ground. We recorded the percent of the screen obscured by vegetation from each of the four cardinal directions at five meters and used the mean as our estimate of ambush cover (Tewes & Schmidly, 1987; Griffith & Youtie, 1988; Dillon, 2005). We then classified ambush cover as high (76-100%), medium (51-75%), low (26-50%), and very low (0-25%). We also estimated the degree of disturbance to the habitat following the criteria of Martínez-Calderas (2009). We classed disturbance as high, medium, or low according to the presence of secondary vegetation, grazing (defined in terms of tracks, feces, and livestock presence), agricultural fields, and habitat clearings. We compared ambush cover and disturbance among classes using Fisher’s exact test (Zar, 1996). We compared cover types at sites of locations with the SLP landscape as described above.
Results
Environmental correlates. We documented 50 reliable records (five Class I and 45 Class II) of jaguarundi around primarily rural communities (but including communities of >10,000 residents), ejidos, and ranches (Table 1). Date of records ranged from 2002-2008. We confirmed the presence of jaguarundi in Zona Huasteca (33), Zona Media (14) and Zona Centro (3) of SLP, but not the Altiplano region. Cover types at the location of occurrences included rainfed and irrigated agriculture (n = 14); tropical forest (including tall evergreen tropical forest, subperennifolious tropical forest, and lowland deciduous forest; n = 10); scrub (including submontane scrub, microphyle desert scrub, and succulent-rosette scrub; n = 10); natural and cultivated grassland (n = 9); temperate forest (including oak, oak-pine, and cloud forest; n = 4); and other (including urban [n = 2] and halophile vegetation [n = 1]).
Table 1 Records of jaguarundi, reliability class of record, municipality and locality within municipality, and type of evidence (and provider for Class II records) of occurrences in San Luis Potosí, Mexico.
| Record | Class1 | Municipality | Locality | Evidence2 |
|---|---|---|---|---|
| 1 | I | Lagunillas | Lagunillas | Pelt |
| 2 | II | Tamuin | Rancho 'El Danes' | Observation (ranch worker) |
| 3 | II | Tamuin | Rancho Zimapan Hidalgo | Observation (ranch worker) |
| 4 | II | San Vicente Tancuayalab | Rancho 'El Cañaberal' | Observation (ranch worker) |
| 5 | II | Tamuin | Rancho 16 | Observation (ranch owner) |
| 6 | I | Tamasopo | San Nicolas de los Montes | Mounted specimen |
| 7 | II | Zaragoza | Valle de los Fantasmas | Observation (ranch workers) |
| 8 | II | Zaragoza | San Francisco | Observation (ranch workers) |
| 9 | II | Rio Verde | Bordo Blanco | Observation (hunter) |
| 10 | II | Rio Verde | Bordo Blanco | Description of the species (ranch owner) |
| 11 | II | San Nicolas Tolentino | Las Milpitas | Observations (hunter) |
| 12 | II | Rio Verde | Ejido San Francisco | Observation (ranch owner) |
| 13 | II | Rio Verde | Los Peroles | Observation (ranch workers) |
| 14 | I | San Ciro | Codornices y Capaderos | Pelt |
| 15 | II | Rayon | Vaqueros | Observation (ranch workers) |
| 16 | II | Chalpuhuacanito | Tempexquititla | Description of the species (ranch owner) |
| 17 | II | San Martin Chalchicuautla | San Martin | Observation (ranch owner) |
| 28 | II | San Martin Chalchicuautla | Lagunillas | Observation (hunter) |
| 19 | II | Tampacan | Carretera salinendo de lagunillas | Observations (hunter) |
| 20 | II | Tanquian | Rancho ‘Lagoleta’ | Observation (ranch workers) |
| 21 | II | Tanquian | El Capricho | Description of the species (ranch owner) |
| 22 | II | Tampomolon | El Naranjo | Observation (hunter) |
| 23 | II | Axtla de Terrazas | Arroyo de en medio | Observation (ranch worker) |
| 24 | II | Xilitla | La Herradura | Description of the species (ranch owner) |
| 25 | I | Cerritos | Joya de Luna | Mounted specimen |
| 26 | II | Guadalcazar | Pozo de Acuña | Observation (ranch workers) |
| 27 | II | Cd. Valles | La Antigua, Rancho Nuevo | Description of species (ranch worker) |
| 28 | II | Cd. Valles | La Calera-Tamunal | Observation (ranch workers) |
| 29 | II | Aquismon | Las Lajas | Observations (hunter) |
| 30 | II | Aquismon | Tampate | Observation (ranch workers) |
| 31 | II | Aquismon | La Caldera | Observation (ranch workers) |
| 32 | II | Aquismon | El Naranjito | Observation (ranch workers) |
| 33 | II | Cd. Valles | Las Granjas | Description of the species (ranch owner) |
| 34 | II | Cd. Valles | Leon Garcia | Observation (hunter) |
| 35 | II | Cd. Valles | Laguna de Mante | Observation (ranch workers) |
| 36 | II | Cd. Valles | Los Sabinitos 2 | Observation (ranch workers) |
| 37 | II | Cd. Valles | Las Pitas | Description of species (ranch workers) |
| 38 | II | Tamuin | San Jose de limon | Observation (hunter) |
| 39 | II | Ebano | Laguna Chica | Observation (ranch owner) |
| 40 | II | Tamuin | Santa Martha | Description of species (ranch workers) |
| 41 | I | Tamasopo | Tamasopo | Pelt |
| 42 | II | Lagunillas | El Cañon | Description of species (ranch workers) |
| 43 | II | Tamasopo | Cabezas | Observation (ranch workers) |
| 44 | II | Tamasopo | Santa Maria | Observation (ranch owner) |
| 45 | II | Cd. Valles | Cerro Alto | Description of species (ranch workers) |
| 46 | II | El Naranjo | El Platanito | Description of species (ranch workers) |
| 47 | II | Cd del Maíz | San Juan del Meco | Observation (ranch workers) |
| 48 | II | San Nicolas Tolentino | Pozo del Aguila | Observation (ranch owner) |
| 49 | II | San Nicolas Tolentino | Ojo de Agua | Observation (ranch owner) |
| 50 | II | Tierra Nueva | La Joyita | Observation (ranch workers) |
1Class I = observations made by a reliable interviewee supported by physical evidence; Class II = detailed description by a reliable and experienced observer (Tewes and Everett, 1986).
2Observation = individual had a priori knowledge of jaguarundi and knew that what they saw was a jaguarundi at time of observation; Description of species = observer was able to correctly describe the species to the interviewer, recognize it by a description given by the interviewer, and/or recognize it from a picture, without a priori knowledge of what a jaguarundi was.
Jaguarundi locations differed (F 11,10037 = 17.2; P < 0.001) from random points for most landscape attributes. Jaguarundi were located closer to water (F 1,10048 = 15.3; P < 0.001), closer to roads (F 1,10048 = 17.4; P < 0.001), at lower elevation (F 1,10048 = 71.6; P < 0.001), and marginally closer to communities (F 1,10048 = 3.5; P = 0.061) than were random points. Human population density was similar (F 1,10048 = 2.4; P = 0.121). Sixty-two percent of occurrences were <500 m from water sources, 70% were <500 m from roads, and 54% were <500 m from the closest community or cluster of buildings. All occurrences were located below 2,000 m (mean = 579 m [range = 16-1900]) in elevation.
Jaguarundi locations also included more edge (16.2 km v. 13.6 km; F 1,10048 = 4.2; P = 0.041), greater edge density (6.2 v. 5.2; F 1,10048 = 4.2; P = 0.041) and marginally more patches (4.8 v. 4.3; F 1,10048 = 2.7; P = 0.100) than did random locations. Patch density (0.18 v. 0.16), patch relative density (0.11 v. 0.11), and relative patch richness (18.6 v. 17.2) were similar (F 1,10048 = 2.4; P ≥ 0.122).
Cover type also differed between jaguarundi locations and random points (Fisher’s exact P < 0.001); jaguarundi were found more frequently in tropical forest (Fisher’s exact P < 0.001) and agriculture (Fisher’s exact P < 0.011) cover types, and less frequently in desert scrub (Fisher’s exact P < 0.001). Jaguarundi presence was marginally positively related to urban (i.e., any mapped community regardless of type or size; Fisher’s exact P < 0.066) and grassland (Fisher’s exact P < 0.071) cover types as well. No other cover types differed (Fisher’s exact P ≥ 0.287) (Fig. 2).
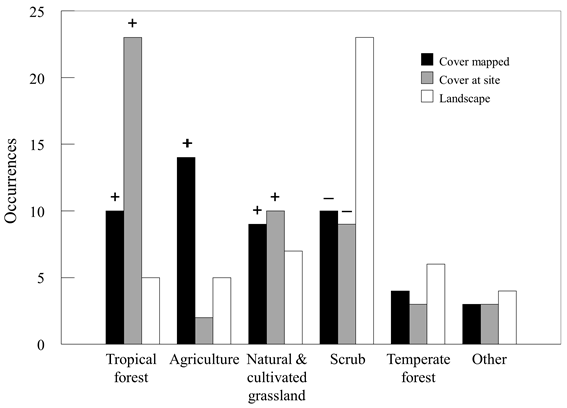
Figure 2 Vegetation cover-land use types associated with occurrences of jaguarundi at the actual site of occurrence (Cover at site), the general area as mapped on vegetation maps (Cover type mapped), and numbers expected based on proportional composition of the San Luis Potosí landscape (Landscape). Proportionately greater (+) or lesser (-) presence than expected (P < 0.10) are noted above bars.
The logistical model including elevation + cover type + distance to roads (χ2 3 = 78.2; P < 0.001; AUC = 0.912; successful classification percentage = 90%) was most strongly related to jaguarundi presence. Likelihood of jaguarundi presence was >0.80 in mosaics of tropical forest, agriculture, and urban cover types below 500 m in elevation within 1.5 km of a road (Fig. 3). Based on partial adjusted R2s, elevation (48%) accounted for the most relative variance in model performance, followed by cover type (40%) and distance to roads (12%).
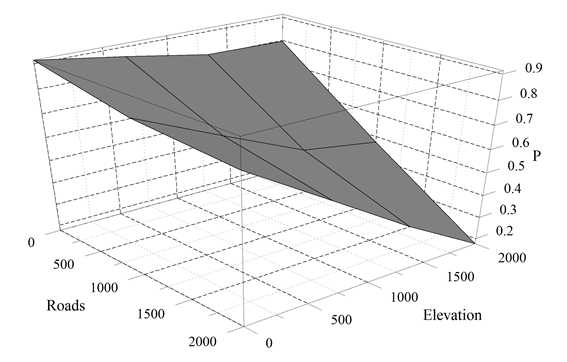
Figure 3 Mean likelihood of jaguarundi presence across preferred cover types (tropical forest, agriculture, grassland, and rural urban) as a function of distance from roads (m) and elevation (m).
The maximum entropy model including cover type, elevation, distance to roads, and human population density provided the most parsimonious fit (P ≥ 0.907 as compared to higher dimensioned, greater AUC models; P ≥ 0.081 compared to lower dimensioned models) and fit data well (AUC = 0.909; SE = 0.027; successful classification percentage = 88%). Model fit was also superior (P = 1.000) to 1,000 random null models. Elevation accounted for the most variance (36%) in model performance, followed by cover type (25%), distance to roads (23%), and population density (16%). Likelihood of presence of jaguarundi was >0.80 in mosaic agriculture-tropical forest, tropical forest-grassland, and tropical forest cover types (1) below 500 m in elevation, (2) <2 km from roads, and (3) in areas with >50 but <150 people/km2 (Fig. 4).
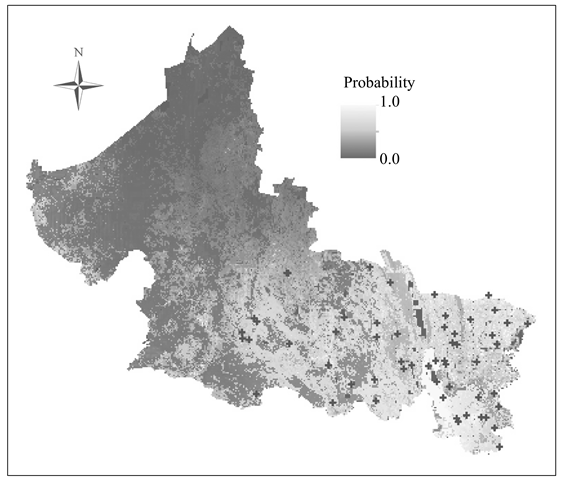
Figure 4 Likelihood of presence of jaguarundi in San Luis Potosí, Mexico, as predicted by maximum entropy modeling. Documented locations of jaguarundi are shown (+).
The above models include identified mosaic associations with the individual cover types identified in the model selection procedures. For cover types identified as having high likelihood of jaguarundi occurrence by either model (i.e., tropical forest, agriculture, grassland, and urban), the predominant associated cover type was either the same cover type in a uniform neighborhood (32% of jaguarundi presence locations) or was a mosaic of tropical forest, agriculture, grassland, and/or urban (44% of presence locations). Thus, tropical forest, agriculture, grassland, and urban were either the sole cover type or in mosaic in 76% of jaguarundi presence locations. In contrast, a uniform neighborhood of the cover type at the location was more common (67%; Fisher’s exact P < 0.001) for random locations on the SLP landscape.
Last, model fit parameters were similar (P ≥ 0.749) between logistical and maximum entropy models (i.e., AUC = 0.912 and 0.909 and successful classification percentage = 90% v. 88%, respectively). Likelihood maps of jaguarundi presence were likewise strongly correlated (I = 0.90).
Site characteristics. Locations of jaguarundi varied in ambush cover (Fisher’s exact P = 0.023); jaguarundi were seldom located in areas with very low ambush cover (N = 2 occurrences) as compared with areas of high (mean = 85.3%; SE = 1.5; N = 19), medium (mean = 67.6%; SE = 1.4; N = 13), and low cover (39.3%; SE = 1.5; N = 16), respectively. Jaguarundi were similarly (Fisher’s exact P = 0.908) located in areas that showed high (30% of the records), medium (32%), and low disturbance (38%) of habitat.
Cover type at the site of occurrences varied compared to random locations (Fisher’s exact P < 0.001; Fig. 2). Jaguarundi were found more frequently in tropical forest (Fisher’s exact P < 0.001) and less frequently in desert scrub (Fisher’s exact P < 0.001) (Fig. 2). Jaguarundi were also found marginally more frequently in urban (Fisher’s exact P = 0.066) and submontane scrub (Fisher’s exact P < 0.054) cover types. No other cover types varied (P ≥ 0.159). When the actual occurrence was in tropical forest, agriculture, grassland, or urban cover types, the aspect dominant of the surrounding area was either the same cover type (17% of occurrences) or was either tropical forest, agriculture, grassland, or urban (66% of occurrences) if a different cover type. Thus, tropical forest, agriculture, grassland, and urban were either the sole cover type or in mosaic in 83% of jaguarundi occurrences, similar to mapped cover type results, above.
Discussion
Jaguarundi were commonly associated with human-altered habitats and were relatively widely distributed throughout the lower elevations of SLP (Fig. 4). These results supported previous work that indicated adaptability of jaguarundi with respect to cover types occupied and disturbance, including human-related disturbance (Caso, 1994, 2013; de Oliviera, 1998; Carrillo et al., 1999; Aranda, 2005; Peña-Mondragón, 2004; Giordano, 2016). Most of our occurrences were in cover types where jaguarundi have been previously recorded, including tropical forests, natural and cultivated grasslands, and oak forests (Guggisberg, 1975; Tewes & Everett, 1986; de Oliveira, 1998; Brown & González, 1999; Peña-Mondragón, 2004; Charré-Medellín et al., 2012; Caso, 2013; Farías et al., 2015; Giordano, 2016). In addition to these, we also documented jaguarundi in several other cover types, including desert scrub and halophytic vegetation. Most relevantly, however, were the frequent locations in agriculture mosaics associated with the cover types noted above (especially tropical forest and grassland) and urban (i.e., communities) areas including communities of >10,000 inhabitants, highlighting the adaptability of jaguarundi to human-altered habitats.
Adaptability of jaguarundi to fragmented mosaic habitats in SLP resulting from human-related disturbance was evident in both landscape correlates and site characteristics of jaguarundi occurrences, i.e. primarily mosaics of tropical forest, agricultural sites (including irrigated agricultural fields, and seasonal croplands), and grasslands (including natural or cultivated pastures). Moreover, most occurrences were along induced edges associated with abrupt changes in land use or cover type, similar to habitat use patterns seen in north-eastern Mexico (Caso, 2013). Additionally, most jaguarundi occurrences were relatively close (<500 m) to water, roads, and communities, attributes which increase induced edge and thus likely presence and abundance of preferred prey (Aranda, 2005). Thus, jaguarundi may actually be benefitted by at least moderate human-induced fragmentation and habitat conversions, rather than merely being tolerant of these activities. Additional work on fitness (i.e., individual condition) and population-level productivity of jaguarundi in human-altered and natural communities is needed to determine to what extent jaguarundi may actually benefit from human-activities (sensuBaldwin & Bender, 2009).
Our results thus support the adaptability of jaguarundi to at least moderate levels of human alterations of natural communities, as well as supporting likely preferences of jaguarundi for ecotones (Cabrera & Yepes, 1960; Jiménez-Guzmán et al., 1997; Giordano, 2016), areas with secondary vegetation (Caso, 2013; Giordano, 2016), and tropical forest (Bisbal, 1989; Caso, 2013; Giordano, 2016), especially fragmented forests associated with agriculture or grassland. Because >50% of jaguarundi occurrences were within 500 m of rural communities, rural human-associated fragmented habitats may provide highly favorable habitat conditions for jaguarundi, and likely their wild and domestic prey (Aranda, 2005; Arias-Alzate et al., 2013). Adaptability of jaguarundi to human-altered habitats was further reflected in the significant associations of roads (logistical and maximum entropy models) and moderate human population density (maximum entropy model) with jaguarundi occurrences, as well as the greater total edge, edge density, and number of patches at actual locations as compared to random points. While we conducted surveys in or near rural communities, ejidos, and ranches, not all occurrences of jaguarundi were adjacent or close to communities, and were reflective of the SLP landscape. For example, distribution with respect to larger communities (i.e., urban areas [communities] included in the most recent cover type map of SLP [INEGI, 2013]) was relatively similar to the SLP landscape; random locations averaged 8 km from such communities, whereas jaguarundi occurrences averaged 7 km, with 34% >8 km from the closest mapped community. Thus, our results provide not only documentation on the adaptability of jaguarundi to human-altered habitats, but also reasonably reflect overall distribution and landscape level environmental correlates of jaguarundi in general. The exception to this may be that our results may underestimate jaguarundi tolerance of larger communities, as our sampling was biased towards rural landscapes.
Most agricultural and other human-altered habitats occur close to water and at low elevation, local attributes we also found associated with most occurrences of jaguarundi, reflecting other work in Mexico. For example, we found all occurrences at elevations <1,900 m, similar to Peña-Mondragón (2004) who found that the distribution of jaguarundi did not exceed elevations >1,500 m in Nuevo Leon, Mexico. These results contrasted with those of Cuervo et al. (1986), who reported some individuals at >3,200 m in elevation in Colombia. The distribution of jaguarundi in Mexico at lower elevations likely relates to increased human-related disturbance, and hence interspersed and edge habitats. Common disturbances associated with these landscapes include roads, narrow waterways (e.g., irrigation ditches), and rights-of-way, which are likely not barriers for jaguarundi movements (Campbell, 2003). Consequently, jaguarundi are generally considered more tolerant of human disturbances than are other threatened felids (Caso et al., 2015). While we did not find a significant relationship between site-level habitat disturbance as we categorized it and the presence of jaguarundi, landscape-level correlates and edge-related metrics support previous studies that showed jaguarundi to be tolerant of landscape disturbances associated with human activities, as long as sufficient low cover remained in those areas (Campbell, 2003; Caso, 2013; Giordano, 2016). Supporting this, we found most (64%) jaguarundi occurrences were in the two densest ambush cover classes. Tewes and Schmidly (1987), Caso (1994) and Caso et al. (2015) noted that jaguarundi were able to inhabit more open terrain as long as it contained patches of structure that facilitated hiding and searching for prey. Thus, regardless of cover types occupied by jaguarundi, dense low cover is likely critical for jaguarundi presence, both to facilitate ambush of prey and to avoid detection by other larger predators. While little is known about jaguarundi prey preferences in Mexico, small rodents were recently identified as their primary prey in the Sierra del Abra Tanchipa Biosphere Reserve, located in northeastern San Luis Potosí (Benítez-Alemán, 2014).
Conclusions
Reliable knowledge of the distribution of species-of-concern, and environmental attributes associated with its presence, is necessary for informed recovery plans. We found jaguarundi were relatively widely distributed in SLP, and were adaptable to and likely benefitted by at least moderate levels of human-related disturbance. This was evidenced by frequent locations in mosaic agricultural-natural vegetation landscapes that were relatively close to communities, especially lower elevations areas characterized by mosaics of forest (especially tropical forest), agriculture, and/or grasslands relatively close to water; as well as locations close to larger (i.e., >10,000 residents) urban centers. Natural resource managers should account for the adaptability of jaguarundi to human-altered habitats and consequently these environmental correlates in their conservation plans for the species in SLP and east-central Mexico. These associations and our likelihood mapping (Fig. 4) of jaguarundi presence provide a framework to identify key recovery areas and areas potentially important for connectivity among recovery areas to facilitate population viability. Relatedly, likelihood mapping also provides a tool to determine areas for more intensive surveying of jaguarundi presence, population demographics, and population status.











 nova página do texto(beta)
nova página do texto(beta)


