Introduction
The majority of native earthworms of Cuba (84%) belong to family Acanthodrilidae, subfamilies Benhamiinae (Eutrigaster) and Acanthodrilinae (Diplotrema, Exxus, Protozapotecia and Zapotecia). Whereas these species are related to central and northeast Mexican high altitude earthworms (Fragoso & Rojas 2016), South American affinities are scarce, being represented only by two species of the family Rhinodrilidae (Rodríguez et al. 2007).
In the more recent checklist of the earthworms of this country, Rodríguez et al. (2007) recorded a total of 47 species, 20 exotics and 23 endemic, eight of these quoted as new non-described species. Preliminary descriptions of some of these species were made by Rodríguez (1999), as part of his Doctoral dissertation. However, and in accordance with article 9.12 of the International Code of Zoological Nomenclature (ICZN 1999), descriptions provided in thesis are considered unpublished. Accordingly, in this paper we are publishing these descriptions and making them widely available.
Materials and methods
Sampling was performed during years 1984, 1994 and 1995 in west and center Cuba. All worms were handsorted by qualitative and quantitative (soil monoliths) manual extraction methods; each worm was fixed in an ethanol-formalin mixture (1:1) and preserved in formalin (4%). For each species (in brackets), individuals were grouped according to the following convention: juveniles (without any sexual character), non clitellate adults (adults with external sexual characters) and clitellate adults. In each description segments are indicated in bold Arabic numbers. All material is deposited at the collection of the Soil Fauna Laboratory, Biology Faculty, Havana University (FBUH). Definition of included families, subfamilies and genera are according to Csuzdi (2010), James & Davidson (2012) and Fragoso & Rojas (2016).
Results
Family Acanthodrilidae
Subfamily Acanthodrilinae
Genus Protozapotecia James, 1993
Protozapotecia angelesae Rodríguez and Fragoso sp.nov.
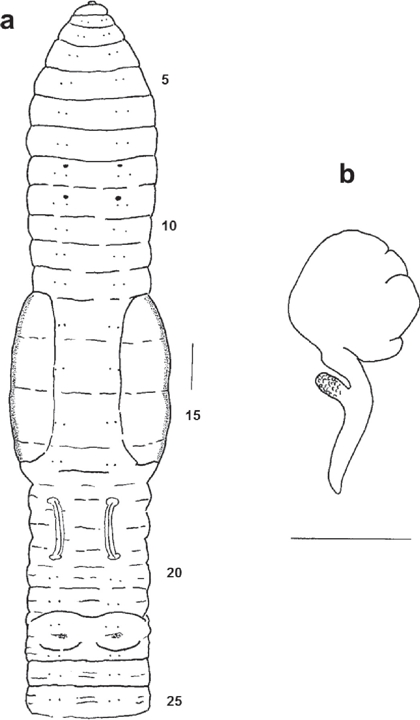
Figure 1 Protozapotecia angelesae sp.nov. (Holotype FBUH 306). a) External ventral view of anterior region (scale 0.9 mm); b) Dorsal view of right spermatheca from segment 9. Scale 1 mm.
Material. Province of La Habana, municipality of Boyeros, Capdevila, Campus of the Institute of Ecology and Systematics, within the soil of a secondary semi-deciduous forest. 23° 02’ 6.51’’ N, 82° 22’ 44.99’’ W, 76 m.a.s.l. Ma. de los Angeles Martínez, coll., October 1994. Holotype: Clitellate adult FBUH 306, Paratype: Juvenile, FBUH 307.
Description
External: Length 40-67 mm, postclitellar diameter 1.5-2.4 mm. Number of segments 212-215. Cylindrical body in cross section throughout. Pigment absent; after preservation in formalin it acquired a yellowish colour. Prostomium open epilobic, bearing a great number of minute papillae. Post-clitellar segments with secondary or tertiary secondary furrows. Setae lumbricine, closely paired throughout, cd lateral, in regular longitudinal rows; setal formula (aa:ab:bc:cd) 10: 3.3:1:3:1, 30: 5:1:9:1. Without penial and genital setae. First dorsal pore 8/9. Nephridiopores at d. Clitellum yellow-orange in 13-16, 17 only dorsally, saddle-shaped without reaching ventral setae and covering setae cd (Fig. 1a). Spermathecal pores paired in 8 and 9, in front of setae a (Figure 1a). Male and female pores inconspicuous. Prostatic pores at the end of nearly straight seminal grooves at AB level in 17 and 19 (Fig. 1a). Genital markings represented by a ventral pair of papillae in segments 22-23 (Fig. 1a).
Internal: Septum 5/6 membranous, 6/7-9/10 gradually increasing thickness and then decreasing. Alimentary canal with two discrete gizzards in 5 and 6, each one divided from the other by a deep furrow. Esophagus apparently with mesially free calciferous lamellae in 11 and 12. Intestinal origin in 14. Typhlosole and caeca were not seen.
Dorsal vessel single throughout, ventral vessel present, subneural vessel absent. Lateroesophageal hearts in 9-12, lateral hearts joined to dorsal and ventral vessels in 6-8, supraesophageal vessel in 8-12.
Excretory system holoic, avesiculate and stomate.
Male sexual system holandric, iridescent testes and funnels free in 10 and 11; a pair of seminal vesicles in 11 and 12, with a fine and spongy texture. Tubular prostates in 17 and 19 extending backwards until 23, the distal portion plicated; the thick, muscular and plicated ducts are covering two segments. Penial setal follicles and penial setae absent. Fan-shaped ovaries in 13. Paired spermathecae in 8 and 9; the voluminous and ovoidal ampulla joins a duct as long as the ampulla diameter. Lateral and near to the ampulla base there is a short digit-form diverticulum with coagulated sperma (Fig. 1b), which is also present in the basal portion of the ampulla.
Etymology. The name of the species is dedicated to our colleague and good friend Dr. Ma. de los Angeles Martínez.
Systematics. The bigiceriate and holoic genus Diplocardia, distributed from USA to northeast Mexico, is characterized by two nearly fused gizzards in segments 5 and 6, tubular prostates open in segments 18 and 20 or backwards, intestine starting in or after segment 17 and a membranous nature of septum 5/6 (Fragoso & Durán 2014, Fragoso & Rojas 2016). Conversely, the three Mexican described species of the genus Protozapotecia possess tubular prostates in segments 17 and 19, intestine beginning in segment 14 or 15 and the two gizzards of 5 and 6 separated by a muscular septum 5/6 (James 1993). By possessing prostatic pores in 17 and 19, an intestine beginning in a more anterior segment (14) and the two gizzards separated by a membranous septum and a clear furrow, the new species is closer to Protozapotecia than to Diplocardia, even though the latter genus shares the condition of a membranous septum 5/6. By the absence of typhlosole and penial setae and by the presence of free lamellae in the esophagus of 11 and 12, P. angelesae sp.nov. is clearly separated from the three Mexican species of Protozapotecia, P. aquilonalisJames, 1993, P. australisJames, 1993 and P. koebeli (Eisen, 1900).
Protozapotecia centralis Rodríguez and Fragoso sp.nov.
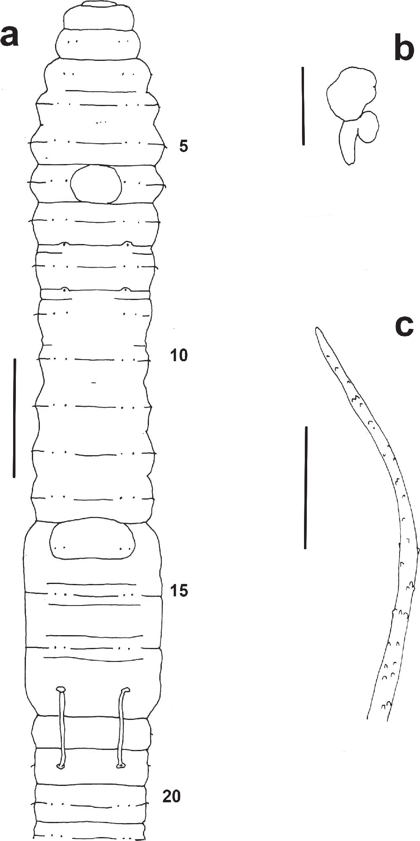
Figure 2 Protozapotecia centralis sp. nov. a) External ventral view of anterior region (Holotype FBUH 155) (scale 1 mm); b) Dorsal view of left spermatheca from segment 9 (scale 0.5 mm); c) Distal part of penial seta from segment 17 (scale 50 μm).
Material. Province of Cienfuegos, Municipality of Palmira, close to the agroindustrial Center “Espartaco”, within soil of a secondary semi-deciduous tropical forest. 22° 16’ 27’’ N, 80° 20’ 43.17’’ W, 67 m.a.s.l. Carlos Rodríguez, coll., May 1994. Holotype: Clitellate adult FBUH 155, Paratypes: Two clitellates, one pre-clitellate and two juveniles, FBUH 156-159.
Description
External. Length 28-70 mm (holotype 48 mm), postclitellar diameter at segment 30 1-2 mm (holotype 1 mm). Number of segments 53-202 (holotype 193). Body cylindrical in cross section. Pigment absent; color after preservation in formalin, clear grey to pinky greyish. Prostomium closed epilobic, covering 1/2-1/3 of peristomium. Secondary annulation characterized by a single furrow, more evident in pre-clitellar segments. Eight setae per segment in lumbricine arrangement, closely paired throughout, visible from segment 2 in regular longitudinal rows; setal formula (aa:ab:bc:cd) 10: 2.5:1:2:1.8, 30: 3.8:1:2.5:1.6. Penial setae present, genital setae absent. First dorsal pore in 4/5. Nephridiopores at c, conspicuous in the clitellum. Clitellum yellow-ochre, saddle shaped, covering segments 14-17, and with the intersegments barely visible (Figure 2a). Spermathecal pores paired in 8 and 9, small and in front of a or ab (Fig. 2a). Male and female pores not visible. Prostatic pores in 17 and 19, located on straight seminal grooves at AB level (Fig. 2a). Genital markings variable, characterized by single midventral papillae in 6 (only in the holotype), 14 (3 ind.) (Figure 2a), 16 and part of 17 (1 ind.) or in 20 (1 ind.).
Internal. Septum 5/6 partially muscular, 6/7-8/9 gradually increasing in thickness until 9/10 which is the thickest; from then septa are gradually more membranous. Two discrete gizzards in 5 and 6, preceded by a proventriculus in 4. Esophagus with intramural mesially free calciferous lamellae in 13. Intestinal origin in 14. Laminar typhlosole starting in 17. Caecae not seen.
Lateroesophageal hearts in 10-12, lateral hearts joined to dorsal and ventral vessels in 6-9, supraesophageal vessel in 10-12.
Excretory system holoic, avesiculate and stomate.
Holandric, testes and large iridescent funnels in 10 and 11 with abundant coagulum; a pair of multi-lobed seminal vesicles in 11 and 12. Paired tubular prostates in 17 and 19, the former limited to its segment, and the posterior ones reaching part of segment 20. Penial setal follicles muscular and anterior to prostatic pores. Penial setae almost straight (0.59-0.63 mm length, 4-9 μ width), with a slight curvature in the distal portion; ornamentation characterized by several and irregular thorns (Fig. 2c). Fan-shaped ovaries in 13, with eggs in rows. Large paired spermathecae in 8 and 9, with the ovoidal ampulla joined to a thick duct, slightly shorter than ampulla diameter; the almost sessile and ellipsoidal diverticulum projects from the ental and lateral portion of duct (Fig. 2b).
Etymology. The name of the species indicates that it was found in the central region of Cuba.
Remarks. The new species belong to the group of Mexican Protozapotecia species with penial setae, typhlosole and seminal vesicles in 11 and 12. It differs from all of them by the more anterior dorsal pore (4/5 vs 6/7-9/10) and the free calciferous lamellae of segment 13 (absent). It also differs from P. aquilonalis and P. australis by the beginning of typhlosole (in 17 vs. 19 in the Mexican species) and the shape of the spermathecal diverticulum (lateral, sessile vs. reniform perpendicular to ampulla axis); from P. koebeli and P. aquilonalis it is also separated by the lack of genital setae. From P. angelesae sp.nov. is separated by the presence of penial setae and typhlosole.
Protozapotecia cubensis Rodríguez and Fragoso sp.nov.
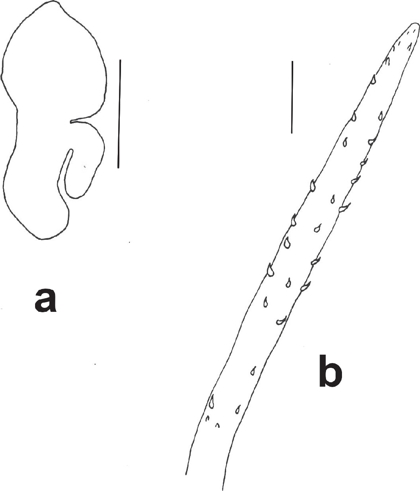
Figure 3 Protozapotecia cubensis sp. nov. (Holotype FBUH 146). a) Dorsal view of right spermatheca from segment 9 (scale 0.5 mm); b) Distal part of penial seta from segment 17 (scale 30 μm).
Material. Province of Cienfuegos, Municipality of Palmira, close to the agroindustrial Center “Espartaco”, within soil of a secondary semi-deciduous tropical forest. 22° 16’ 27’’N, 80° 20’ 43.17’’W, 67 m.a.s.l. Carlos Rodríguez, coll., May 1994. Holotype: Non-clitellate adult FBUH 146, Paratypes: 8 Juvenile (6 fragmented), FBUH 147-154.
Description
External. Length 95-130 mm (holotype 105 mm), postclitellar diameter at segment 30 2.5 mm (holotype). Number of segments 218-242 (holotype 245). Body cylindrical in cross section throughout. Pigment absent; color after preservation in formalin clear grey to pinky greyish. Prostomium closed epilobic, covering 1/3 of peristomium. Two or three secondary furrows from segment 5 or 6. Eight lumbricine setae per segment, closely paired throughout, visible from segment 2 in regular longitudinal rows, a and b conspicuous and large; setal formula (aa:ab:bc:cd) 10: 2.2:1:1.8:1.3, 30: 1.9:1:1.7:1.4. Penial setae present, genital setae absent. First dorsal pore in 4/5. Nephridiopores at c. Clitellum not yet developed; genital marks not seen (absent or not developed?). Spermathecal pores paired in 7/8 and 8/9, in a. Male and female pores inconspicuous. Prostatic pores at the end of straight seminal grooves in 17 and 19 each pore with two associated (a and b) penial setae.
Internal. Septum 5/6 membranous, 6/7-10/11 muscular, 11/12 less muscular. Two clearly delimited gizzards in 5 and 6, the anterior preceded by a proventriculus in 4. Esophagus with mesially free calciferous lamellae in segments 13, 14 and anterior region of 15. Intestinal origin in 17. Laminar typhlosole visible from 20/21, occupying 1/2-1/3 of intestinal lumen. Intestinal caeca absent.
Supraesophageal vessel in 9-13. Latero-esophageal hearts in 10-13, lateral hearts joined to dorsal and ventral vessels in 6-9.
Excretory system holoic, avesiculate and stomate.
Holandric, incipient funnels in 10 and 11; Acinous seminal vesicles in 11 and 12; not fully developed, they cover the dorsal part of the esophagus. Two pairs of tubular prostates in 17 and 19, small and coiled. Penial setae within muscular setal follicles, measuring 0.5 mm in length and 17-19 μ width (Holotype); ornamentation limited to the distal region, characterized by several irregular thorns (Fig. 3b). Ovaries not seen. Paired spermathecae in 8 and 9; ovoidal heart-shaped ampulla and duct of equal length; a sessile digit-form diverticulum, ectally oriented, projects from the ental part of the duct (Figure 3a).
Etymology. The name of the species refers to the country where it was found.
Remarks. Protozapotecia cubensis sp.nov. and P. centralis sp.nov. are unique in the genus by the anterior location of dorsal pores (4/5); from the latter species it is separated by the beginning of intestine (17 in P. cubensis vs 14 in P. centralis) and typhlosole (20/21 vs 17). The new species shares some morphological features that relate it with both Diplocardia and Protozapotecia. With the latter it shares the position of prostatic pores in 17 and 19 and seminal vesicles in 11 and 12, whereas with (some) Diplocardia it shares the last hearts in 13, calciferous lamellae in segments 13-15 and beginning of intestine and typhlosole in 17 and 20/21, respectively (Fragoso & Rojas 2016). However the first dorsal pore in 4/5, only shared with P. centralis sp.nov. and never recorded for any Diplocardia or any other Protozapotecia species suggest a common ancestor for both species. This common ancestor should have had seminal vesicles in segments 11 and 12, a character shared by all Protozapotecia species.
A recent molecular analysis (COI gene) of some holoic Mexican species of Protozapotecia and Diplocardia (two gizzards) and Zapotecia (three gizzards) (Cervantes et al. 2014), revealed that Protozapotecia and Zapotecia cluster together, clearly separated from Diplocardia. Based on this phylogenetic analysis, these authors suggested that the trigiceriate Zapotecia and the bigiceriate Protozapotecia correspond to the same lineage; by priority the name of this lineage should be Zapotecia.
However, and in spite of the high support values of the Bayesian inference analysis, Cervantes et al. (2014) recommended further analysis with more genes to confirm the observed topology. In the meantime we prefer to maintain both genera separated, hoping that future molecular analysis with more genes and including the Cuban species will definitively set the relationships of Mexican and Caribbean multigiceriate acanthodriline earthworms.
Genus Trigaster Benham, 1886
Trigaster setarmata Rodríguez and Fragoso sp.nov.
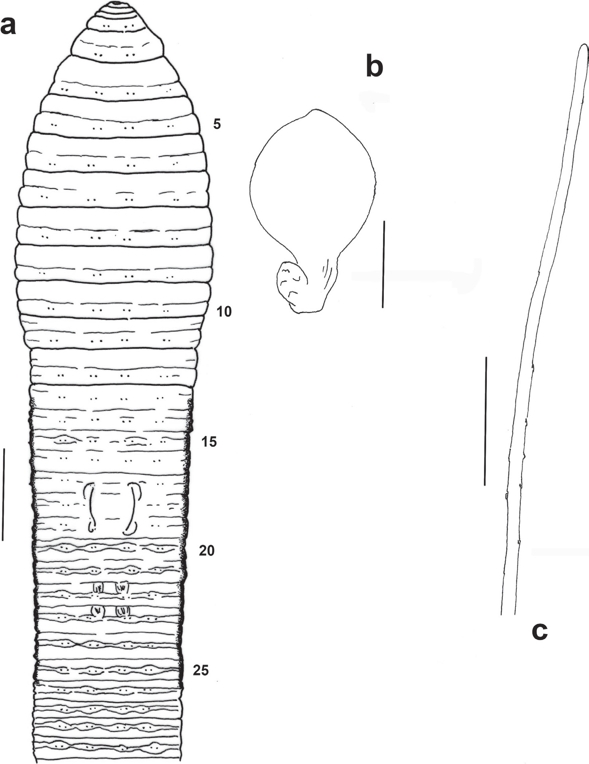
Figure 4 Trigaster setarmata sp. nov. (Holotype FBUH 003). a) External ventral view of anterior region (scale 4 mm); b) Dorsal view of right spermatheca from segment 9 (scale 1 mm); c) Distal part of penial seta from segment 17 (scale 100 μm).
Material. Province of Camaguey, Municipality of Sierra de Cubitas, within soil of a semideciduous microphile forest from “Sierra de Cubitas”. 21° 40’ 27.1” N, 77° 46’ 1.8” W, 84 m. a.s.l. L. F. de Armas, coll. June 1984. Holotype: Clitellate adult FBUH 003; Paratype: one juvenile FBUH 004.
Description
External. Length 305 mm, postclitellar diameter 6.8 mm. Number of segments 584. Body cylindrical in cross section, throughout. Pigment absent, color after preservation in formalin greyish. Prostomium prolobous. Secondary furrows mainly in faint clitellum and post-clitellum regions. Setae in lumbricine arrangement, minute, closely paired throughout, and in regular longitudinal rows; setal formula (aa:ab:bc:cd) 10: 1.6:1:3.8:1.3, 30: 5:1:9:1. Penial setae present. First dorsal pore in 11/12. Nephridiopores inconspicuous. Clitellum slightly developed in ½ 13-½ 26; yellowish. Spermathecal, female and male pores inconspicuous. Prostatic pores in 17 and 19, at the end of mesially curved seminal grooves at ab (Figure 4a). Barely developed, ventral paired genital marks in 13 in AB line, and in 22 and 23, in A (Fig. 4a).
Internal. Septa 5/6-11/12 muscular, funnel shaped and displaced backwards, 12/13 and 13/14 slightly muscular. Alimentary canal with three gizzards in 5, 6 and 7; anterior part of gizzard of segment 5 apparently forming a proventriculus, but a septum was not seen. Intestinal origin in 20. Typhlosole and caeca lacking.
Dorsal vessel single throughout, supraesophageal visible only in 10-13. Lateral hearts in 10-11, lateroesophageal in 12-13, last two pairs voluminous; ventral vessel lacking in 5.
Excretory system meroic; a pair of dense fringes of micronephridia on segments 3 and 4; in 30 there are 32 to 36 avesiculate and astomate nephridia per segment.
Male sexual system metandric, characterized by scarcely developed free testes and male funnels in 11; a pair of small, granular and digit-shaped seminal vesicles in 12. Two pairs of incipient racemose prostatic glands in 17 and 19, with slender and short ducts. Slightly curved penial setae present in segments 17 and 19; they are thin, 1.7-2.3 mm by 0.01 mm, with some dispersed sub-apical notches (Fig. 4c). Penial setal follicles, just anterior to ducts. Fan shaped ovaries in 13. Paired spermathecae in segments 8 and 9, projecting from posterior face of septa 7/8 and 8/9, near mid-ventral line and just in front to setae ab. Ampulla nearly spheroidal, with a duct shorter than the ampulla diameter and measuring 1/3 of the spermatheca length. Located near the ectal portion of the duct there is a small, spheroidal diverticulum with some incipient chambers (Fig. 4b).
Etymology. The species name refers to the presence of penial setae.
Systematics. James (1991) divided the multigiceriate and meroic genus Trigaster in four lineages. In the new arrangement, Trigaster was restricted to metandric species with one seminal vesicle in 12, tubular prostates in 17 and 19, three gizzards and eight setae per segment; the genus so defined was restricted to Virgin Islands and Puerto Rico. The new species shows several morphological characters that relate it to Trigaster: three gizzards, metandry, absence of ventral vessel in some portion of the body (in 5 in this species) and spermathecae in 8 and 9. From all the species of the genus (T. lankesteri, T. calwoodi, T. intermedia from Virgin Islands and T. longissimus from Puerto Rico), T. setarmata sp.nov is separated by the presence of penial setae and racemose prostatic glands. Accordingly the definition of the genus should be modified to include tubular or racemose prostates and with or without penial setae.
Blakemore (2007) suggested that this species should be placed in the highly debated family Exxidae, because it is assumed that racemose prostates in an acanthodrilid arrangement constitute an evolutionary novelty that appeared only once (in the ancestor of Exxidae). Considering that tubular and racemose prostates are also present in bigiceriate genera of Cuba (Exxus) and North México (Zapatadrilus, Cervantes et al. 2016), we prefer to maintain Trigaster in Acanthodrilidae because current evidence supports (e.g. molecular phylogenies) that the number of gizzards is more conservative than the type of prostates.
Discussion
The four species described in this paper share the common character of multiple gizzards, two gizzards in the species of Protozapotecia and three in Trigaster setarmata sp.nov. Within Acanthodrilidae, the multiple gizzard condition is observed in two main lineages: subfamily Benhaminae (tribe Benhamiini) and the so called diplocardins of south USA, northeast and central Mexico and Caribbean islands (Fragoso & Rojas, 2016). Whereas in the first case the majority of species present two gizzards (there are some genera with a single gizzard) in combination with kidney-shaped calciferous glands in the region of segments 14-19 (Csuzdi 2010), in the diplocardins there are two or three gizzards but no extramural calciferous glands occur. All Benhamiinae species are meroic; in diplocardins, on the other hand, both meroic and holoic species occur.
The most recent molecular studies (Buckley et al. 2011 and James & Davidson 2012) indicate that Benhamiinae represents a phylogenetic independent lineage; in the case of the diplocardine worms (for the entire list of species see the recent paper of Fragoso & Rojas 2016) all the evidence points out that they constitute also an independent lineage. A future morphological and molecular revision should be made in order to confirm the relationship of all these diplocardine multigiceriate worms; if a single origin is demonstrated, then, and as suggested by Blakemore (2005) and James & Davidson (2012), all these worms should be grouped in the resurrected subfamily Diplocardinae (in the sense of Eisen 1900).
All the species described in this study were found in west (Protozapotecia angelesae sp.nov.), central (Protozapotecia centralis sp.nov., Protozapotecia cubensis sp.nov.) and east Cuba (Trigaster setarmata sp.nov.) (Figure 5). Before being tempted to speculate on their biogeographical origin (dispersal vs vicariance), what we can state without any doubt is that the new Protozapotecia species are more closely related to the North American Diplocardia and north Mexican Protozapotecia than to any other acanthodriline worm of southern Mexico or elsewhere. In the case of Trigaster setarmata sp.nov. it should be clarified why this genus occurs in Cuba, Puerto Rico and Virgin Islands, without any record in Jamaica and Hispaniola. When and how these worms arrived to Cuba? The answer to this question should wait until robust molecular phylogenies of the entire native Cuban earthworms clarify their relationship with continental genera and species.











 nueva página del texto (beta)
nueva página del texto (beta)



