Introduction
Spiders are a diverse, widely distributed group in all terrestrial ecosystems and even freshwater (Turnbull, 1973; Foelix, 2011). At the trophic level, the spiders are important generalist predators due to their abundance, biomass, species diversity and life strategies. Their feeding habits influence the density and activity of detritivores and fungivores, affecting the litter decomposition process (Wise et al., 1999; McNabb et al., 2001), and they are a key factor to the mortality of crop pest insects (Liljesthröm et al., 2002).
A cadaver can represent a trophic and/or reproductive resource, among others, to the associated fauna. Most scavenger species are insects, belong to the orders Coleoptera, Diptera and Hymenoptera, but also arthropods such as spiders (Araneae), millipedes (Diplopoda), centipedes (Chilopoda) and isopods (Isopoda), are commonly associated with that resource and/or ephemeral habitats, due to their predatory or saprophagous habits (Norris, 1965; Seastedt et al., 1981; Keh, 1985; Smith, 1986; Lord, 1990; Catts & Goff, 1992). Considering the ecological role of the cadaveric entomofauna, such arthropods are regarded as adventitious or accidentals species, that is, those which use the cadaver as an extension of their own normal habitat (Catts & Goff, 1992; Goff, 2009). In forensic cases, the presence of spiders can confirm the compatibility of the specimens with the environment (Garcia Rojo et al., 2009) and, together with other opportunistic insects, could provide clues about the type and location of the crime scene (Chin et al., 2011).
Several factors can influence the abundance, distribution and composition of spiders, such as: the type and structure of the vegetation (Scheidler, 1990; Rubio et al., 2008); the type of habitat (Hatley & MacMahon, 1980; Uetz, 1991); the pattern of land use (Weeks & Holtzer, 2000); seasonality (Lubin, 1978; Sudhikumar et al., 2005; Mineo et al., 2010); climatic conditions (Crouch & Lubin, 2000; Kwon et al., 2014); amongst others. The latter are considered environment indicators (Clausen, 1986; Maelfait et al., 1990; Willett, 2001; Pinkus-Rendón et al., 2006; Tsai et al., 2006). Certain species are associated to environments with a certain degree of disturbance. Some of them are related to urban environments and can be found inside homes or in the peridomicile, and are indicators of anthropic impact (Durán-Barrón et al., 2009; Greene et al., 2009; Lima Silveira, 2009; Silva de Miranda & Ponce Leão Giupponi, 2011; Desales-Lara et al., 2013; Cramer, 2015). Other species can disperse and/or broaden their geographic location, adapting to new environments and becoming introduced or invasive species that usually displace native ones, eventually resulting in a loss of biodiversity, due to human activity (Taylor & Doran, 2001). Therefore, because of farming and cattle breeding activity, the anthropic action has caused changes in the ecosystems (Barnes et al., 1998), modifying populations, species distribution, structure and functioning of communities, and even more leading to eventual extinctions (Meffe & Carroll, 1994).
In Argentina, census or studies of the community or different patterns of spiders were performed with different crops and in different places (Liljesthröm et al., 2002; González et al., 2009; Armendrano & González, 2010, 2011a, b; Almada et al., 2012; Avalos et al., 2013). Also, spiders associated with other ecosystems of Argentina were studied under different conditions (Avalos et al., 2007; Rubio & Moreno, 2010; Pompozzi et al., 2011).
The purpose of this study was to know and characterize the composition, diversity, abundance and seasonality of the epigeal spiders of a semi-rural area of Bahía Blanca, in the temperate region of Argentina, using the data obtained from a cadaveric decomposition and succession study, which contributed to the analysis of the coleopterofauna of forensic interest of the area.
Materials and methods
The study was conducted in natural fields (38°41'52" S, 62°14'47ˮ W, 51 m.a.s.l.), in 4 randomly selected plots, of approximately 20 × 30 m, located at 300-500 m from buildings and at 100 m from a branch of the Napostá stream, enclosed by Populus alba L. 1753 and Tamarix sp. plantations (Fig. 1). The rest of the vegetation was dominated by Eucalyptus globulus Labill. 1800, Prosopis alpataco Phil. 1862, Chenopodium quinoa Willd. 1797 subsp. melanospermum Hunz. 1943, and pastures (Fig. 1). The study area is located within the Pampa biogeographic province (Morrone, 2002). The climate is continental warm and sub-humid (Campo de Ferreras et al., 2004; Campo & Zapperi, 2010) with high thermal and rainfall variability through the year. According to Köppen, it can be classified as "pampeano", because in the warmer season the average temperature exceeds 22 °C, and there is no dry season. The annual average rainfall is 600 mm, but there are variations in the area (Gil et al., 2008; Campo et al., 2009; Gabella et al., 2010). The maximum and minimum average temperature, and accumulated rainfalls recorded during the study periods were: 20.2 °C, 7 °C and 19.1 mm, respectively (winter); 23.3 °C, 11.6 °C and 40.3 mm, respectively (spring); 27.4 °C, 15.8 °C and 35.1 mm, respectively (summer); and 18.2 °C, 6.8 °C and 18.3 mm, respectively (autumn). These values were calculated from data provided by the CERZOS-CONICET weather station.

Figure 1: Map of Argentina with the location of Bahía Blanca (point), study area (arrows) and vegetation (Chenopodium quinoa subsp. melanospermum, pastures, and plantations of Populus alba, Tamarix sp. and Eucalyptus globulus, can be seen).
The methodology used was part of a cadaveric decomposition and succession study which contributed to the analysis of the coleopterofauna of forensic interest (Zanetti et al., 2014). Samplings were carried out every season in selected plots, starting in winter 2010 and finishing in spring 2011. The average length (Mean ± SE) of each experiment (total time to cadaver decomposition) was: 168 ± 7 days (winter), 39 days (spring), 31 days (summer), and 72 days (autumn). In the selected plots, vegetation was cut at 4-5 cm of soil brim. Then, in three of the four plots we placed a cage of wire mesh containing a pig cadaver. Six pitfall traps were installed surrounding each cage. In the remainder plot six traps with the same disposition were used as control. The pitfall traps were made from plastic containers of 500 mL volume and 8.5 cm diameter, each buried to the rim of the soil. Each trap had two containers, one inside the other: the inner container had a solution of 90% distilled water and 10% coolant. The outer container remained buried in the ground while the solution with the arthropods was filtered with a plastic funnel and a piece of fine mesh cloth, according to (Zanetti, 2013). The pitfall traps and cadavers were examined daily between 9:00 and 12:00 AM, during experiment time. I followed the criterion established by (Centeno et al., 2002) to define the stages of decomposition. The biological material collected was transferred to a plastic container with 70% ethanol for later determination in the laboratory and storage.
The adult specimens were determined to family and species level according to (Schiapelli & Gerschman de Pikelin, 1963, Goloboff, 1995, Ramirez, 1999, Ferretti et al., 2010), and validated with (World spider Catalog, 2015), and when it was not possible, they were separated into morphospecies; juveniles were only identified to family level due to the absence of genitalia and the identification at level species the adults specimens are necessary.
Relative abundance of each family, species, state of development of specimens and seasonal abundance was estimated as: RA = (ni (100)/N), where ni is the number of specimens collected at each site and N is the total of specimens collected at the site (Flórez-D., 1998). Species richness (S) was considered as the number of species per family and per season. Density of each family and species was calculated per week. Diversity per season was assessed and compared with the Shannon Index (H'), in a binary logarithm scale, and the species evenness using the J (Pielou) index (J' = H'/ H'max). To determine if the environment has been enough sampled was used the estimator CHAO 1 (Colwell & Coddington, 1994): S1= Sobs + (a²/2b), where Sobs is the number of species/morphospecies observed in the study area, a is the number of species/morphospecies with one individual in the study area and b is the number of species/morphospecies with two individuals in the study area. For this analysis was used the EstimateS 9.1.0 version (Colwell, 2013).
Spiders were grouped into eight guilds after (Cardoso et al., 2011) to see if families may have similar ecological roles and for future studies of ecological change in the area. The specimens collected were deposited in the collection of Laboratorio de Zoología de Invertebrados II, Universidad Nacional del Sur, Bahía Blanca, Argentina.
Results
A total of 972 spiders were collected, belonging to 22 families and 65 species/morphospecies (20 of them were identified to species level) and, 189 individuals (19.44%) were identified at family level because they were juveniles. The most abundant families were: Lycosidae (n = 254, 26.13%), Zodariidae (n = 162, 16.67%), Thomisidae (n = 149, 15.33%), Theridiidae (n = 74, 7.61%) and Salticidae (n = 59, 6.07%), amounting to 71.81% of the total of the collected individuals (Table 1). Lycosidae, Thomisidae and Theridiidae were more abundant in spring; Zodariidae and Salticidae were more abundant in summer followed by spring. Some families were only found in one season (Table 1).
Table 1: Density of spiders of each family and species/morphospecies per week during each season, and absolute and relative abundance of them. Juveniles were not included in the species/morphospecies analysis.

* Grammostola doeringi Holmberg, 1881 was not included because was found only as juvenile (1 individual in summer).
All the families with exception of Theraphosidae (n = 5, 0.51%), Actinopodidae (n = 7, 0.72%) and Nemesiidae (n = 11, 1.13%), because they live in burrows, were grouped into eight guilds (Table 2). The greatest number of individuals was represented by ground hunters (n = 362; 37.24%), followed by specialists (n = 162; 16.67%) and ambush hunters (n = 149; 15.33%). The rest of the guilds had percentages lower than 10.08% (Table 2). The guild of ground hunters also exhibited the greatest species richness (S = 20).
Adults (n = 738, 75.93%) predominated over juveniles (n = 234, 24.07%), and males were more abundant (n = 523, 70.87%) than females. When seasonality was evaluated, males were the most abundant in all seasons. Figure 2 shows the relative abundance of adults and juveniles of each family. Lycosidae was the family most strongly represented by juveniles (n = 140; 55.12%).
The specific richness (S) registered was of 65 species. The total specific diversity and the evenness were high in all seasons (Fig. 3). The families with the greatest number of species were Lycosidae (S = 10) and Theridiidae (S = 7) (Table 2). The former had the greatest number of species in winter (S = 7) and spring (S = 8), and the latter in winter (S = 4) (Table 1). The most abundant species (Table 1) were Leprolochus birabeni Mello-Leitao, 1942 (Zodariidae) (n = 120), Steatoda sp. 1 (Theridiidae) (n = 62), Thomisidae sp. 33 (n = 56), Thomisidae sp. 32 (n = 47), Ostearius melanopygius O. P.-Cambridge, 1879 (Linyphiidae) (n = 45) and Metaltella simoni Keyserling, 1878 (Amphinectidae) (n = 41). Leprolochus birabeni (Zodariidae) was almost constant in number of individuals between seasons; Steatoda sp. 1 was more abundant in spring followed by autumn; Thomisidae sp. 33 had the greatest number of individuals in winter; Thomisidae sp. 32 and O. melanopygius were more abundant in spring; and Metaltella simoni was more abundant in autumn (Table 1). For the more abundant families the relative abundance of Lycosidae was due to Lycosa thorelli Keyserling, 1877 and Hogna bivitatta Mello-Leitão, 1939; for Thomisidae it was due to Thomisidae sp. 1, and for Theridiidae, it was due to Steatoda sp. 1. During summer and spring the relative abundance of Zodariidae was due to L. birabeni, and of Salticidae it was due to Salticidae sp. 1. The total number of species was highest in winter and lowest in summer. Some species were only found in one season (Table 1). When the abundance of the six most abundant species was analysed per sex (Fig. 4), it was registered that contrary to the general finding, L. birabeni females were more abundant than males particularly in summer and autumn; Thomisidae sp. 32 females were more abundant than males in spring; Steatoda sp.1 males were very abundant in spring as well as autumn; and O. melanopygius both sexes were almost in equal amounts during seasons.
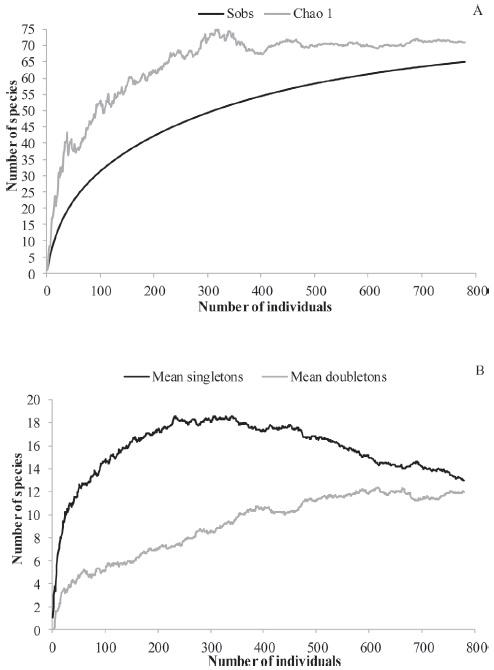
From the total of the species registered, 13 were "singletons" (species represented by one individual in the sample) (20% of all the species) and other 12, "doubletons" (species represented by two individuals in the sample) (18.46%). The observed species and the expected number of species curves reached an asymptote (Fig. 5A). Chao 1 mean was 70.99. The accumulative curve of singletons tends to decrease and of doubletons tends to stability (Fig. 5B).
Discussion
The geography, vegetation, and climatic variables, amongst other factors may result in differences in the distribution of members of some groups of spiders and on habitat selection (Gerschman de Pikelin & Schiapelli, 1963; Comstock, 1965; Uetz, 1992). Several authors have described a greater diversity and biomass of spiders in undisturbed natural areas than in agro-ecosystems (Nyeffeler & Benz, 1987; Alderweireldt, 1989; Desender et al., 1989; Heideger & Nentwig, 1989; Liljesthröm et al., 2002; Avalos et al., 2007, 2009; Beltramo et al., 2006; Almada, 2010; Almada et al., 2012), such as is the semi-rural area used in this work.
The spider families found in this study represented 34% of the total of the families described in Argentina and the species, to 5.5% (World spider Catalog 2015). The families Amphinectidae, Nemesiidae, Theraphosidae, Zoridae, Ctenidae and Segestriidae were not registered in agro-ecosystems of Santa Fe (Beltramo et al., 2006; Almada et al., 2012), and neither Amphinectidae nor Nemesiidae were recorded in the ecological Reserve "El Pozo" (Almada, 2010), before this survey. Moreover, these two families were not found in degraded woods of the humid Chaco in Corrientes province (Avalos et al., 2007). These differences might be due to the geography, type of environment, climate or the sampling technique. In relation to the sampling technique, may be differences could also be attributed to the fact that some spiders are commonly associated with ephemeral habitats such as cadavers (which are significant feeding and/or reproducing resource for other arthropods such as insects), due to their predatory or saprophagous habits (Norris, 1965; Seastedt et al., 1981; Keh, 1985; Smith, 1986; Lord, 1990; Catts & Goff, 1992).
Regarding abundance, the families with a greatest number of individuals were Lycosidae, Zodariidae, Thomisidae, Theridiidae and Saliticidae; Lycosidae, followed by Theridiidae, had the greatest number of species. Several studies also reported Lycosidae as the most abundant family in natural areas (Rubio et al., 2008), and agro-ecosystems (Beltramo et al., 2006; Armendano & González, 2010) as well as the most abundant in the ground substrate of those disturbed environments in Argentina (Minervino, 1996; Liljesthröm et al., 2002; Beltramo et al., 2006). Because they are active spiders that move above ground and under fallen leaves in search of their prey, and do not spin webs to hunt (Nyffeler & Benz, 1988). The families Zodariidae and Salticidae are active hunters who must also move to find their prey. Such findings could also be related to the fact that spiders can use cadavers as a refuge or hunting places (Watson, 2004; Gill, 2005).
Individuals were grouped into eight guilds, of which the greatest number of individuals corresponded to the ground hunters followed by specialists. This could be explained by the sampling methods as well as the type of environment. (Cardoso et al., 2011) mentioned that the precise proportions per guild (and family) found are specific to the methods available. They also suggested that functional diversity is positively related with habitat complexity, with more complex habitats being more functionally diverse.
Adults were more abundant than juveniles, and adult males more abundant than females, which could be due to the sampling technique used. Furthermore, males are probably more active in their search for mates; consequently, they have a higher probability of being captured in the traps (Mineo et al., 2010; Ferretti et al., 2012). Because the highest proportion of adults and juveniles were trapped during spring, it cannot be concluded that many species have their reproductive period during that season.
The species richness was lower than that recorded in degraded woods of the humid Chaco in Corrientes (Avalos et al., 2007), but greater than that observed in agro-ecosystems of Buenos Aires (Grismado 2007; Armendano & González, 2010) and Santa Fe (Almada et al., 2012). The same factors cited for differences in developmental time and abundance may be affecting species richness. The prey availability and the risk of predation or parasitism of spiders could be other important factors influencing the species richness of spiders (Crouch & Lubin, 2000). These factors could influence species richness or other parameters of the spiders, alone or in combination with other factors (Halaj et al., 1998; Bell et al., 2001; New, 2005; Spears & MacMahon, 2012; Diehl et al., 2013). In this way, the great diversity and abundance of arthropods observed (Coleoptera, Diptera, Hymenoptera, amongst other) during the decomposition and succession study, could contribute to the results found in this work.
Leprolochus birabeni (Zodariidae) was the most abundant species, which is coincident with the results reported by (Pompozzi et al., 2011) for La Pampa province. These spiders belong to the specialists guild, so they capture their prey moving over the ground (Grismado et al., 2011). The second in abundance was Steatoda sp. 1 (Theridiidae), which was probably due to the activity of males (Mineo et al., 2010; Ferretti et al., 2012). Metaltella simoni (Amphinectinae), which was fifth in abundance, are ground hunters common in places such as cities, gardens and vacant lots (Grismado et al., 2011).
The number of singletons was lower than 50%, thus it is unlikely to be underestimation of species (Colwell & Coddington, 1994) as reported in other studies (Avalos et al., 2007, 2009; Pompozzi et al., 2011). The values of Sobs and of Chao 1 were very similar and the expected number of species curve and the curve of doubletons were asymptotic, and the curve of singletons tended to decrease. This could indicate that the effort was enough and the study area was sufficiently sampled (the number of species/morphospecies of the area would not increase with the number of samplings) (Villarreal et al., 2004). This was not unexpected because the high proportion of "singletons" is a characteristic of the tropical arthropod fauna in woods and tropical and temperate savannahs (Florez, 1998; Sørensen et al., 2002; Withmore et al., 2002). Such areas are characterized by a high number of species that usually exhibit low population densities or are rare (Florez, 1998; Sørensen et al., 2002). Some studies have shown that seasonality influences the abundance, growth rate, and size of spiders (Gaston et al., 1993; Gasnier et al., 2002). This could be related to different factors, as mentioned above, as well as foraging and/or an increase in prey density, which can vary over the season in response to environmental conditions (Kiritani et al., 1972; Mahalakshmi & Jeyaparvathi, 2014). These factors could account, at least partially, for the increase recorded in the spider density during spring. In this study, climate variables did not affect the spider abundance differently depending on the sex and age of the spiders. The fact that some families were found in certain seasons could be due to juveniles or eggs that hibernate during those periods, as well as to some organisms having two reproductive periods and entering diapause in the adult stage (Jimenez & Navarrete, 2010).











 text new page (beta)
text new page (beta)