Services on Demand
Journal
Article
Indicators
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta zoológica mexicana
On-line version ISSN 2448-8445Print version ISSN 0065-1737
Acta Zool. Mex vol.27 n.2 Xalapa Aug. 2011
Artículos originales
A new species of Dahlibruchus Bridwell, 1931 (Coleoptera: Bruchidae) from an archaeological site in Texcoco, Mexico, with some comments about history of the site and bionomics of the insect
Una nueva especie de Dahlibruchus Bridwell, 1931 (Coleoptera: Bruchidae) de un sitio arqueológico en Texcoco, México, con comentarios acerca de la historia del sitio y bionomía del insecto
Jesús ROMERO NÁPOLES & María ROMERO RAMÍREZ
Programa de Entomología y Acarología, Instituto de Fitosanidad, Colegio de Postgraduados, Montecillo, Estado de México C.P. 56230, México. E–mail: jnapoles@colpos.mx.
Recibido: 21/06/2010.
Aceptado: 08/11/2010.
ABSTRACT
A new species of the Dahlibruchus genus is described and information is given about its bionomics and host plants, also are mentioned general aspects about the archeological site where the insect was found.
Key words: Archeology, Mexico, Bruchidae, new species.
RESUMEN
Se describe una nueva especie del género Dahlibruchus y se brinda información sobre su biología y plantas hospederas, también se mencionan aspectos generales del lugar arqueológico en donde se encontró el insecto.
Palabras clave: Arqueología, México, Bruchidae, nueva especie.
INTRODUCTION
Bruchids are insects specialized only in plant seeds. Adults are free living organisms. A few days after females copulate they start to lay eggs on pods or directly onto seeds. The larvae feed in the seeds, and adults may or not feed before reproduction; if they feed, they can use pollen or nectar of flowers of the same or different host plant. There are in the world 62 genera in the family Bruchidae; of these genera there are 21 known from Mexico (Kingsolver 1989, Romero 2002). In 2004, Romero published a very interesting discovery, he found in Tetzcotzinco, an archeological place near Texcoco, Estado de México, México a very typical bruchid associated with a wild dahlia flowers.
The genus Dahlibruchus Bridwell, 1931 in Mexico. For more than 30 years the U. S. Department of Agriculture (USDA) has been exploring the world to find useful plants in agriculture. There are almost 90,000 kinds of seeds that have been considered for introduction. The USDA has the biggest seed collection of economically important plants (12,000 species). In the seeds of Dahlia sp. that were imported from Mexico, H. Y. Gouldman found specimens of Bruchidae; later, the insects were described as a new species by Bridwell (1931), but because of their unusual form it was necessary to erect a new genus, Dahlibruchus, to include the species D. conradti Bridwell. In the same paper the author included D. sharpianus Bridwell, which initially was described as Bruchus longulus Sharp, but because the specific name was preoccupied in Bruchus it had to be replaced. The species was collected in Dahlia maxonii Saff. seeds from Antigua, Guatemala. Actually, the genus Dahlibruchus contains only two species.
Host plants. Between Bruchidae and their host plants there is a very dependent relationship, and for this reason the family has great importance, firstly as a natural regulator of plant populations because they destroy the dispersion forms (seeds), and secondly because many bruchids feed on seeds that are economically important to man. Approximately 1200 plant species have been registered as hosts for bruchids, 900 belonging to Fabaceae; however, there is information showing that these insects may attack 34 plant families in the world. In Mexico Bruchidae have been reported from only 12 families, including about 520 species (Romero 2002).
The relationship between bruchids and plants of the family Asteraceae is not very extensive, and we have only two records: Dahlibruchus spp. in the genus Dahlia, and Cosmobruchus russelli in Cosmos sp. In both groups, the insects have the unusual elongate form, which corresponds to the form of the seeds (Bridwell 1931).
The genus Dahlia is a group of plants that diversified in Mexico, includes 35 species, and all can be found in Mexico (Saar 2003, Sorensen 1969). In Mexico these plants have three main uses: 1) food (the root is edible), 2), as medicine (almost all the structures have curative properties, such as for stomach problems), and 3) ornamental.
Whitely (1985) stated that the big variety of dahlias were in the past a very important source of food (as a root culture) and medicaments that used to be utilized by the pre–Columbian indigenous peoples from central México, Yucatán and Guatemala. For this reason the flower was the sun symbol to King Moctezuma and other nobility. The same author suggested that dahlia root may be used in the modern world as a valuable supplement food, mainly in subtropical areas.
The Tetzcotzinco forest
The forest is located approximately 7 km east of Texcoco, Estado de México; it belongs to the municipality of Tlaminca (Fig. 1). According to Martínez (2000) Tetzcotzinco is a hill where there used to be a magnificent garden containing many kinds of rare trees and flowers, some brought from very far places. In the same area there used to be rustic showers and some caverns fixed as field houses. Of the many recreative places in ancient Texcoco, the Tetzcotzinco forest was preferred by King Nezahualcoyotl.

The forested hill used to be fenced, and in order to ascend to the top people had to climb 520 steps. The steps were built with mortar or just formed directly on the rock. Because there was no water to irrigate the garden, the king ordered to build an aqueduct. This system had a main stream that fed side channels which led to reservoirs (Fig. 2). In the first reservoir there was a big rock where the ancient Mexicans wrote all the main feats of Nezahualcoyotl's life (1402–1472). Unfortunately this relic was destroyed in 1528 by Fray Juan de Zumárraga, the first bishop of Mexico, who thought that the stone could be an idol.

From the first reservoir of carved rock, the water was distributed to both sides of the forest; even in this superior esplanade there were constructions that simulated towers that at the top were finished with a plant pot which contained tufts of feathers that provided the etymology of the forest's name. Underneath the rock reservoir there was a sculpture like a lion with wings and feathers, laid down and looking to the East, and through his mouth the king's face showed. Down slope there were three more reservoirs with diverse carved images on the rock: three branches meant the great lake, and three heads the empire or alliance, the name and shield of Tollan, capitol of the Toltecas, and of Tenayuca, capitol of the old dominion Chichimeca. From the last reservoir a spurt of water splashed over some large stones and fell as rain into a garden of fragrant flowers. Beside this garden were the famous Nezahualcoyotl's baths. The baths are formed by reservoirs excavated in the solid rock (Figs. 3 and 4). A series of steps, also carved into the rock and burnished (polished) as a mirror, led from the baths to the palace and to the royal house that the king had in the forest, a place where he used to retire to meditate and fast. Also there were many rooms and toilets; and there was a courtyard that was used to receive gentlemen (masters) of Mexico and Tlacopan, and some dances and other performances were given there.


O'Gorman (1972) quoted Fernando de Alva Ixtlilxochitl, where he gave a detailed description of what in his time was the Tetzcotzinco forest. He translated it from Spanish: "There was this fortress built in so admirable and wonderful a way, and with a great variety of rocks, that did not seem made by human industry. The room where the king slept, was round: everything else in that forest, as I have said, was planted with a variety of trees and odoriferous (fragrant) flowers; and with that a diversity of birds, that the king had in cages from many parts, that made a harmony and chant so that people weren't heard; out of the flowers, that were split by a wall, the mountain entered in which were deer, rabbits and hares, that if each thing in particular was described, and of the other forest of this kingdom, it would be necessary make a very particular history."
Another version of Tetzcotzinco was established by Miguel Medina (1997) who indicated that, toward the year 1450, a terrible dry season occurred and lasted for seven years. King Nezahualcoyotl solved the problem of the dry season and the hunger it caused by building a hydraulic system. Not only did this prepare the soil for agricultural production, but at the same time this master work provided an aesthetic phenomenon or, as Miguel Medina suggested, landscape architecture.
According to the latter author the construction of Tetzcotzinco started in 1453. The royal palace and Tetzcotzinco forest were formed as a wholeness of human life: of habitation, recreation, bathing, walking, contemplation, meditation, celebration, mystic observation, apprenticeship, education, reflection, dance, mysticism, sculpture, flower ornamentation, feathering, art, crop cultivation, common life with the birds and other animals, and the experience of love. Originally the hill of Tetzcotzinco was conical, lengthened in an east–west direction that presented a convex slope toward the north side, a semi–convex slope toward the south side, and two sides that overlook to the east and to the west. On the north side, since time immemorial, a mature forest of oaks extends mixed with extensive brush and rocks. The south hillside, on the other hand, suffered transformations. For example, there was established the xochitepancalli or the king's plant collection that can be considered an adaptation laboratory, and for vegetable production (Fig. 5).

MATERIAL AND METHODS
At Tetzcontzinco on September 26, 1999 we collected the lilac colored flowers of Dahlia rudis Sorensen, on some of which there were bruchids eating pollen. At the time we identified them as Dahlibruchus conradti. Later we found another dahlia with flowers of a different color, varying from yellow to orange to red, and it was identified as Dahlia coccinea Cav. The bruchid associated with its seeds proved to be different, a new species. For the next six years we continued studying those insects with the purpose to obtain more specimens and information about their life history. To determine the distribution of both species of Dahlibruchus, we examined material that we collected in other areas, and specimens deposited at Centro de Entomología y Acarología, Colegio de Postgraduados, Montecillo, México (CEAM).
For the study of the species we used the methods described by Kingsolver (1970) and Kingsolver and Whitehead (1974). For interpretation of genitalia we follow Romero and Johnson (1999). We used the terminology and taxonomic characters used by Johnson (1983, 1990).
Most specimens of D. conradti are deposited at CEAM, and type material of the new species is deposited at Florida State Collection of Arthropods (FSCA), United States National Museum of Natural History (USNM), Coleccion Nacional de Insectos (CNIN), and CEAM.
RESULTS
Plants and bruchids. Dahlia rudis characteristically has lilac colored flowers (some may be white) and D. coccinea has yellow to orange to reddish flowers; both are perennial plants that grow in the Tetzcotzinco forest, flowering between July and October. According to Sorensen (1969) the former occurs in the states of Hidalgo, Mexico, Morelos, and in Distrito Federal; the latter has a much wider distribution, occurring practically all over the country, and it is enormously variable.
According to our observations, now we know that the bruchids are perfectly adapted to the dahlia life cycle. By July, just when the dahlias start to bloom, the two species of Dahlibruchus emerge from their burrows, in which they were hibernating, and start to eat pollen on dahlia flowers (Fig. 6). Soon they start to copulate, which act may last from two to four minutes. By the time that the female is ready to oviposit, the bracts of the flower heads are dry and the females can place their eggs (Fig. 7). Approximately 30 days later, the eggs hatch and the larvae burrow into the involucral bracts until they reach the seeds, where they feed (Fig. 8). One seed has enough food only to feed one larva, so from each seed can be developed only a single adult. Adults of the new generation remain in the flowers until September or October, by which time Dahlia spp. flowers are dried and the insects hide, possibly between the withered leaves or the fissures of nearby trees, and they stay there until the next year when the dahlias start to bloom again. A similar behavior was seen when some heads of D. coccinea were collected at Laguna de Servin, Querétaro and brought to the laboratory for observation. The collection was made October 11, 2001 and the bruchids stayed alive until August 10, 2002. Generally they remained hidden between the leaves, seeds and bracts. They were provided small drops of water, and periodically they drank small amounts then hid again.



Dahlibruchus nezahualcoyotli Romero & Romero, new species
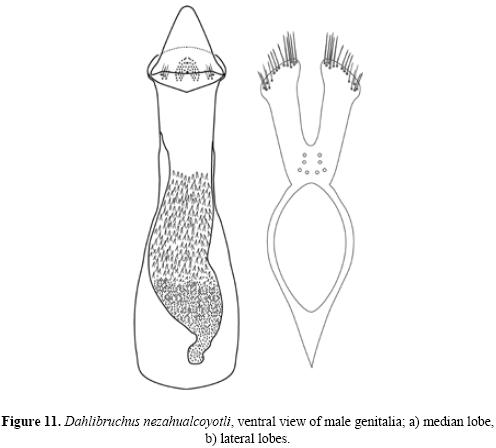
Description. Male. Measurements. Length (pronotum–elytra) 1.65–2.58 mm; width 0.87–1.38 mm; maximum thoracic depth 0.81–0.93 mm. Vestiture. Pronotum, elytra, and pygidium with whitish pubescence; metepisternum, apical portion of metacoxa, and apical lateral abdominal sternites with white tufts of pubescence, forming a lateral whitish stripe. Integument color. Body dark except antenna and legs yellowish; procoxa, mesocoxa, and apical portion of hind femur dark brown. Some specimens may have antennal segments 5–11 dark. Structure. Body elongate, about twice as long as broad; head short, densely micropunctulate; frons with weak inpunctate median carina; distance between eyes about 0.60 to 0.80 as wide as eye width; eye cleft 0.62 to 0.66 its length by ocular sinus; posterior margin of eye protruding from adjacent surfaces; postocular lobe rounded; distance from base of antennae to apex of labrum 0.41 to 0.48 as long as distance from upper limits of eyes to apex of labrum; antennal segment 1 filiform, 2–4 moniliform to filiform, 5 subserrate, 6 to 10 serrate, 11th subovate; antenna extending to humerus. Pronotum subquadrate with anterior angles rounded, dorsum slightly convex, surface even, median lobe slightly impressed medially, posterior angles slightly acute, lateral margin acute, carinate posteriorly to the middle, ampliate in the middle, obsolete anteriorly; cervical sulcus moderately deep, extending from near coxal cavity to about 0.66 to 0.76 distance to pronotal midline; pronotal disk microfoveolate; propleura glabrous and finely striate; prosternum acute at apex, separating the procoxae for about one half their length. Scutellum small, subquadrate, emarginately bidentate at apex. Each elytron about three times as long as broad, with small teeth at base of stria 3, 4, and 5, striae finely impressed, without visible punctures, 4 and 5 abbreviated at apex, humeral callus and humeral lobe feeble, apices covering the pygidium at base. Foretibia unarmed, hind coxa and metepis–ternum punctulate; hind femur constricted basally and apically, expanded medially to about width of coxa and unarmed, hind tibia straight without carinae, tibial corona with 4 spinules, mucro 0.10–0.16 as long as first tarsomere, first hind tarsomere slightly arcuate and without carina. First visible abdominal sternum slightly flattened medially, posterior margin straight, longer than remaining sterna; sterna 2 to 4 similar in size; fifth emarginate medially; pygidium finely punctate without foveolae, convex in lateral view (Figs. 9a, b (10)). Genitalia. Median lobe moderate in length; in ventral view, ventral valve triangularly rounded, arcuate in lateral view; internal sac very long and folded, armature of internal sac with a small mass of fine spicules apically and a pair of large masses composed of fine spicules extending from near middle to near base (Fig. 11a). Lateral lobes elongate, expanded at apex, cleft to about 0.28 their length (Fig. 11b).

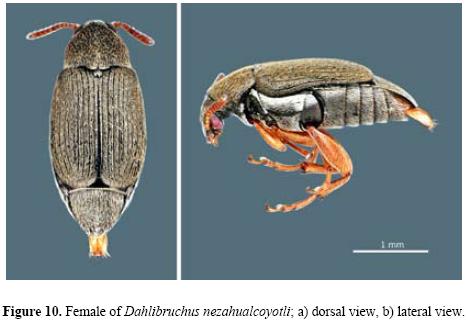
Female. Similar to male, except the 5th abdominal sternum is longer, about as long as 3rd and 4th together. Length (pronotum–elytra) 1.5–2.67 mm; width 0.78–1.44 mm; maximum thoracic depth 0.60–1.2 mm (Figs. 10a, b).
Type series. Holotype male, allotype: MEXICO, Estado de México, 7 km E Texcoco, Tlaminca, 16/IX/2002, 2315 m, Romero N.J. Paratypes. MEXICO, Chiapas: ECOSUR San Cristobal de las Casas, 23/IX/2003, Luna Cozar J., Dahlia imperialis Roezl ex Ortgies. Distrito Federal: Jardines del Instituto de Ecología, UNAM, 12/X/2004, Romero N.J., Dahlia coccinea Cav.; Pedregal de San Angel, 15/IX/1978, Castillo M.L., 19°17'N, 99°09'W; Pedregal de San Angel, 27/IX/1980, 2600 m, Arias M., 19°17'N, 99°09'W. Estado de México: Cerro Tetzcotzinco, 7 km E Texcoco, Tlaminca, 16/IX/2002, 2315 m, Romero N.J.; Cuajimalpa, 20/VIII/1977, Hendrichs S.J.; Km 17 carr. fed. México–Toluca, Cuajimalpa, 2/IX/2004, Romero N.J. Jalisco: Km 30 autopista Guadalajara–Tepic, 8/VIII/2003, 1565 m, Romero N.J., Dahlia sp., 20°49'36''N, 103°48'11''W; Parque Nacional de Volcán, 11 mi. from hwy., 11/VII/1984, Woolley J.B. Michoacan: Km 168 carr. Zinapécuaro–Huajumbaro, 24/ VIII/2002, 2360 m, Romero N.J., 19°42'32''N, 100°44'58''W; Km 24 carr. Zinapécuaro–Huajumbaro, 24/VIII/2002, 2113 m, Romero N.J., 19°48'45''N, 100°48'52'' W; Los Cantiles, km 209 carr. Zinapécuaro–Morelia, 24/VIII/2002, 2248 m, Romero N.J., 19°39'42''N, 100°55'21''W; Patzcuaro, 9/VIII/1949, Bottimer L.J. Oaxaca: 3 km SSE Llano Verde, km 124 carr. Oaxaca–Huajuapan, 8/X/2003, 2280 m, Romero N.J., 17°16'49''N, 97°04'26''W; Km 112.5 carr. fed. Miahuatlan–Puerto Angel, 21/ IX/2008, 2088 m, Romero N.J., Dahlia sp., 16°14'59.5''N, 96°32'30.3''W; Km 120 carr. fed. Miahutlan–Puerto Angel, 3/X/2009, 2402.7 m, Romero N.J., Dahlia imperialis Roezl ex Ortgies, 16°12'40.5''N, 96°32'09.0''W; Km 125 carr. fed. Miahuatlan–Puerto Angel, 21/IX/2008, 2419 m, Romero N.J., Dahlia imperialis Roezl ex Ortgies, 16°12'38.5''N, 96°32'13''W; Km 162 carr. fed. Teotitlan–Oaxaca, 20/IX/2008, 1971.5 m, Romero N.J., Dahlia coccinea Cav., 17°32'48''N, 96°56'56.1''; Km 188.5 carr. Teotitlan–Oaxaca, 20/IX/2008, 2230 m, Romero N.J., Dahlia pteropoda Sherff, 17°22'29.4''N, 96°55'15.8''W; Km 35.5 carr. Nochixtlan–Huajuapan, on Hwy 191, 9/X/2003, 1985 m, Romero & Westcott, 17°41'31''N, 97°35'24''W; La Venta, km 127 carr. fed. Miahutlan–Puerto Angel, 3/X/2009, 2402.7 m, Romero N.J., Dahlia aff. coccínea, 16°11'43.9"N, 96°31'13.6''W; La Venta, km 127 carr. fed. Miahutlan–Puerto Angel, 3/X/2009, 2402.7 m, Romero N.J., Dahlia imperialis Roezl ex Ortgies, 16°11'43.9''N, 96°31'13.6''W; Santiago Naranjas, km 108 carr. fed. Putla–Huajua–pan de León, 24/IX/2008,1744 m, Romero N.J., Dahlia campanulata Saar, P.D. Sorensen & Hjert., 17°16'18''N, 98°00'37.1''W. Querétaro: 2 km S Laguna de Servin, Amealco, 11/X/2001, 2500 m, Romero N.J., Dahlia sp., 20°15'8''N, 100°19'4''W; Laguna de Servin, Amealco, 17/VIII/2001, Luna Cozar J.; Laguna de Servín, Amealco, 23/VIII/2002, 2545 m, Romero N.J., 20°15'44'N, 100°14'16''; Laguna de Servin, NW Amealco, 23/VIII/2002, 2590 m, Bellamy C.L., 20°15'46''N, 100°14'16''W. Tlaxcala: Km 77 carr. fed. México–Tlaxcala, 27/VIII/2005, Romero N.J. Holotype deposited at National Museum of Natural History (USNM), Washington, DC; allotype and paratypes at Florida State Collection of Arthropods (FSCA), Gainesville, paratypes at Colección Entomologica del Instituto de Fitosanidad, Montecillo, Estado de Mexico (CEAM) and Colección Nacional de Insectos, UNAM (CNIN), México.
Diagnosis. Dahlibruchus nezahualcoyotli can be separated from the other two described species in the genus by the lateral whitish body stripe, male protibia without spine, elytra and pronotum with whitish pubescence; the median lobe of male genitalia with different sort of spines in the internal sac, and the lateral lobes with specific structures for this new species.
Discussion. Now the genus Dahlibruchus contains three species: D. conradti is easy to separate from the other two because the male protibia has a spine; the others share the character of the absence of a spine on the male protibia, however they can be separated by the pubescence on elytra and pygidium, which is yellowish in D. sharpianus but whitish in D. nezahualcoyotli. Another good character to separate the latter two species is the lateral whitish body stripe that is present in D. nezahualcoyotli and absent in D. sharpianus. However, the best way to identify both is by the male genitalia.
Distribution. México: Chiapas, Distrito Federal, Estado de México, Jalisco, Michoacán, Oaxaca, Querétaro, and Tlaxcala.
Host Plants. Dahlia campanulata Saar, P.D. Sorensen & Hjert., Dahlia coccinea Cav. Dahlia imperialis Roezl ex Ortgies, Dahlia pteropoda Sherff.
Etymology. The specific epithet refers to Acolmiztli Nezahualcóyotl (1402–1472), King of Tezcoco, known commonly as the Poet King.
Key to species of Dahlibruchus Bridwell.
1. Male protibia with an erect acute tooth at middle beneath .... D. conradti Bridwell
1'. Male protibia without an erect acute tooth at middle beneath..............................2
2. Propleura pubescent and finely foveolate..........................D. sharpianus Bridwell
2'. Propleura glabrous and finely striate..................D. nezahualcoyotli, new species
General discussion
An interesting question arises: did dahlias grow naturally at Tetzcotzinco before Nezahualcóyotl developed the Tetzcotzinco forest, or were they introduced by the King for his botanic garden? First, it is important to indicate that the Tetzcotzinco vegetation is quite unusual. Pulido and Koch (1988) did an inventory of the vascular plants and revealed the presence of 375 species, 234 genera, and 70 families, a surprising diversity for an area of only 50 hectares. They mentioned three species of dahlias, D. coccinea, D. merckii Lehman, and D. rudis; however, D. merckii is not present now in the area and the populations of the other two species are low. Sorensen (1969) stated that the latter two species have a wide distribution, including the State of Mexico. So with this information at least we can surmise that those plants are native to the Tetzcotzinco forest. Another important point is that bruchids and dahlias are very closely associated. Based upon our extensive observations both the insects and the plants always are together in their natural condition. Where the bruchids are absent, the dahlias have been cultivated. However, it is difficult to discard that there used to be many more species of dahlias in the Tetzcotzinco forest, that some grew naturally and others may have been planted by the King. It is interesting to point out that dahlia flowers used to be the sun symbol to King Moctezuma and other nobility.
ACKNOWLEDGMENTS
We thank to Rick L. Westcott for the review of the manuscript. We are also grateful to Jorge Valdez Carrasco for taking the pictures.
LITERATURE CITED
Bridwell, J.C. 1931. Bruchidae infesting seeds of Compositae, with descriptions of new genera and species (Coleoptera). Proceedings of the Entomological Society of Washington, 33: 37–42. [ Links ]
Johnson, C.D. 1983. Ecosystematics of Acanthoscelides (Coleoptera: Bruchidae) of Southern Mexico and Central America. Miscellaneous Publications of the Entomological Society of America, 56: 1–248. [ Links ]
Johnson, C.D. 1990. Systematics of the seed beetle genus Acanthoscelides (Bruchidae) of Northern South America. Transactions of the American Entomological Society (Philadelphia), 116: 297618. [ Links ]
Kingsolver, J.M. 1970. A study of male genitalia in Bruchidae (Coleoptera). Proceedings of the Entomological Society of Washington, 72: 370–386. [ Links ]
Kingsolver, J.M. 1989. New World Bruchidae past, present, future, pp. 121129. In: Fujii, K., A.M.R. Gatehouse, C.D. Johnson, R. Mitchell, and T. Yoshida (Eds.). Bruchids and Legumes: Economics, Ecology and Coevolution. Proceedings of the Second International Symposium on Bruchids and Legumes (ISBL2) held at Okayama (Japan), September 69, 1989. Kluwer Academic Publishers, Dordrecht, Boston, London. [ Links ]
Kingsolver, J.M. & D.R. Whitehead. 1974. Classification and comparative biology of the seed beetle genus Caryedes Hummel (Coleoptera: Bruchidae). Transactions of the American Entomological Society (Philadelphia), 100: 341–436. [ Links ]
Martínez, José Luis. 2000. Nezahualcóyotl, vida y obra. Fondo de Cultura Económica, México. 9ªreimpresión. 334 pp. [ Links ]
Medina, Miguel A. 1997. Arte y Estética de El Tetzcotzinco: arquitectura de paisaje en la época de Nezahualcóyotl. UNAM. 217 pp. [ Links ]
O'Gorman, Edmundo. 1972. Fernando de Alva Ixtlilxochitl: Nezahualcóyotl Acolmiztli. Gobierno del Estado de México, México. 160 pp. [ Links ]
Pulido Ma. T. and S.D. Koch. 1988. Inventario florístico en el cerro Tetzcotzinco, Texcoco, Estado de México. Boletín de la Sociedad Botánica de México, 48: 81–94. [ Links ]
Romero, J., and C. D. Johnson. 1999. Zabrotes sylvestris, a new species from the United States and Mexico related to Z. subfasciatus (Boheman) (Coleoptera: Bruchidae: Amblycerinae). The Coleopterists Bulletin, 53: 87–98. [ Links ]
Romero N., J. 2002. Bruchidae. In: J. Llorente B. y J.J. Morrone (Eds.). Biodiversidad, Taxonomía y Biogeografía de Artrópodos de México, Hacia una síntesis de su conocimiento. Vol. III, UNAM, pp. 513–534. [ Links ]
Romero N., J. 2004. Tetzcotzinco, cuando las dalias florecen. Ciencia y Desarrollo, CONACYT 30(177): 17–21. [ Links ]
Saar, Dayle E., Neil Q. Polans, and Paul D. Sorensen. 2003. A Phylogenetic Analysis of the Genus Dahlia (Asteraceae) Based on Internal and External Transcribed Spacer regions of Nuclear Ribosomal DNA. Systematic Botany, 28: 627–639. [ Links ]
Sorensen, P.D. 1969. Revision of the genus Dahlia (Compositae, Helianthae, Coreopsidinae). Rhodora, 71: 309–352. [ Links ]
Whitely, G.L. 1985. The medical and nutritional properties of Dahlia spp. Journal of Ethnopharmacology, 14: 75–82. [ Links ]














