Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta zoológica mexicana
versão On-line ISSN 2448-8445versão impressa ISSN 0065-1737
Acta Zool. Mex vol.26 no.2 Xalapa Ago. 2010
Artículos originales
Burrow systems of Iranian jerboa (Allactaga firouzi Womochel, 1978)
Sistema de túneles del jerbo iraní ( Alloctaga firouzi Womochel, 1978)
Saeed MOHAMMADI1 *, Mohammad KABOLI2 **, Mahmoud KARAMI2 *** and Gholamreza NADERI3
1 Department of Environmental Sciences, Sciences & Research Branch, Islamic Azad University, Tehran, IRAN. E–mail: mohammadi7517@yahoo.com.
2 Department of Environmental, Faculty of Natural Resources, University of Tehran, Karaj, IRAN. E–mail: **mkaboli@ut.ac.ir, ***mkarami@ut.ac.ir
3 Department of Environmental Sciences, Sciences & Research Branch, Islamic Azad University, Tehran, IRAN, E–mail: ghnaderi@yahoo.com
*Corresponding author:
Saeed Mohammadi
Recibido: 22/09/2009
Aceptado: 22/03/2010
ABSTRACT
Iranian jerboa was recorded as a new species for Iran near village of Shah–Reza, Isfahan province. It is considered as a data deficient species according to IUCN criteria. Since, No data have been yet reported, on the relationship between architecture of burrows and the social organization of this species, this study aimed to identify the burrow systems of the species. We excavated 15 burrows of Iranian jerboa in the type locality of the species. The burrow system of Iranian jerboa is composed of three types including: temporary, summer and winter burrows. The length of tunnels were significantly different (P=0.00) in winter burrows. General burrow described for Small Five–toed jerboa Allactaga elater was similar with these burrows except having reproduction burrow. Results show that depth of nest chamber in third type of burrow was deeper than in temporary and summer (P=0.00, P=0.003 respectively).
Key words: Iranian jerboa, summer, winter and temporary burrow, Iran.
RESUMEN
El jerbo iraní fue registrado como nueva especie para Irán cerca del poblado de Shah–Rezam, provincia Isfahan, y de acuerdo con los criterios de la IUCN esta información es considerada como deficiente. Desde su descripción, ningún dato ha sido publicado sobre interrelaciones entre la arquitectura de sus túneles y la organización social de la especie. El presente estudio identifica el sistema de túneles de esta especie. El sistema de túneles del jerbo iraní está compuesto de tres tipos de túneles: temporal, de verano y de invierno. El análisis ANOVA mostró que la longitud media de los túneles es más grande en el invierno (P = 0.00). En general, los túneles descritos para el pequeño jerbo de cinco dedos Alloctaga elater fue parecido con los túneles de A. firouzi, excepto túnel de reproducción. Los resultados muestran que la profundidad de la cámara nido en el tercer tipo de túnel fue más profunda que los túneles temporal y de verano (P = 0.00, P =.003, respectivamente).
Palabras clave: jerbo iraní, datos deficientes, túneles temporal, de verano e invierno, Irán.
INTRODUCTION
Arid zone rodents are typically subject to harsh environmental condition which may impose severe physiological stress on them, affecting both their water–and thermo–regulation (Jackson 2000). Whilst many of these species show physiological adaptations to cope with these stresses, including a relatively low metabolic rate, a lower critical temperature and improved water retention (Buffenstein 1984, Buffenstein & Jarvis 1985, Buffenstein et al. 1985). Another adaptation may be to retreat into underground refugees that provides relatively stable microclimate and buffers outside condition (Du Plessis et al. 1992). Desert rodents typically escape from climatic extremes by retreating into the more moderate microclimate within their refuges. Underground refuges, with their relatively stable microclimate, provide protection for small animals from extreme temperatures on the surface (Shenbrot et al. 2002). A common refuge type is the underground burrow, which can vary in architecture from simple to complex structures (Hinze et al. 2006). In some cases these may take the form of nest chambers constructed under rocks (e.g. Dassie Rat Pettromys typicus and Namaqua Rock Mouse Aethomys namaquensis, Skinner & Smithers 1990), large and complex stick nests (e.g. Karoo Bush Rat Otomys unisulcatus, Du Plessis and Kerley 1990; Desert Wood Rat Neotoma lepida, Cameron and Rainey 1972), or burrow system (e.g. Short Tailed Gerbil Desmodillus auricularis, Nel 1967; Pouched Mouse Saccostomus campestris, Ellison 1993; Small Five–toed Jerboa Allactaga elater, Williams's Jerboa A. williamsi and Euphrates Jerboa A. euphratica Çolak & Yigit 1998; Mongolian Gerbil Meriones unguiculatus Scheibler et al. 2006). Simple burrows comprise of a single nest chamber and one or two entrance holes (Hinz et al. 2006); such as in the several gerbil Gerbillurus species (Downs and Perrin 1989). Complex burrow comprise of several aboveground entrance holes joined to many interconnected tunnels belowground (Brett 1991, Goyal & Ghosh 1993, Mankin & Getz 1994). These complex systems may contain one or more nesting, hoarding and nursery chambers, or a combination of these structures (Hoogland 1995, Khalidas & Hansell 1995). In addition to providing protection against predators and the weather extremes (Downs & Perrin 1989), burrow systems have other functions, such as raising offspring (e.g., Black–tailed Prairie Dog Cynomys ludovicianus; Hoogland 1995) and gaining access to high–quality feeding sites through numerous entrance holes (e.g., Brant's whistling Parotomys brantsii; Jackson 2001). The major advantage of burrow as cover is to buffer the rodent against environmental extremes of both temperature and humidity (Jackson 2000). Iranian jerboa A. firouzi was discovered from a single locality in a flat plain with a gravel substrate and sparse mountainous steppe vegetation (Womochel 1978). The population is restricted to an area near the village of Shah–Reza in Isfahan Province. This species is one of poorest known species of the genus Allactaga and jerboas in general. It belongs to Dipodidae family. Population of this species is estimated to be less than 250 individuals (Nowak 1999). We studied the burrow system of Iranian jerboa A. firouzi in order to gain some insight into the life of this species. The aim of the present study was to document the architecture and field characteristics of the burrow systems of this endemic species in which they spend most of their life.
MATERIAL AND METHODS
Study area
Research was conducted at 22 km south of Shah–Reza village in Isfahan Province, Iran, on an area of approximately 2200 ha (31°47'57"N, 52°01'51"E). The study area is an unprotected area and located approximately 1 Km from a main road (Shah–Reza to Abadeh) with heavy traffic and used for grazing and recreation activities. The area is semi–arid the, mean annual temperature is approximately 12°C ranging from 38 °C (in summer) to –17 °C (in winter). The mean annual precipitation is about 68 mm. Soil is hard–packed, gray, sandy which contains little organic matter with sparse vegetation dominated by Anabasis aphylla, a small herbaceous mixturing with Artemisia aucheri, A. herba–alba, Astragalus canadensis, Kochia dana. Average vegetation height is about 20–40 cm. In totally 70% of the ground is covered by herbaceous plants and no tree or shrub is found.
Data collection
Fifteen burrows of Iranian jerboa were excavated in the study area during July and Aug of 2008, Jan and Feb 2009. Burrows were carefully excavated by a spade and small shovel so as to maintain the original organization of tunnels and associated structure. We studied active burrows namely the burrows that jerboas entered it (Shenbrot et al. 2002). Data on length of tunnels, their junctions and chambers, maximum length of tunnels, depth of chambers below the ground height, length, width and position of chambers were recorded and mapped as well as the number of entrances for each system. Volume of ellipsoid chambers was calculated using the formula V = a × b2 × π × 1/6 and expressed in liters (Scheibler et al. 2006). All measurements were made using a coil meter to the nearest 0.5 cm. We limited number of excavated burrows to minimize disturbance to the studied population.
Data Analysis
Statistical analysis to determine significant mean differences among inter–substrate and surface differences in burrow systems including; depth of nest chamber, number of entrance holes and the length of tunnels. Data were tested for normality and homogeneity of variance using Kolmogorov–Smirnov and Shapiro–Wilk test respectively. Kruskal–Wallis tests were employed on non–normal data and ANOVA on normal data. Significance was measured as P<0.05. If significant differences were detected, multiple comparison tests (Tukey) was used. Analyses were performed using SPSS Package version 10 (Chicago, IL, USA).
RESULTS
Our observations in three burrow showed that the number of Iranian jerboa A. firouzi inhabiting a burrow system was one individual. The number of entrance holes in excavated burrows ranged from 1 to 9 per system. Furthermore, the number of tunnels varied between 2 to 15, depending on the burrow system type. General characteristics of burrow systems are depicted in Fig. 1. The simplest type was the temporary or escape burrow (Philps et al. 1997), which had the function of an escape burrow. These were burrows with one nest chamber and a few number (1–2) of entrance holes per burrow. The length of tunnels for this burrow system was short compared to the summer or winter burrows (Table. 1). Summer burrows contained one nest chamber with total length between one to three meters. Total length of each burrow system reach 4m, ending in a spherical nest chamber of 3.5 cm in diameter, and descending gradually to as deep as 23 cm without branching. There was no stored food at the nest chamber. Dry foliage and seeds of A. aphylla and A. aucheri were found only in tunnels. This suggests that tunnel material may periodically be cleaned out and replaced with fresh material. Winter burrows had a single nest chamber at various depths and the nest chambers had been dug deeper than those of summer and temporary burrows. Numbers of entrance holes were compared by Kruskal–Wallis test in three burrow system. There was a significant difference between winters with summer and temporary burrows (Table. 1).
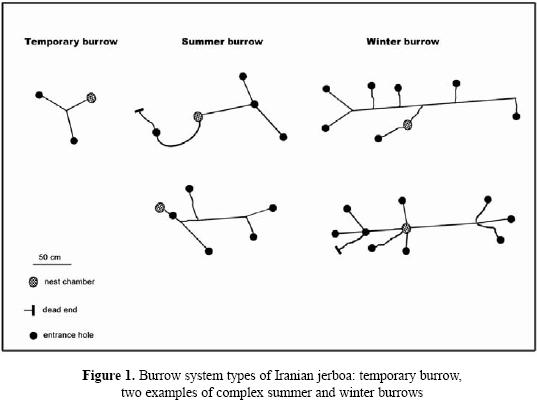
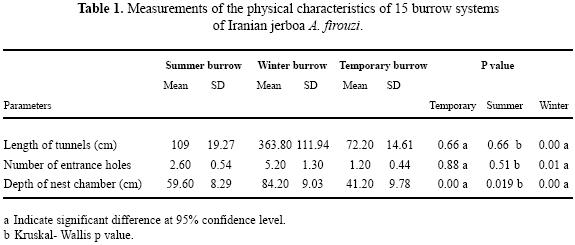
Mean length of three types burrow systems were statistically different. However, there was no difference between temporary and summer burrows (P=0.66). Results showed that depth of nest chamber in winter burrow is more than in temporary and summer (P=0.00, P=0.003 respectively). Also, there was a significant difference between summer and temporary burrow in depth of nest chamber (P=0.019).
The nest chamber without the stored food consists of dry plant material such as A. aphylla and A. aucheri. The size of nest chambers varied from a minimum size of 10 × 8 x 15 cm to maximum size of 17 × 14 × 15 cm. This is equivalent to 0.3 to 1.7 liters.
DISCUSSION
The burrow systems of Iranian jerboa A. firouzi are comprised of temporary, summer and winter types. The general burrow described by Çolak & Yigit (1998) for A. elater is similar with these burrows except having the reproduction burrow. Scheibler et al. 2006 recognized three types of burrow for M. unguiculatus including temporary, summer and winter burrow. The different usage of different burrow types and their advantages have been examined. Temporary burrow of A. firouzi has the function of an escape burrow with one nest chamber. It was similar to the type of temporary burrow system of A. elater (Çolak & Yigit 1998) but differed in its structure. They determined temporary burrow of A. elater with no marked nest chamber. Mankin & Getz (1994) concluded that escape burrow that is slightly smaller than nesting burrows, had numerous (2–9) entrance holes to allow for rapid entry or exit. Rongstad (1965) reported two hiding burrows for Thirteen–lined Ground Squirrel Spermophilus tridecmlineatus, one with a single entrance and the other with two entrances. Temporary burrows for M. unguiculatus were burrows without a chamber and a low number of entrance holes (Scheibler et al. 2006). No data have been reported, however, on the relationship between architecture of burrows and the social organization of A. firouzi. Summer burrow had a nesting chamber that similar to summer burrow of A. elater.
Çolak & Yigit (1998) determined two types of summer burrows for A. elater; one had a lateral passage leading to the surface other than the main gallery. The second was a burrow with a single exit. Three types were located on the open area with sparse vegetation. Winter burrow had a nest chamber. Burrow system in winter burrow had a larger surface area and were deeper. All of the three types of burrow of A. firouzi had a nest chamber without feeding chamber. Also determined that winter burrow of A. elater had a single nest chamber and without the stored food (Çolak & Yigit 1998), suggesting that in all three of burrow system, nest chambers are adequately buffered from above ground temperature conditions. We could not confirm which burrow types were used for reproduction. However since pregnant female were found in summer burrows we assume these are used for reproduction. In conclusion, the structure of burrows reflects the short hibernating time of this species and their burrow systems provide a suitable microhabitat in which to escape from adverse environmental conditions, particularly during summer. Buildings, roads and heavy traffic machines in the zone have become a critical environmental challenge and needs careful management efforts in order to conserve the habitat and burrow systems of this endemic species in Iran.
AKNOWLEDGEMENT
Our thanks to the Fakhri Mirabadi family for their hospitality, Mohammad Javidkar for his advice, Mahdi Mostafavi, Shahab Cheraghi and Dr. Mostafa Tarahomi for their assistance in the fieldworks, Zahra Yahaghi for drawing figures. We also appreciate to Dr. Pedro Reyes Castillo for his assistance during the preparation of the manuscript. The manuscript was much improved by two anonymous referees. Authors wishes to thank the Iran National Science Foundation (INSF) for their financial support.
LITERATURE CITED
Brett, R. A. 1991. The ecology of naked mole–rat colonies: burrowing, food, and limiting factors. In: The biology of the Naked Mole–rat. Ed. By P.W. Sherman, J. U. M. [ Links ]
Buffenstein, R. 1984. Energy and water balance during torpor and hydropenia in the Pygmy gerbil (Gerbillus pusillus). Journal of Comparative Physiology, 154B:535–544. [ Links ]
Buffenstein, R. & Jarvis, J. 1985. The effect of water stress on growth and renal performance of juvenile Namib rodents. Journal of Arid Environment, 9:232–236. [ Links ]
Buffenstein, R., Campbell, W.E. and Jarvis, J.U.M 1985. Identification of crystalline allantoin in the urine of African Cricetidae (Rodentia) and its role in their water economy. Journal of Comparative Physiology, 155B:493–499. [ Links ]
Cameron, G.N.and Rainey, D. G. 1972. Habitat utilization by Neotoma lepida in the Mohave desert. Journal of Mammalogy, 53:251–266. [ Links ]
Çolak, E & Yigit, N. 1998. Ecology and biology of Allactaga elater, Allactaga euphratica and Allactaga williamsi (Rodentia: Dipodidae) in Turkey. Turkish Journal of Zoology, 22:105–117. [ Links ]
Downs, C. T. & Perrin, M. R. 1989. An investigation of the macro– and micro–environments of four Gerbillus species. Cimbebasia, 11:41–54. [ Links ]
Du Plessis, A. & Kerley, G. I. H. 1990. Refuge strategies and habitat segregation in two sympatic rodents, (Otomys unisulcatus) and (Paratomys brantsii). Journal of Zoology, 224:1–10. [ Links ]
Du Plessis, A., Kerley, G.I.H. & Winter, P. E. D. 1992. Refuge microclimates of rodents: a surface nesting Otomys unisulcatus and a burrowing Paratomys brantsii. Acta Theriologica, 37: 351–358. [ Links ]
Ellison, G. T. H. 1993. Group size, burrow structure and hoarding activity of pouched mice (Saccostomus campestris: Cricetidae) in southern Africa. African Journal of Zoology, 31:135–155. [ Links ]
Goyal, S. P. and Ghosh, P. K 1993. Burrow structure of two gerbil species of Thar desert, India. Acta Theriologica, 38:453–456. [ Links ]
Hinz, A., Pillay, N. & Grab, S. 2006. The burrow of the African ice rat (Otomys sloggetti robertsi). Mammalian Biology, 71:356–365. [ Links ]
Hoogland, J. L. 1995. The Black–tailed Prairie Dog: social life of a burrowing mammal. Chicago: University of Chicago Press. [ Links ]
Jackson, T. P. 2000. Adaptation to living in an open arid environment: Leassons from the burrow structure of the two southern African whisling rats, Paratomys brantsii and P. littleedalei. Journal of Arid Environment, 46:345–355. [ Links ]
Jackson, T. P. 2001. Factors influencing food collection behavior of Brant's whilstling rat (Parotomys brantsii): a central place forager. Journal of Zoology (London), 255:15–23. [ Links ]
Khalidas, K. & Hansell, M. H. 1995. Burrowing behaviour and burrow architecture in Apodemus sylvaticus (Rodentia). Zeitschrift fur Säugetierkunde, 60:246–250. [ Links ]
Mankin, P. C. & Getz, L. L. 1994. Burrow morphology as related to social organization of Micotus ochrogaster. Journal of Mammalogy, 75: 492–499. [ Links ]
Nel, J. A. J. 1967. Burrow systems of Desmodilus auricularis in the Kalahari Gemsbok National Park. Koedoe, 10:118–121. [ Links ]
Nowak, R. M (Ed.). 1999. Walkers Mammals of the World. Sixth edition. The Johns Hopkins University Press, Baltimore and London. [ Links ]
Phillips, J., Kearney, T., Pillay, N. & Willan, K 1997. Reproduction and postnatal development in the Angoni vlei rat (Otomys angoniensis) (Rodentia, Muridae). Mammalia, 61:219–229. [ Links ]
Rongstad, O. J. 1965. A life history study of thirteen–lined ground squirrels in southern Wisconsin. Journal of Mammalogy, 46:76–87. [ Links ]
Scheibler, E., Liu, W., Weinandy, R. & Gattermann, R. 2006. Burrow systems of the Mongolian gerbil (Meriones unguiculatus Milne Edwards, 1867). Mammalian Biology, 71:178–182. [ Links ]
Skinner, J. D. & Smithers, R. H. N. 1990. The mammals of the African subregion. University of Pretoria, Pretoria, South Africa. [ Links ]
Shenbrot, G. I., Krasnov, B., Khokhlova, I., Demidova, T. & Fielden, L. 2002. Habitat–dependent differences in architecture and microclimate of the burrows of Sundevall's jird (Meriones crassus) (Rodentia: Gerbillinae) in the Negev Desert, Israel. Journal of Arid Environment, 51: 265–279. [ Links ]
Womochel, D. R. 1978. Anew species of Allactaga (Rodentia: Dipodidae) from Iran. Journal of Fieldiana Zoology, 72:65–73. [ Links ]














