Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex vol.26 no.1 Xalapa abr. 2010
Artículos originales
Using population viability analysis for management recommendations of the endangered endemic peninsular pronghorn
Recomendaciones de manejo usando análisis de viabilidad poblacional del berrendo peninsular endémico
Jorge CANCINO1 *, Ricardo RODRÍGUEZ–ESTRELLA1 and Philip MILLER2
1 Centro de Investigaciones Biológicas del Noroeste, S. C. Mar Bermejo 195. Col. Playa Palo de Santa Rita. La Paz, Baja California Sur. 23090, MÉXICO. E–mail: jcancino04@cibnor.mx.
2 Conservation Breeding Specialist Group (SSC/IUCN) 12101 Johnny Cake Ridge Road. Apple Valley, Minnesota, EUA, 55124–8151, US. E–mail: pmiller@cbsg.org
*Autor para correspondencia
Recibido: 16/06/2009
Aceptado: 17/02/2010
ABSTRACT
A case study on the viability of small populations with a restricted distribution and reduction in habitat quality is addressed using the peninsular pronghorn (Antilocapra americana peninsularis) of Baja California Peninsula, Mexico. The present size of its wild population is less than 250 individuals, being in an IUCN "Critically Endangered" status. Captive management of peninsular pronghorn began in 1998 in El Vizcaino Desert with 22 founders. We predicted future trends in the pronghorn population, and assessed the risk of extinction through population viability analysis (PVA) using VORTEX. Deterministic and stochastic factors designed to simulate human activity on the landscape were evaluated for their impact on this endemic taxon. The concept of "supportive breeding" was assessed. The results of PVA simulations indicate that removal of founder animals to initiate the captive breeding did not significantly reduce the viability of the wild population. However, a population size <100 individuals greatly increase the risk of extinction. Also, one of the most important factors for the viability of the peninsular pronghorn population is the survival of fawns. The risk of extinction can be significantly reduced using "supportive breeding". We propose that the likelihood of successful population management of peninsular pronghorn could be increased establishing a number of subpopulations across the species' historic range and, even more importantly, the establishment of ecologically functional connections between these subpopulations to create a proper metapopulation. Captive breeding can be an important factor to decrease the probability of extinction of this subspecies.
Key Words: Antilocapra americana, endangered, management, PVA, recovery.
RESUMEN
Se evaluó el riesgo de extinción de la población del berrendo peninsular (Antilocapra americana peninsularis) en la península de Baja California, México que presenta distribución restringida y deterioro en la calidad de su hábitat. En el año 2000, el tamaño de la población silvestre del berrendo peninsular era menor a 250 individuos. Se desarrolló un análisis de viabilidad de poblaciones (PVA) usando VORTEX, que incluyó variables determinísticas y estocásticas, e información de 25 años para proyectar cambios en la población, y evaluar su riesgo de extinción. Con el PVA se evaluó el concepto de "Reproducción de Apoyo". En 1998 se inició el manejo en cautiverio del berrendo peninsular con 22 animales fundadores. Los modelos sugirieron que una población de menos de 100 individuos incrementa considerablemente el riesgo de extinción, siendo uno de los factores más importantes para la viabilidad de la población la sobrevivencia de las crías. Se propone que el éxito del manejo de la población del berrendo peninsular puede incrementarse estableciendo subpoblaciones dentro de su rango histórico de distribución con una perspectiva metapoblacional donde se conecten las subpoblaciones. Y se concluye que la reproducción en cautiverio puede ser un factor importante para disminuir la probabilidad de extinción de esta subespecie.
Palabras clave: Antilocapra americana, amenazado, manejo, PVA, recuperación.
INTRODUCTION
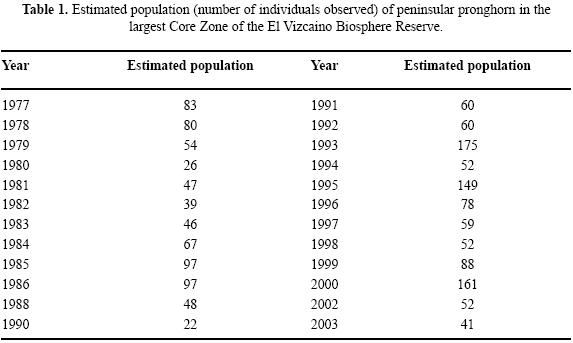
The peninsular pronghorn (Antilocapra americana peninsularis) is an endemic ungulate with a significant risk of extinction that is recognized both nationally (NOM–ECOL–059 2001), and internationally (IUCN 2003). This taxon has a very narrow distribution in the middle portion of Mexico's Baja California peninsula, located within an area of approximately 500 000 ha (Fig. 1). In 2000, the size of the wild population was estimated to be less than 250 individuals, with most animals living in the natural protected area known as the El Vizcaino Biosphere Reserve (SEDUE 1988). This Reserve was officially decreed in 1988 and, although a few herds and some individuals have been recorded exploring outside the Reserve, it contains nearly the entire peninsular pronghorn population. A portion of this population is being managed in captivity (Cancino et al. 2005). Estimates of population size within the core area of the Reserve have fluctuated considerably over the past 30 years, ranging from just 20 animals to nearly 200 (Table 1).
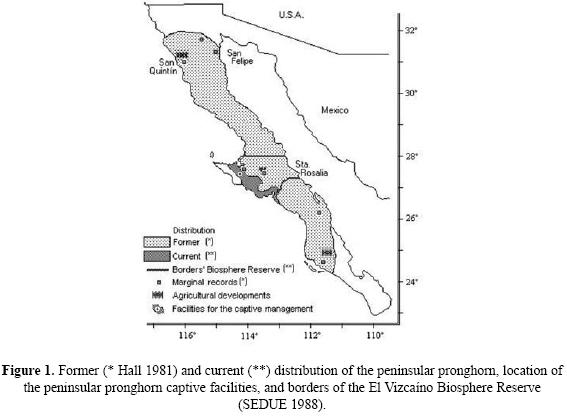
The low number of individuals and restricted distribution of this population, in combination with human pressures like habitat modification and poaching, make this subspecies susceptible to extinction in the short term, as has been found for other species with these characteristics (e.g. Apps & McLellan 2006). Because of this critical conservation status, a Peninsular Pronghorn Recovery Plan was established by the Mexican government in 1983. Nevertheless, the viability of this population should be urgently evaluated in order to design more effective conservation strategies. Towards that end, the El Vizcaino Biosphere Reserve initiated actions from the Recovery Plan in 1994, first organizing a workshop with the objectives of evaluating the condition of the peninsular pronghorn habitat and assessing the status of the population. These activities would constitute significant steps in updating the species' Recovery Plan. The 1994 workshop was conducted under the guidance of the Conservation Breeding Specialist Group, of the IUCN – World Conservation Union's Species Survival Commission, in a format known as the Population and Habitat Viability Assessment (PHVA) process (Cancino et al. 1995; Westley & Miller 2003). This process is thoughtfully designed with the specific goals of bringing together conservation scientists and practitioners, along with their information, in order to create scientifically rigorous practical recommendations for managing endangered species. Much of the rigor derives from computer simulation of alternative future management scenarios and their efficacy of reducing extinction risk – a technique known broadly as Population Viability Analysis or PVA (Soulé 1987; Beissinger & McCullough 2002). The models serve as a tool to make better decisions in the management. An advantage is to simulate scenarios and the impact of the variables on the studied population. This process had been used with many species; Miller & Lacy (2005) cited more than 150 cases. The advantages and disadvantages of using PVA models when comparing with subjective judgment or between PVA's have already been discussed elsewhere (Brook et al. 1999; Coulson et al. 2001, Croos & Beisinger 2001; McCarthy et al. 2004).
In 1998, after reviewing the status of the peninsular pronghorn and considering its continued high risk of extinction, in part because of drought and poaching (Cancino et al. 2005), the managers of the Reserve decided to capture a number of wild animals and start a captive management program based on studies as that of Rodríguez–Clark & Sánchez–Mercado (2006) for Andean bears (Tremarctos ornatus). Harvest of the animals was made without the support of a tool such as PVA. A new species assessment was conducted in 2004, using demographic and ecological data through 2003. The resulting simulation model considered only the size of the wild population prior to the captures used to initiate the captive population (Cancino et al. 2005). Here, we present the results of a PVA considering: a) the current status of the wild population, b) the a posteriori analysis and effects on the wild population of extraction of individuals to begin the captive program, and c) the feasibility of establishing a "new" subpopulation in the Biosphere Reserve considering the potential release of captive animals. This release is not yet a case of supportive breeding as described by Ryman et al. (1994) because the release will likely be made into an "artificial island". This is also not a marooning case (Wilson & Stanley 1994) because this zone was part of the original range of the species. Thus, conditions defining this potential release of adults and young during captive management are planned mainly to avoid predation and to provide an adequate food supply. Supportive breeding is planned for future releases: some animals produced in captivity would reinforce an existing wild population (Ryman et al. 1994). This practice has being successfully used in different taxa (e.g. Brightsmith et al. 2005). In this PVA, we first wanted to determine the intrinsic and extrinsic factors that influence peninsular pronghorn population dynamics. Second, we wanted to evaluate the effects of the extraction of the 22 animals from the wild population in order to establish the captive herd. Third, we wanted to evaluate the effects of increasing the wild population size with individuals produced by the captive herd. Lastly, we wanted to evaluate the effects of taking animals from the new "semi–wild" pronghorn population for future use in reintroduction programs in different areas of the Baja California peninsula where the subspecies has disappeared.
MATERIAL AND METHODS
Population Viability Analysis (PVA) has been developed to assess extinction risk and to compare management options. PVA is a methodology for predicting the future fate of wildlife populations based on demographic, environmental and genetic parameters, most commonly with the use of computer simulation (Brook et al. 1999). A Population Viability Analysis requires information on the demography, ecology and habitat requirements of a species (Beissinger & McCullough 2002; Miller & Lacy 2003). More accurate information on these parameters will permit researchers to more realistically simulate alternative future population scenarios (Durant & Mace 1994; Brook et al. 2000; Ellner et al. 2002). Most information on the peninsular pronghorn ecology and biology is known from several studies in the area (Cancino et al. 1995; Cancino 2003; Cancino et al. 2005). However, in cases where data were absent we used the abundant literature on the biology and ecology of the pronghorn species (O'Gara 1978; Lee et al. 1998; O'Gara & Yoakum 2004). In particular, we used particular useful data from surrogate subspecies present in similar habitat (Hosack et al. 2002; Bright & Hervert 2005). We also convened a meeting of pronghorn experts from USA and Mexico to use a Delphi method to determine some values of the variables required (Table 2). The meeting was done on April, 2004 at La Paz, Baja California Sur, Mexico. Three main sets of analyses were performed to: a) Assess the probability of survival for the wild source population in the Biosphere Reserve; b) Assess the probability of survival for the wild source population given the "harvest" of 16 fawns and six wild adults to establish a captive herd; and c) Evaluate the likelihood of successfully establishing a new wild population derived from the captive herd under different management possibilities.
For simulations and analyses of the PVA we used VORTEX Version 9.45 (Lacy et al. 2003; software available at http://www.vortex9.org). This population dynamics model is designed specifically for stochastic simulation of the extinction process in small wildlife populations. VORTEX incorporate age (or stage) structure, demographic and environmental stochasticity, density dependence, inbreeding depression, systemic pressures such as habitat decline, catastrophic events and metapopulation structure. The baseline input parameters for our analyses were as follows:
Breeding System: Pronghorn are polygynous species. The social system in the reproductive season can be territorial or harem breeding. We did not consider the potential multiple paternity in this subspecies as Carling et al. (2003) found for the species.
Age of First Reproduction: VORTEX considers the age of first reproduction as the age of the first parturition, not simply the onset of sexual maturity. Female pronghorn can be pregnant as early as seven months (Mitchel 1967) but the most common age is in the second year (Lee et al. 1998). As a gregarious species, young males are often excluded so we therefore used data from Hosack et al. (2002) to establish age of first breeding in males at five years of age.
Age of Reproductive Senescence: In its simplest form, VORTEX assumes that animals can reproduce at the normal rate throughout their adult life. A reproductive female of 16 years old was recorded in the Minnesota Zoo (T. Hill pers. comm.). However, as we do not have real data on senescence in the wild population, we set it as 10 years, assuming that the rigors of life in the wild would reduce the longevity of individual adult animals.
Offspring Production: Production of triplets has been documented in the literature (O'Gara 1978). However, we assumed that most females (90%) would produce two fawns and the remainder would successfully bear only one offspring. This choice of parameter values is based on the low probability of survival of at least one individual in triplet births. The sex ratio at birth was set at 1:1 (Zimmer & Lindzey 2002).
Male Breeding Pool: There are species in which some adult males may be socially restricted from breeding despite being physiologically capable. This is the case for the pronghorn. This can be modeled in VORTEX by specifying a portion of the total pool of adult males that may be considered "available" for breeding each year. We used 40% in our baseline for the wild population analysis, and 100% for the simulations involving population re–establishment because the plan includes only one male.
Mortality: Detailed estimates of age–sex–specific mortality rates do not yet exist for wild populations of peninsular pronghorn in the Biosphere Reserve. Consequently, we were forced to use data from other subspecies and expert opinion to guide our choice of model parameters. Fawn survival among pronghorn populations in North America can be heavily influenced by predation, primarily by such predators as coyotes (Canis latrans) and bobcats (Felis rufus). For example, fawn mortality due to coyote predation in Alberta, Canada was nearly 50% (Barrett 1984). This level of mortality was in an area of relatively low coyote density, estimated conservatively at one per 13 km2. Research in the Vizcaino Desert suggests that coyote densities may be much higher. In addition, data from American pronghorn populations in zoos indicate juvenile mortalities can approach 50%. Taken together, we chose peninsular pronghorn fawn annual mortality to be 70%. No specific data are available for adult mortality of peninsular pronghorn, but data from Sonoran pronghorn populations indicate that adult mortality is about 10% for females and perhaps slightly greater for males as they compete for breeding opportunities.
Catastrophe: VORTEX can consider one or more catastrophes with different characteristics: type, dimension, severity, and frequency. Considering the Sonoran experience (Hosack et al. 2002; Bright & Hervert. 2005) we included a simulated severe five year drought event that impacts the reproductive output of adult females. We assume that this type of event occurs on average once approximately every 15 years, thereby giving an annual probability of occurrence of 0.066. This was simulated through the use of a complex function to define the percent of adult females breeding in a given year. If a drought is called at any point in the simulation, the percentage of adult females that are expected to breed is decreased cumulatively by 10% each year for five years. Therefore, if 95% of adult females breed in the absence of a drought, only 45% are expected to breed by the end of a five–year drought event.
Inbreeding Depression: Specific data on the presence and/or severity of inbreeding depression in peninsular pronghorn do not yet exist. However, we suspect that inbreeding could lead to additional mortality, perhaps concentrated among fawns, as has been described in the broad population genetics literature. In its simplest form, VORTEX simulates inbreeding depression through the reduction of juvenile survival as a function of an individual's inbreeding coefficient. The severity of inbreeding depression in mammal populations can be measured as the number of "lethal equivalents" contained in the genome of the population of interest. Data for some captive ungulate species suggests that these species harbor about three lethal equivalents, a value very close to the median value of 3.14 obtained in a larger dataset of 40 captive mammalian species analyzed by Ralls et al. (1988). Consequently, we modeled inbreeding depression using this median lethal equivalent value.
Initial Population Size: Census size estimates for the extant peninsular pronghorn population in the Vizcaino Desert refer only to the core zone of the Reserve. Because of the uncertainty in these estimates (Cancino et al. 1995) we present the results of seven different initial size estimates for our simulated peninsular pronghorn population. For the analysis of population size on persistence probabilities we studied N0 = 50, 100, 125, 200, 250, and 300 individuals. Our first baseline model had an initial population size of 150 individuals with a stable age structure calculated from the life table.
Carrying Capacity (K): This is used to define the upper limit for the population size. Above this limit, additional mortality is imposed randomly across all age classes in order to return the population to the value set for K. The estimation of carrying capacity is quite difficult (Dhondt 1988) and has not yet been formally assessed for the Biosphere Reserve population of peninsular pronghorn. Based in part on past observations of pronghorn census estimates in and around the Reserve, we set our baseline value of K at 500 individuals.
Population Augmentation and Harvest: An important issue for the recovery and management of the peninsular pronghorn is the feasibility of increasing the size of the original Reserve population or perhaps to produce one or more new populations in situ. In our case the meaning of in situ management is to develop a reintroduction program within the species' original range in order to increase the number of subpopulations (Bretagnolle et al. 2004). To address this issue, we developed a set of scenarios that included the potential release of some animals to the wild population which was the original source. The current plan is to release a group of 18 females with one male into an "artificial island" (Biosphere Reserve "El Vizcaino" R. Castellanos pers. comm.). Therefore, the simulations included this "potential" release to assess some management practices including one additional model with some extractions after the fifth year. There is no legal hunting of peninsular pronghorn but poaching is suspected. Also, if a plan of reintroduction through the former peninsular pronghorn habitat is considered, it is important to simulate the effects of extractions from the "new population". With time and several reintroductions, we consider feasible to create a metapopulation dynamics into the Vizcaino region and adjacent areas.
Iterations and Years of Projection: All population projections (scenarios) were simulated 500 times for 50 years. Each projection has demographic information obtained at annual intervals.
Although simulations produce a suit of data sometimes with a wide variation within a range, we obtained from VORTEX the average value of predictions for each parameter in order to present the trends of population growth. Table 2 summarizes the baseline input dataset upon which all subsequent VORTEX models are based for the wild and the new population. Table 3 shows the sources for each parameter used in the simulation process.
RESULTS
We will not present the full suite of models that represent all combinations of the different parameter values that were run, but instead will focus on only the key analyses of viability for the original wild population and for the potential new population, according to the way in which this population was initiated. We focused only on those parameters that may be crucial to the permanence of the species and its management.
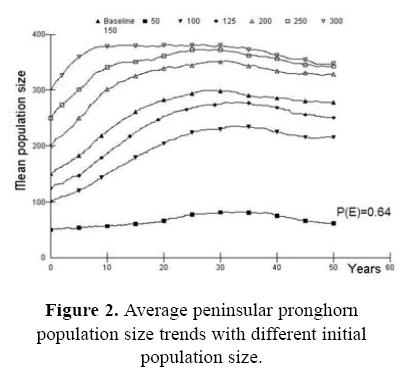
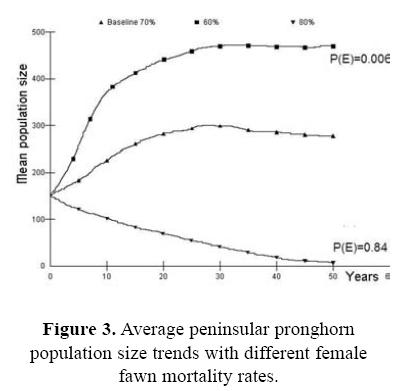
For the first case, assessing the probability of survival for the wild source population in the Biosphere Reserve as part of the risk analysis, we present the effect of the initial population size (Fig. 2). If the initial population size is <100 animals (Nt = 50) the population do no grow up, the probability of extinction is high P(E) = 0.642, and genetic issues become pronounced. Fawn mortality was also a very important parameter in determining overall pronghorn population dynamics in our simulations. With small changes in the baseline values the trend in stochastic population growth changed in a very significant way (Fig. 3). When fawn mortality was reduced from the baseline value of 70% to just 60%, the stochastic population growth rate increased to 0.097 with a very low extinction risk [P(E) = 0.006]. On the other hand, if mortality increased to 80% the stochastic population growth rate became strongly negative (r = –0.044) and the risk of population extinction is high [P(E) = 0.842]. Similarly, our models showed strong sensitivity to changes in pronghorn reproductive rates (Fig. 4). In our analysis, the impact of variability in adult mortality depended strongly on sex: perturbations in adult male mortality produced almost imperceptible changes in population dynamic behavior, while identical changes in female mortality caused quite strong impacts (Fig. 6). Probabilities of extinction were P(E) = 0.010 and P(E) = 0.572, for the 5 and 15% of female mortality, and P(E) = 0.090 and P(E) = 0.124 for the 10 and 20% of male mortality.
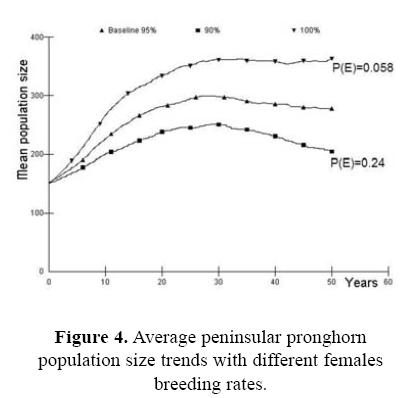
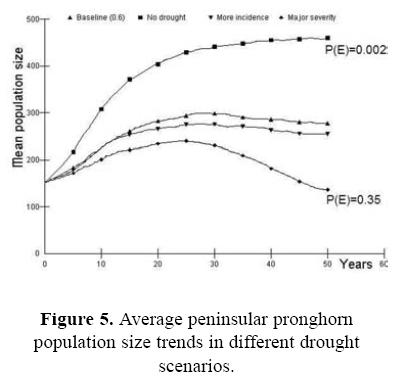
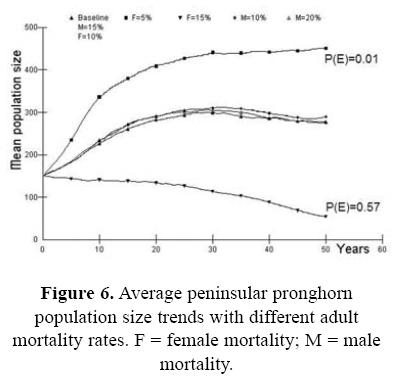
As part of our overall sensitivity analysis, the effect of drought was evaluated as a function in the reproductive rate among breeding females, with two levels of severity. In addition, we changed the carrying capacity as an effect of the drought and vice versa (Fig. 5). The increment in the mortality of breeding females is important when it is combined with the initial population size and the presence of inbreeding (Fig. 6). The probabilities of extinction are from P(E) = 0.25 to P(E) = 0.96 in the extreme values. It seems that factors act synergistically in severe conditions.
For the second case, assessing the probability of survival for the wild source population given the "harvest" of 16 fawns and six wild adults to establish a captive herd, and after analyzing the VORTEX simulations, it seems that there was not a relevant negative effect of that "harvest". It seems also that the collect of the 16 fawns did not significantly change the population growth after some years later.
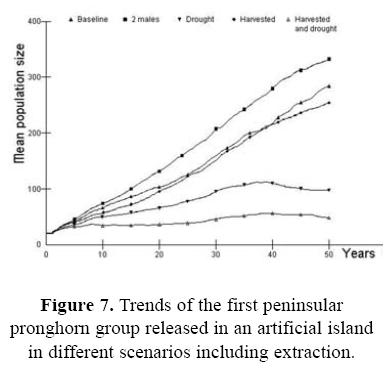
For the third case, to evaluate the likelihood of successfully establishing a new wild pronghorn population on an artificial island, Figure 7 shows four scenarios designed to investigate this proposed program under different associated conditions. First, it should be considered to release at least two males, in order to increase the population size higher and faster. Second, two of these scenarios include potential extraction. In the presence of extraction under captive management, there is no additional increase in the probability of extinction for this newly–established population.
DISCUSSION
Respect to the population size analysis (Fig. 2), if the initial population size is <100 animals (Nt = 50) the population do not grow up and the probability of extinction is high P(E) = 0.642. Hosack et al. (2002) obtained a similar response in their scenarios with a small initial population size (N = 100) with the Sonoran pronghorn. The probability of extinction of the population increased to 12% within 50 years. The recommendation would be that, if the initial population size is probably one of the most important parameters, it is important to obtain more precise estimates of initial population size with the best available technology, perhaps including conventional radio or satellite telemetry (Song 1996; Ticer et al. 1999; Bright et al. 2001). It is also important to maintain the Peninsular pronghorn population size above 200 individuals in order to keep the risk of extinction as low as possible according to our simulations (Fig. 2).
Another factor, fawn mortality was one of the most important parameters in the demographic scenarios for the Peninsular pronghorn. It is important to note that similar findings on the importance of fawn mortality on pronghorn numbers was found for both Peninsular and Sonoran pronghorns populations (Hosack et al. 2002). The rate of fawn mortality could be modified through different actions. Keeping a captive herd, the predator control access to the captive population could be an effective action to control fawn mortality (Cancino et al. 2005). Also fawn mortality could be decreased by increasing food supply. These factors should be considered in planning the methods by which the first group of individuals is to be released in order to reinforce the wild population. Rosemarino (2001) and Robinson et al. (2002) documented the importance of fawn predation in the population dynamics of other ungulate species. The results of our simulations using the removal of 22 individuals (founders) from the original population apparently did not show relevant effects that could increase the probability of extinction of the wild population (Fig. 3). Although it seems that this management practice did not apparently affect the previous peninsular pronghorn population status, caution should be taken when new potential extractions of the wild population would be planned. In the future when extractions of fawns would be considered, harvest should be analyzed independent of fawn natural mortality. It means that harvest of fawns should be analyzed in simulations as an additive factor in mortality rates of this population parameter instead of as included in the natural mortality parameter. We were unable to perform these analyses independently because official extractions were done without previous simulation analysis.
After some years of the Vizcaino Desert experience, and their simulations, Hosack et al. (2002), started to suggest "the establishment of a population in a secure environment (perhaps captive or semi–captive in a fenced area)…" in order to increase the numbers of Sonoran pronghorn, and then Krausman et al. (2005) pointed out the collaborative effort to replicate the Mexican experience. Thus, we believe that for this objective the captive management has been a successful practice.
Mortality in different ages and sex is important. Studies in other species have assessed the effects of mortality reducing the reproductive rate and considered that its importance is similar to other factors such as emigration (Albon et al. 2000; Clutton & Coulson 2002; DelGiudice et al. 2002). The management of the wild Peninsular pronghorn population should consider control mortality in order to maintain the survival of the taxon.
Regarding the effect of drought, some studies assessed its impact on the carrying capacity for other ungulates as the mule deer, white–tailed deer and bison (Wakelin 2001; Hudson & Jeon 2003; and Sweitzer et al. 2003, respectively). All of these studies concluded that the first effect of drought is on the habitat, defined as changes in food availability, and secondarily as a direct impact in the population itself. Management actions for the Peninsular pronghorn should consider to increase food availability during droughts.
As part of the captive management strategy for this population, it was considered a release of an initial group of pronghorns onto an artificial island. We propose that the likelihood of successful population management of peninsular pronghorn could be increased through an increase in the number of subpopulations across the species' historic range and, even more importantly, the establishment of ecologically functional connections between these subpopulations to create a proper metapopulation. We propose to consider an analogous way to that of van Aarde & Jackson (2007) for the elephant (Loxodonta africana), and Hellgren et al. (2005) for the black bear (Ursus americanus). Animals to be used for such a strategy would ideally come from both multiple captive facilities as well as from other newly–repopulated areas. Caution should be taken when considering the use of captive individuals for release in the wild. Care should be taken of not impronting captive animals to be released in the wild. In fact, the program for the management of the peninsular pronghorn in captivity was planned taking into account that the new generation produced after the second generation had a behavior similar to wild animals.
It is urgent to determine the genetic structure of the current captive population. This will avoid problems of inbreeding and will let to select the best individuals for the proposed reintroduction. We also recommend that a continuous monitoring of released animals should be implemented to record survival and behavioral changes (White et al. 2003, Steury & Murray 2004, Molony et al. 2006). Wild population management strategies that are supported by a captive breeding program and release should be considered with caution, due in large part to their high financial costs and the significant efforts required for their success (Mathews et al. 2005).
ACKNOWLEDGEMENTS
The authors thank the participants of the PHVA workshop held in La Paz, Baja California Sur, April 1–4, 2004, and in particular those of the El Vizcaino Biosphere Reserve. Important support for the captive program comes from Ford Motor Co., Espacios Naturales y Desarrollo Sustentable (a Mexican NGO). Los Angeles Zoo and CIBNOR are also involved in this recovery project. Comments of two anonymous reviewers substantially improved the clearness and organization of the paper.
LITERATURE CITED
Albon, S.D., T.N. Coulson, D. Brown, F. E. Guinness, J. M. Pemberton & T. H. Clutton–Brock. 2000. Temporal changes in key factors and key age groups influencing the population dynamics of female red deer. Journal of Animal Ecology, 69:1099–1110. [ Links ]
Apps, C.D. & B.N. McLellan. 2006. Factors influencing the dispersion and fragmentation of endangered mountain caribou populations. Biological Conservation, 130:84–97. [ Links ]
Barrett, M.W. 1984. Movements, habitat use, and predation on pronghorn fawns in Alberta. Journal of Wildlife Management, 48:542–550. [ Links ]
Beissinger, S.R. & D.R. McCullough (Eds). 2002. Population viability analysis. The University of Chicago Press. Chicago and London. [ Links ]
Bretagnolle, V., P. Inchausti, J.F. Seguin & J.–C. Thibault. 2004. Evaluation of the extinction risk and of conservation alternatives for a very small insular population: the bearded vulture Gypaetus barbatus in Corsica. Biological Conservation, 120:19–30. [ Links ]
Bright, J.L. & J.J. Hervert. 2005. Adult and fawn mortality of Sonoran pronghorn. Wildlife Society Bulletin, 33:43–50. [ Links ]
Bright, J., J. Hervert, L. Piest, M. Brown, & R. Henry. 2001. Sonoran pronghorn home ranges and habitat use. Pp. 112. In: Cancino, J. (Ed.). Proceedings of the Nineteenth Biennial Pronghorn Antelope Workshop. La Paz, Baja California Sur, Mexico. 2000. La Paz, Baja California Sur. [ Links ]
Brightsmith, D., J. Hilburn, A. Campo, J. Boyd, M. Frisius, R. Frisius, D. Janik, & F. Guillen. 2005. The use of hand–raised psittacines for reintroduction: a case study of scarlet macaws (Ara macao) in Peru and Costa Rica. Biological Conservation, 121:465–472. [ Links ]
Brook, B.W., J.R. Cannon, R.C. Lacy, C. Mirande, & R. Frankham. 1999. A comparison of the population viability analysis packages GAPPS, INMAT, RAMAS and VORTEX for the Whooping Crane (Grus americana). Animal Conservation, 2:23–31. [ Links ]
Brook, B.W., J. O'Grady, A.P. Chapman, M. A. Burgman, H. R. Akçakaya, & R. Frankham. 2000. Predictive accuracy of population viability analysis in conservation biology. Nature, 404:385–387. [ Links ]
Bustamante, J. 1996. Population viability analysis of captive and released bearded vulture populations. Conservation Biology, 10:822–831. [ Links ]
Cancino, J., P. Miller, J. Bernal Stoopen, & J. Lewis (Eds). 1995. Population and habitat viability assessment for the peninsular pronghorn (Antilocapra americana peninsularis) International Union for Conservation of Nature. Species Survival Commision. Conservation Breeding Specialist Group, Apple Valley, Minnesota, U.S.A. [ Links ]
Cancino, J. (Ed). 2003. Libro preparativo para el Taller de Evaluación del Plan de Recuperación del Berrendo Peninsular, PHVA. INE–CIBNOR. La Paz, B. C. S., México. [ Links ]
Cancino, J., V. Sánchez, & R. Castellanos. 2005. Capture, hand raising, and captive management of peninsular pronghorn. Wildlife Society Bulletin, 33:61–65. [ Links ]
Carling, M.D., P.A. Wiseman & J.A. Byers. 2003. Microsatellite analysis revels multiple paternity in a population of wild pronghorn antelope (Antilocapra americana). Journal of Mammalogy, 84:1237–1243. [ Links ]
Clutton, T.H. & T. Coulson. 2002. Comparative ungulate dynamics: the devil is in the detail. Philosophical Transactions of the Royal Society: Biological Sciences, 357:1285–1298. [ Links ]
Coulson, T., G. Mace, E. Hudson, & H. Possingham. 2001. The use and abuse of population viability analysis. Trends in Ecology and Evolution, 16:219–221. [ Links ]
Cross, P.C. & S.R. Beissinger. 2001. Using Logistic Regression to Analyze the Sensitivity of PVA Models: A Comparison of Methods Based on African Wild Dog Models. Conservation Biology, 15:1335–1346. [ Links ]
DelGiudice, G., M. Riggs, P. Joly, & W. Pan. 2002. Winter severity, survival, and cause–specific mortality of female white–tailed deer in North–Central Minnesota. Journal of Wildlife Management, 66:698–717. [ Links ]
Dhondt, A.A. 1988. Carrying capacity: a confusing concept. Acta OEcologica, 9:337–346. [ Links ]
Durant, S.M. & G.M. Mace. 1994. Species differences and population structure in population viability analysis. Pp. 67–91 In: Olney, P. J. S., G. M. Mace, and A. T. C. Feistner (Eds.). Creative conservation. Interactive management of wild and captive animals. Chapman and Hall. London, UK. [ Links ]
Ellner, S.P., J. Fieberg, D. Ludwig, & C. Wilcox. 2002. Precision of Population Viability Analysis. Conservation Biology, 16:258–261. [ Links ]
Hall, E. R. 1981. The mammals of North America. Vol. II. Second edition. John Wiley and Sons. New York. [ Links ]
Harwood, J. 2000. Risk assessment and decision analysis in conservation. Biological Conservation, 95:219–226. [ Links ]
Hellgren, E.C., D.P. Onorato & J.R. Skiles. 2005. Dynamics of a black bear population within a desert metapopulation. Biological Conservation, 122:131–140. [ Links ]
Hosack, D. 1997. Population Viability Analysis Workshop for Sonoran Pronghorn. Pronghorn News, 1:2. [ Links ]
Hosack, D.A., P.S. Miller, J.J. Hervert, & R. C. Lacy. 2002. A population viability analysis for the endangered sonoran pronghorn, Antilocapra americana peninsularis. Mammalia, 66:207–229. [ Links ]
Hudson, R.J. & B.T. Jeon. 2003. Nutrition of farmed deer: Lessons from the wild. http://www.deer.rr.ualberta.ca/library/lessonwild/quebec.htm (29 July 007). [ Links ]
IUCN. 2003. IUCN Red List of Threatened Species. www.redlist.org (29 July 2007). [ Links ]
Krausman, P.R., J.R. Morgart, L.K. Harris, C. S. O'Brien, J. W. Cain III & S. S. Rosenstock. 2005. Introduction: management for the survival of Sonoran pronghorn in the United States. Wildlife Society Bulletin, 33:5–7. [ Links ]
Lacy, R.C. 1993/1994. What is population (and habitat) viability analysis? Primate Conservation. 14/15:27–33. [ Links ]
Lacy, R., M. Borbat & J.P. Pollak. 2003. VORTEX: A stochastic simulation of the extinction process. Version 9. Chicago Zoological Society, Brookfield, IL. [ Links ]
Lee, R., J.D. Yoakum, B.W. O'Gara, T. Pojar & R. A. Ockenfels. (Eds). 1998. Pronghorn Managements Guides. 18th Pronghorn Workshop, Prescott, Arizona. [ Links ]
Mathews, F., M. Orros, G. McLaren, M. Gelling & R. Foster. 2005. Keeping fit on the ark: Assessing the suitability of captive–bred animals for release. Biological Conservation, 121:569–577. [ Links ]
McCarthy, M.A., Keith D, Tietjen J, Burgman MA, Maunder M, Master L, Brook BW, Mace G, Possingham HP, Medellin R, Andelman S, Regan H, Regan T, & Ruckelshaus M. 2004. Comparing predictions of extinction risk using models and subjective judgement. Acta Oecologica, 26: 67–74. [ Links ]
Miller, P.S. & R.C. Lacy. 2003. VORTEX: A Stochastic Simulation of the Extinction Process. Version 9.21 User's Manual. Conserv. Breed. Spec. Group (SSC/IUCN), Apple Valley, MN. [ Links ]
Miller, P.S. & R.C. Lacy. 2005. VORTEX: A Stochastic Simulation of the Extinction Process. Version 9.5 User's Manual. Apple Valley, MN; Conserv. Breed. Spec. Group (SSC/IUCN). [ Links ]
Mitchel, G.J. 1967. Minimum breeding age of female pronghorn antelope. Journal of Mammalogy, 48:489–490. [ Links ]
Molony, S.E., C.V. Dowding, P.J. Baker, I. C. Cuthill & S. Harris. 2006. The effect of translocation and temporary captivity on wildlife rehabilitation success: An experimental study using European hedgehogs (Erinaceus europaeus) Biological Conservation, 130:530–537. [ Links ]
O'Gara, B. 1978. Antilocapra americana. Mammalian species. American Society of Mammalogists, No. 90. 7 pp. [ Links ]
O'Gara, B.W. & J.D. Yoakum. 2004. Pronghorn. Ecology and Management. Wildlife Management Institute. University Press of Colorado, Boulder, Colorado. [ Links ]
Ralls, K., J.D. Ballou & A.Templeton. 1988. Estimates of lethal equivalents and the cost of inbreeding in mammals. Conservation Biology, 2:185–193. [ Links ]
Reed, J.M., L.S. Mills, J.B. Dunning Jr, E. S. Menges, K. S. McKelvey, R. Frye, S. R. Beissinger, M. Ch. Anstett & P. Miller. 2001. Emerging issues in population viability analysis. Conservation Biology, 16:7–19. [ Links ]
Robinson, H.S., R. B. Wielgus & J. C. Gwilliam. 2002. Cougar predation and population growth of sympatric mule deer and white–tailed deer. Canadian Journal of Zoology, 80:556–568. [ Links ]
Rodríguez–Clark, K.M. & A. Sánchez–Mercado. 2006. Population management of threatened taxa in captivity within their natural ranges: Lessons from Andean bears (Tremarctos ornatus) in Venezuela. Biological Conservation, 129:134–148. [ Links ]
Rosemarino, W.J. 2001. Proposal for a coyote/mule deer study and recommendations for interim mule deer management. Rocky Mountain Animal Defense. Boulder, Co. http://www.ourcolorado.org/alerts/RMADstudy.PDF (15 March 2007). [ Links ]
Ryman, N., O.E. Jorde & L. Laikre. 1994. Supportive breeding and variance effective population size. Conservation Biology, 9:1619–1628. [ Links ]
Seal, U.S. 1993. Population and Habitat Viability Assessment Reference Manual. IUCN/SSC Conservation Breeding Specialist Group, Apple Valley, MN. [ Links ]
SEDUE. Secretaría de Desarrollo Urbano y Ecología. 1988. Decreto por el que se declara la Reserva de la Biosfera "El Vizcaíno", ubicada en el Municipio de Mulegé, B. C. S. Diario Oficial de la Federación. Tomo CDXXII. No. 22: 2–27. [ Links ]
Song, Y. 1996. Population viability analysis for two isolated populations of Hainan Eld's deer. Conservation Biology, 10:1467–1472. [ Links ]
Soulé, M.E. (Ed). 1987. Viable Populations for Conservation. Cambridge University Press, Cambridge. [ Links ]
Steury, T.D. & D.L. Murray. 2004. Modeling the reintroduction of lynx to the southern portion of its range. Biological Conservation, 130:84–97. [ Links ]
Sweitzer, R., J. Constible & D. van Vuren (2003) Population ecology and ecological effects of bison on Catalina island, California. http://www.catalinaconservancy.org/ecology/research/BisonStudy.pdf (29 July 2007). [ Links ]
Ticer, C., S. Boe, R. Ockenfels & J. deVos Jr. 1999. Factors affecting home ranges and movements of pronghorn on a shortgrass praire of Northern Arizona. Pp. 84–90 In: Ockenfels, R. (Ed). Proceedings of the 18th Biennial Pronghorn Antelope Workshop. Prescott, AZ, March 23–27, 1998. Prescott, Arizona. [ Links ]
van Aarde, R.J. & T.P. Jackson. 2007. Megaparks for metapopulations: Addressing the causes of locally high elephant numbers in southern Africa. Biological Conservation, 134:289–297. [ Links ]
Wakelin, B. 2001. Mule deer demographic responses to select climatic variables in Arizona. http://www.usgs.nau.edu/proceedings/5thBiennial/6%20Wakeling_muledeer.pdf In: van Riper III, C., K.A. Thomas, and M.A. Stuart (Eds.). Proceedings of the Fifth Biennial Conference of Research on the Colorado Plateau. U.S. Geological Survey (15 March 2007). [ Links ]
Westley, F.W. & P.S. Miller (Eds.) 2003. Experiments in Consilience: Integrating Social and Scientific Responses to Save Endangered Species. Island Press, Washington, DC. [ Links ]
White, P.C.L., C.J. McClean & G.L. Woodroffe. 2003. Factors affecting the success of an otter (Lutra lutra) reinforcement programme, as identified by post–translocation monitoring. Biological Conservation, 112:363–371. [ Links ]
Wilson, A.C. & M.R. Stanley Price. 1994. Reintroduction as a reason for captive breeding. Pp. 243–262 In: Olney, P.J.S., G.M. Mace and A.T.C. Feistner (Eds.). Creative conservation. Interactive management of wild and captive animals. Chapman and Hall. London, UK. [ Links ]
Yoakum, J.D. & B.W. O'Gara. 2000. Pronghorn. Pp. 559–577. In: Demarais, S. and P. R. Krausman (Eds.). Ecology and management of large mammals in North America. Prentice Hall. Upper Saddle River, NJ, U.S.A. [ Links ]
Zimmer, J. & F. Lindzey. 2002. Fetal rates and sex ratios in three pronghorn populations in Wyoming. Pp. 24. In: J. S. Abegglen & W. S. Fairbanks (Eds.) Proceedings of the 20th Biennial Pronghorn Workshop. Kearney, NE, USA. [ Links ]














