Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta zoológica mexicana
On-line version ISSN 2448-8445Print version ISSN 0065-1737
Acta Zool. Mex vol.25 n.3 Xalapa Dec. 2009
Ensayos
Darwin's pigeons and the evolution of the columbiforms: recapitulation of ancient genes
Los pichones de Darwin y la evolución de los columbiformes: recapitulación de genes ancestrales
Luis Felipe BAPTISTA1 †, Juan Esteban MARTÍNEZ GÓMEZ2 and Helen Mary HORBLIT3
1† Department of Ornithology and Mammalogy. California Academy of Sciences. 55 Music Concourse Drive, Golden Gate Park. San Francisco California 94118. USA.
2 Red de Interacciones Multitróficas. Instituto de Ecología, A. C. Apartado Postal 63. Xalapa, Veracruz, 91000. MÉXICO.
3 Island Endemics Foundation. P.O. Box 320097, San Francisco California 94132. USA. socorro.dove@island–endemics.org
* Autor para correspondencia:
juan.martinez@inecol.edu.mx
Recibido: 14/09/2009
Aceptado: 21/09/2009
ABSTRACT
To commemorate the sesquicentennial of Charles Darwin's "On the Origin of Species by Means of Natural Selection", we address an essential topic in this publication. Domestic pigeons were extremely important in shaping Darwin's theory of evolution: pigeons featured prominently not only in his "Origin of Species", but also in his treatise on "Variation under Domestication", in his "Descent of Man" and finally in his "Expression of Emotions". Darwin saw the process of domestication as solid evidence demonstrating the power of selection. He argued convincingly that all domestic pigeon breeds (some 150 in his day) descended from one ancestral species, the Rock Dove (Columba livia), and that from this single species, humans selected directionally for colors, sizes, shapes, peculiarities of bill shape and length, plumage characteristics and voice qualities. While these domestic races achieved remarkable morphological differentiation under selection in the course of human generations, extant genera of pigeons (Columbiformes) have attained similar traits during the course of natural selection in the wild. We present a comparison of such characters between modern domestic breeds of the Rock Dove, the original Darwin's Pigeons plus new breeds, and wild pigeon species to encourage further studies on their evolution in the light of molecular techniques not available at Darwin's time.
Key words: Darwin's pigeons, domestication, natural selection, Columbia livia, Columbiformes.
RESUMEN
Dentro del marco conmemorativo del 150 aniversario de "El Origen de las Especies por Medio de la Selección Natural " de Charles Darwin, se presenta un ensayo de un tema esencial de esta publicación. Los pichones domésticos tuvieron un lugar destacado en la conformación de la teoría de evolución de Darwin: no sólo jugaron un papel fundamental en "El Origen de las Especies", sino también en su tratado sobre "Variación en Domesticación", en "La Descendencia del Hombre" y finalmente en "Expresión de Emociones". Darwin vio en el proceso de domesticación una evidencia sólida para demostrar el poder de la selección. Él arguyó de manera convincente que todas las razas de pichón (unas 150 en su época) provenían de una sola especie ancestral, la paloma doméstica (Columba livia). De esta especie única, los humanos seleccionaron direccionalmente colores, tamaños, formas, particularidades en la forma y longitud del pico, características del plumaje y cualidades vocales. Mientras estas razas domésticas adquirieron una diferenciación morfológica impresionante bajo selección en el curso de generaciones humanas, los géneros actuales de los pichones (Columbiformes) han adquirido rasgos similares durante el curso de la selección natural en la vida silvestre. En este trabajo se hace una comparación entre las razas actuales de la paloma doméstica, los pichones de Darwin originales más otros obtenidos posteriormente, y varias especies de pichones silvestres. De esta forma se desea alentar el estudio, en razas domésticas y especies silvestres, sobre la evolución de los rasgos morfológicos que cautivaron a Darwin, a la luz de técnicas moleculares que aún no se desarrollaban en su tiempo.
Key words: Pichones de Darwin, domesticación, selección natural, Columbia livia, Columbiformes.
INTRODUCTION
Many of the morphological features found in the domestic breeds used by Darwin to provide evidence of the efficiency of selection (Darwin 1859, 1868, 1871, 1872) can be seen in extant pigeon species in diverse genera, e.g. giantism and dwarfism, elongated and shortened bills, feathered tarsi, supernumerary tail feathers, hypertrophied nose and eye ceres, frontal and occipital crests, colors of irises and orbital rings, sexual dichromatism as well as various plumage patterns. Certain elements of nuptial displays and vocalizations selected for in domestic pigeon breeds are also to be found in wild species (e.g. Goodwin 1960, 1983 Gibbs & Barnes 2000).
Darwin wrote about the biology of domestic pigeon breeds in four of his works: in his "Origin of Species" (Darwin 1859), in "Variation under Domestication" (Darwin 1868), in his "Descent of Man" (Darwin 1871) and briefly in his "Expression of Emotions" (Darwin 1872). He saw the process of domestication as analogous to that of natural selection, noting that in the first process, humans single out individuals possessing certain morphological or behavioral traits and use those individuals as breeders, thus perpetuating those features (= artificial selection). In the speciation process, natural selection operates on individuals to fix or eliminate certain characteristics. Although Darwin did not understand the underlying genetic mechanisms involved, he nonetheless saw the study of the domestication process as instructive as to how natural selection shapes the characters of natural populations (Mayr 1992), especially if the (wild) ancestral forms are still available for comparison with the domestic breeds. Darwin (1868) saw domestic pigeon breeds as ideal subjects for such studies, stating..."that the races have descended from one known source is far clearer than with any other anciently domesticated animal" (p. 131). He and other naturalists in his day singled out the Rock Dove (Columba livia) as the ancestor of all domestic pigeon breeds.
Darwin had available to him many treatises on domestic pigeons which enabled him to trace the history of several breeds. He noted that since antiquity, humans have selected for certain shapes, sizes, peculiarities of bill shapes, plumage characteristics, and distinct voice qualities. Some of these domestic breeds, he noted, differed "fully as much from each other in external characters as do the most distinct natural genera." (Darwin 1868, p. 139) (Fig.1).
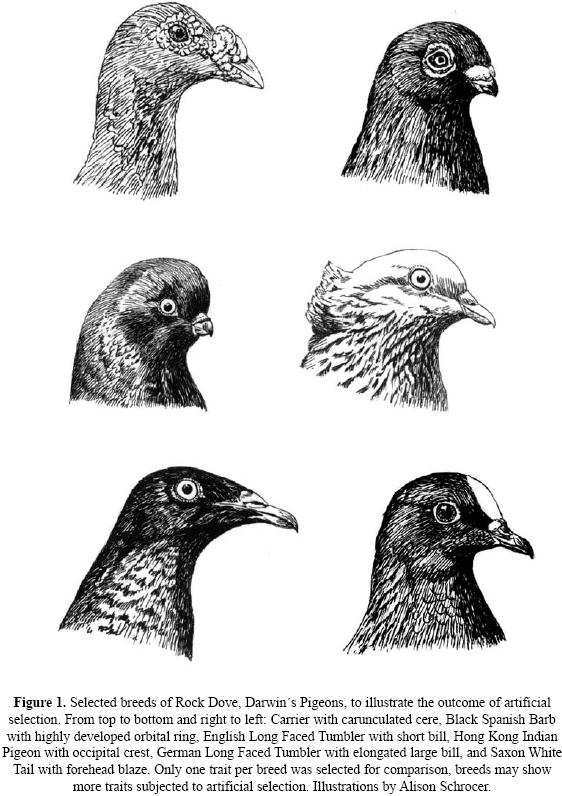
Darwin estimated that there were more than 150 breeds of domestic pigeons in his day. Since his time, humans have continued to select and produce new breeds so that some 350 breeds are now known today (D. McElvey, pers. comm.). In examining the breeds described by Darwin (1868) as well as other breeds not available to Darwin (Levi 1965), we have noted that morphological traits selected by humans are also to be found in many unrelated lines of living pigeons and doves. Possibly, these morphological and behavioral similarities between extant columbiform species and domestic pigeon breeds result from the action of genes found in the (ancestral) protocolumbiform that are normally suppressed and are recalled from time to time by natural or artificial selection. Patterns of occurrence can help in elucidating mechanisms of inheritance as well as the adaptive value of these morphological changes in this extremely successful avian family. Moreover, some of the patterns found in pigeons may apply to other avian families such as ducks (Anatidae), Cardueline finches (Fringillidae) and estrildid finches (Estrildidae) as well.
This essay calls attention to morphological, behavioral and vocal characters in domestic pigeon breeds and similar characters appearing in several columbiform species. Mechanisms are then suggested whereby these parallels between domestic pigeon breeds and extant columbiform species may have been brought about.
RADIATION IN PIGEONS
Phylogenetic relationships of the columbiformes have been recently established (Johnson & Clayton 2000a, 2000b, Johnson et al. 2001, Shapiro et al. 2002, Pereira et al. 2007). Recent evidence suggests that the group is conformed by three major groups: New World pigeons and allies, Neotropical ground doves, and Afro–Eurasian and Australasian pigeons and doves (Pereira et al. 2007). Pigeons as a group are readily recognized. The typical pigeon tends to have a compact body, short legs, a small head and a small bill. The bill usually has a horny distal portion and a tumid basal portion covered with soft skin and a cere. The middle portion of the bill tends to be constricted. The nostrils are overhung by a valve–like scale or operculum. A ring of bare skin of various colors, depending on species surrounds each eye, the eye cere. Columbiforms may be referred to as pigeons or doves, and the two terms are often interchangeable. Pigeons feed their young on a nutritious cottage cheese–like substance known as crop–milk. This consists of epithelial cells engorged with nutrients sloughed from the crop lining. Crop–milk contains an as yet unidentified growth factor which accelerates the development of chicks (Baptista et al. 1997).
Columbiforms may be found on all continents except for Antarctica (Goodwin 1983, Gibbs & Barnes 2000). They tend to have larger wings, lower wing loadings and larger flight muscles in comparison to other groups (Hartman 1961), characteristics that have rendered them excellent dispersers. Kingdon (1990) examined dispersal capabilities in some 20 avian families of birds and concluded that pigeons are the most effective colonists. They occur in oceanic islands off Africa (Kingdon 1990), Ecuador (Grant & Grant 1979), Mexico (Baptista et al. 1983, Jehl & Parkes 1983), Europe (Bannerman & Bannerman 1966), as well as in Micronesia, Macronesia, New Guinea and surrounding islands (Beehler et al. 1986, Pratt et al. 1987).
Pigeons have radiated into over 322 species and may occupy some of the harshest environments in the world. For example the Golden–spotted Ground–dove (Metriopelia aymara) may occur from 3000 to 5000 m in the high Andes (Fjeldsä & Krabbe 1990). The Snow Pigeon (Columba leuconota) thrives in the thin air of the Himalayas (Ali & Ripley 1969). The Spinifex Pigeon (Geophaps plumifera) survives harsh conditions of the Australian desert (Frith 1982, Williams et al. 1995), and the White–winged (Zenaida asiatica) and Mourning Doves (Z. macroura) are often denizens of New World deserts. The Mourning Dove may breed in temperatures of 44°C. Eggs and young are no longer warmed by the attending parent but cooled by a combination of shading with the body and gular fluttering to dissipate heat (Weathers 1983). Columbiforms rank among the most successful of avian taxa (Baptista & Trail 1992).
Columbiforms may be roughly divided into seed and fruit–eating forms. The seed–eating forms (Columbinae) tend to be dull browns and grays with perhaps some iridescent greens or purples around the neck or breast region. The true fruit–doves (Ptilinopus) tend to be more gaudily attired in greens, purples, oranges and reds. Pigeons swallow their food whole and fruit eating forms tend to have enormous gapes enabling them to ingest whole fruit or berries and most (or perhaps all) are capable of grasping sites with their feet and hanging upside down to reach fruits hanging from sites beneath them. The species that eat grain or fruit–pits (e.g. Treron spp., Phapitreron spp., Caloenas) ingest gravel which is held in the gizzard to help grind these hard food items. Many pigeons also feed on some invertebrates such as snails and insects, and some do this more so than others (e.g. Gallicolumba and Geotrygon spp.). The Atoll Fruit–dove (Ptilinopus coralensis) who lives in treeless atolls has been observed feeding on lizards (Baptista et al. 1997). Also, columbiforms show traits that parallel those found in Darwin's Pigeons (Fig. 2).
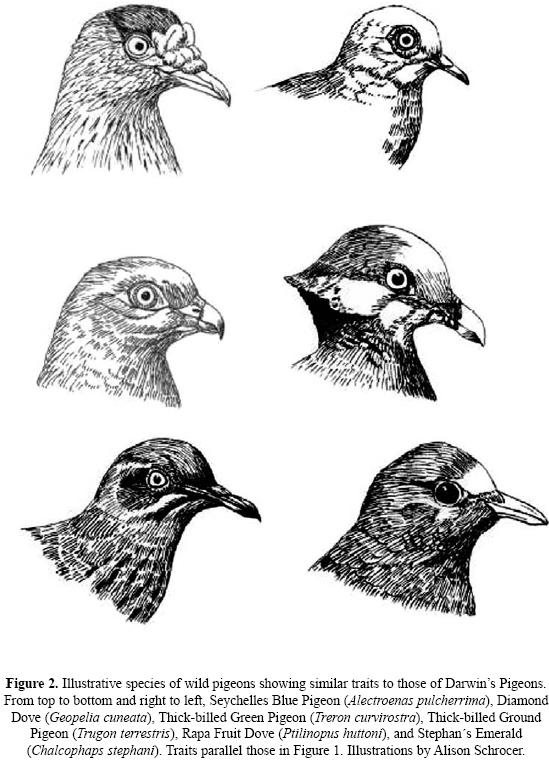
MORPHOLOGICAL PARALLELS BETWEEN SOME EXTANT PIGEON SPECIES AND DOMESTIC PIGEON BREEDS
Orbital rings. All pigeons and doves possess a naked ring of skin around their eyes known as the "orbital ring" or "eye cere". In the race of domestic pigeon known as the "Carrier" Darwin noted that this eye ring is prodigiously developed. He noted also that this hypertrophied eye ring is to be found in the related breed known as the "Barb." Large eye wattles are also to be found in several other breeds not kept by Darwin, e.g. the Steinheimer Bagdad, Berne Half Beak or Stralsund Highflier (Levi 1965). An hypertrophied red orbital ring is present in one subspecies of Rock Dove, namely Columba livia gymnocyclus of Africa (Goodwin 1983).
A highly developed orbital ring has appeared independently in several unrelated lines of columbiforms. It is to be found, for example, in the diminutive Diamond Dove (Geopelia cuneata) of Australia, the Bare–eyed Pigeon (Columba corensis) of South America, the Speckled Pigeon (Columba guinea) of Africa and in the Blue Pigeons (Alectroenas spp.).
Nose cere. Pigeons also possess a slightly swollen naked area surrounding the nostril known as the "cere" or "nasal cere". Darwin noted that in the breed known as the "Barb" the cere is much more swollen than that in C. livia and moreover it is sometimes carunculated, i.e. suffused with small bumps on the surface. In the breed known as the "Dragoon" the swelling and carunculations are even more exaggerated. Finally, in Darwin's "Carriers" the nose cere is so highly developed as to appear like a piece of cauliflower perched on the bill. In comparing the illustration of the Carrier in Darwin's Treatise with modern day Carriers (in Levi 1965) one sees that humans have further selected for the hypertrophy of the carunculated cere so that extant carrier pigeons appear to have cauliflower completely surrounding the bill.
Although no extant pigeon species possesses the exaggeratedly hypertrophied cere of Dragoons and Carriers, different degrees of development of the bill cere may also be found in different columbiform species (e.g. Delacour 1980). The cere is developed into a small protruding lump in the Nicobar Pigeon (Caloenas nicobarica). A cere has developed into a spherical protrusion in the fruit dove Red–knobbed Fruit–dove (Ptilinopus insolitus) of the Bismarck Islands, New Guinea (Diamond 1973). Its close relative with identical plumage color and pattern, the Orange–bellied Fruit–dove (Ptilinopus iozonus) of mainland New Guinea, has a "normal" pigeon cere. A swollen lump is also associated with the cere in the Red–knobbed Imperial–pigeon (Ducula rubricera). The closely–related mainland Ducula species do not exhibit such a cere development. The hypertrophied nasal cere reaches its peak in development in the Seychelles Blue pigeon (Alectroenas pulcherrima) and the Carunculated Fruit–dove (Ptilinopus granulifrons). The hypertrophied ceres in these pigeons are also carunculated and thus reminiscent of Darwin's Carriers and Dragoons. Other fruit doves in the genus Ptilinopus do not have inordinately swollen ceres.
Bill lengths. Darwin noted that the bill in some domestic pigeon breeds such as the Carrier, Scanderoon, and Runt, are longer than that in the ancestral Columba livia (Darwin 1868). He also illustrated skulls of two Carrier breeds along with that of a C. livia to show the difference in the proportion of the elongated mandible in relation to the skull. The bill is also elongated in the extant Bacska Tumbler, a breed not available to Darwin (Levi 1965).
The bill is especially long in the New Guinea Bronzewing (Henicophaps albifrons) (Coates 1985), reminiscent of the condition in Darwin's Carrier pigeons. No extant pigeon species possesses a bill as short as Darwin's "Barbs"; however, shortening of the bill does occur in pigeon species, e.g. the bill in the Thick–billed Green–pigeon (Treron curvirostra) is much shorter and stubbier than that in other members of the genus.
Size. According to Darwin (1868) the ancestral Rock Dove weighs about 414 g. He noted that humans have selected for large and small breeds, i.e. giantism and dwarfism. The smallest breed known is the Short–faced Tumbler which weighs 170 to 200 g. In contrast, he noted that the Runt may weigh as much as five times the above Tumbler breed. A modern day Runt weighs 1371 g (Levi 1965). A breed known as the Valencian Giant may weigh up to 2286 g (Levi 1965:414), more than five times the weight of the ancestral Rock Dove.
The Columbiformes have radiated into species with enormous extremes in size ranging from the Common Ground–dove (Columbina passerina) which weighs 31 g to the crowned–pigeons (Goura spp.) of New Guinea which weigh 2000 g. (Dunning 1992).
Feathered Tarsi. Tarsi of the original Columba livia are naked as are the tarsi in most domestic pigeon breeds. However, in several domestic breeds the tarsi are feathered. Darwin (1868) noted that the tarsi in the "Trumpeter" breed are "...so heavily feathered, that they almost appear like little wings." This is the extreme in tarsal feathering and is dubbed the "muffed" condition by pigeon breeders. Other pigeon breeds not described by Darwin possess feathered tarsi known as "leggings". This condition is to be found, for example, in the Lahore, the Tumbler of Craiora and the Shack Kee of China. Oftentimes the same breed may exist as feathered or unfeathered ("clean") tarsal mutants.
Although tarsi of most pigeon species are devoid of feathers, those in fruit pigeons of the genus Ptilinopus are heavily feathered, as are the tarsi in the closely related Cloven Feathered Dove (Drepanoptila holosericea) of New Caledonia.
Frontal and Occipital Crests. Darwin reported that his trumpeter pigeon possessed a "tuft of elongated feathers, which curls forward over the base of the beak, and which is possessed by no other breed" (Darwin 1868). Frontal crests are to be found in several extant domestic pigeon breeds including 9 breeds of Trumpeters, the Double–crested Priest and the Tung Koon Pak from China. Some extant pigeon breeds have crests of modified feathers on the occiput and part of the nape region, e.g. the American Crest, Russian Highflier, Archangel, to name a few. Breeding studies indicate that the occipital crest is a recessive character in domestic pigeons (e.g. Johansson 1927). Some trumpeter breeds have both a frontal and an occipital crest.
The Topknot Pigeon (Lopholaimus antarcticus) of Australia possesses both a frontal and an occipital crest reminiscent of domestic Trumpeters (Frith 1982). Occipital crests have evolved independently in several unrelated lines of columbiforms; for example, the Jamaican Crested Quail–dove (Geotrygon versicolor) possesses an occipital crest as does the Thick–billed Ground–pigeon (Trugon terrestris) of New Guinea (Coates 1985) and the Sulawesi race (paulina) of the Green Imperial–Pigeon (Ducula aenea) (L. F. Baptista, pers. obs.). A conspicuous occipital crest is to be found in the nominate form of the Pheasant Pigeon (Otidiphaps nobilis nobilis) from western New Guinea, whereas the crest in O. n. cervicalis of southeastern New Guinea is much shorter (Delacour 1980).
Number of Rectrices. The original Rock Dove possesses 12 rectrices or tail feathers. Darwin noted that occasionally individuals belonging to several breeds in his collection would have supernumerary rectrices, e.g. he found 14 or 15 tail feathers in some of the Pouters he possessed, a "Nun" with 13 and a "Helmet" with 15 (Darwin 1868). A breed known as the Fantail which lacks an oil gland on its rump and has the habit of spreading and raising its tail has been selected for extra numbers of tail feathers. Darwin noted that although the standard number of rectrices in the fantail is 32, individuals may have as many as 42. He called attention to a breed known as a "Java Fantail" which is intermediate between fantails and C. livia. The Java Fantail possesses an oil gland and has 18 to 24 rectrices. Moreover, Java Fantails do not spread or raise their tails to the extent of regular Fantails.
Doves in the genus Zenaida, with only one exception, have 14 tail feathers. The Zenaida Dove (Zenaida aurita) has 12. Two distant relatives, the crowned–pigeons (Goura spp.) of New Guinea have 16 rectrices and the Pheasant Pigeons (Otidiphaps nobilis) possess 20 or 22 (Glenny & Amadon 1955) and are thus reminiscent of Darwin's Java Fantails.
Tail length. The tail of the domestic pigeon breed known as the swift is longer than that in other breeds. Elongated tails have appeared independently in several unrelated taxa of pigeons, e.g. the cuckoo–doves in the genera Macropygia and Reinwardtoena, the pintailed green–pigeons of the genus Treron and the diminutive Namaqua Dove (Oena capensis) of Africa. The Namaqua Dove's closest relatives, members of the genus Turtur, do not have elongated tails (Delacour 1980).
Fat quills. Two domestic pigeon breeds, the Nuremberg Swallow and the South German Shield are unique in possessing fat quills that secrete oily substances (Eiselen 1939, Menon 1984). Fat quills are present in the Pied Imperial–pigeon (Ducula bicolor) of Australasia (Berthold 1967).
Orbital skin color. The color of the orbital skin varies between subspecies of Rock Dove. It is generally blue gray to gray in most subspecies, but red in C. l. gymnocyclus (Levi 1965). There is greater variation in color of the orbital skin in domestic pigeon breeds than in the wild C. livia e.g. it is blue in the Damascene, bright red in the barb, plum or white in other breeds.
Color of orbital skin varies considerably between columbiform species (Goodwin 1983, Gibbs & Barnes 2000). It is light blue in the Jambu Fruit–dove (Ptilinopus jambu), and bright blue in the White–winged Dove during the breeding season. The orbit may be bright yellow, golden or orange in the Bare–faced Ground–dove (Metriopelia ceciliae) of South America. The orbit is red in the Diamond dove, gray in the Luzon Bleeding–heart Dove (Gallicolumba luzonica) and white in the White–crowned Pigeon (Columba leucocephala) of the Caribbean. In the Zebra Dove (Geopelia striata) the orbit is blue but is yellow in the congeneric from Timor (G. maugei).
Iris color. Iris color of the Rock Dove is a dark red, but varies greatly in domestic pigeon breeds. The iris is orange in the Racer, yellow in the Squabbing Homer, white in the Baldhead Tumbler, light pearl in the Shak Kee. The Chinese have bred for different eye colors in a number of breeds. For example the Tung Koon Pak may have irises of purple, gold, light purple, bloody red and blue, and a much prized dark eye color which they call "rat" (Levi 1965).
Irises of columbiform species differ even more than those of domestic pigeons, e.g. they are red in the African Collared–dove (Streptopelia roseogrisea), white in the Speckled Wood Pigeon (Columba hodgsonii) blue in the Luzon Bleeding–heart (Goodwin 1983, Baptista et al. 1997), yellow in the Grey–chested Dove (Leptotila cassini) and olive–green in the Black–naped Fruit–dove (Ptilinopus melanospila) (L. F. Baptista pers. obs.).
Color patterns. The Rock Dove is blue–gray in general body color with two black wing bars and a greenish–purplish sheen around the neck and upper breast. The shiny throat/breast feathers are an important feature during the courtship display (Goodwin 1983). Humans have selected for many colors and color patterns during domestication both between and within breeds. The Italian Modena, e.g., exists in 152 color varieties and the Catalonian Tumbler in 318 colors and combinations thereof (Levi 1965).
Remarkably, several color patterns peculiar to certain domestic pigeon breeds appear also in pigeon species other than C. livia. For example the white forehead blaze in the breed known as the Spot is to be found in the Brown–backed Emerald Dove (Chalcophaps stephani) of New Guinea. The dark head and back and white face, throat and underparts of the Lahore breed (reminiscent of a penguin) are almost identical to the pattern found in the male Tambourine Dove (Turtur tympanistria) of Africa. Although iridescent green and purple feathers are usually confined to the throat and breast region in most domestic pigeon breeds, in breeds such as the White–tail this iridescence is spread throughout the body except the immaculate white tail. The Nicobar Pigeon is metallic purple green throughout except for its white tail. Although he did not name any specific breeds, Darwin (1868) noted that the colors and color patterns of several pigeon breeds were reminiscent of the Snow Pigeon. The color pattern of the extant Gazzi Modena, Strasser, Florentine and Prachen Kanik breeds are remarkably similar to that of the Snow Pigeon (L. F. Baptista pers. obs.).
Preen glands. Darwin (1868) noted that the oil or preen gland in domestic pigeon breeds "varies in development and is sometimes quite aborted (p. 187)." He examined many specimens of Fantails from different countries and found no trace of the oil gland. However, he also noted that a sub–race called the Java Fantail possessed a well–developed oil–gland. The oil–gland is also absent in the Oriental Roller, and White Carneau (D.W. Johnston 1988). The heritability of this character in domestic pigeon breeds has been studied by Johansson (1927).
Oil glands are absent in all the crowned–pigeons (Goura spp.), in many species of fruit–doves in the genera Treron, Ptilinopus and in Mountain Imperial–pigeon (Ducula badia). In three fruit–dove species Coroneted Fruit–dove (Ptilinopus coronulatus), Crimson–Capped Fruit–dove (Ptilinopus pulchellus), and White–breasted Fruit–dove (Ptilinopus rivoli) glands may or may not be present (D.W. Johnston 1988). There seems to be variation in the Tooth–billed Pigeon (Didunculus strigirostris) with regard to this character. Jacob and Ziswiler (1982) reported this gland in two individuals examined; however, D.W. Johnston (1988) noted its absence in three specimens.
Sex dichromatism. Darwin (1868, 1871: 466) also called attention to color dimorphism between the sexes in some pigeon varieties. He noted for instance that males of a wine–colored variety of the Pouter are generally checkered whereas females are not. Two extant breeds, the auto sex King and auto sex Pioneer are sexually dichromatic. In the King the male is white with red flecking on the neck and the female is light blue. In the male Pioneer red flecking is distributed throughout its white plumage but the female is all red (Levi 1965).
Sexual dimorphism in color may vary greatly within genera of pigeons. The male Tambourine Dove is white in front and brown behind, whereas his female is mostly grayish–brown. Other members of the genus Turtur are not chromatically dimorphic between the sexes. Although Darwin (1868:171) himself did not find any marked color dimorphism in pigeon species; he cites a personal communication from Alfred Russel Wallace who noted that sexes of various fruit dove species (Treroninae) "often differ considerably in color." The Black–backed Fruit–dove (Ptilinopus cinctus) is not color dimorphic between the sexes. In contrast, the male Orange Dove (Ptilinopus victor) of Fiji is bright orange whereas the female is green (Pratt et al. 1987). The diminutive New World ground–doves in the genus Columbina usually differ only slightly in color between the sexes. However the Croaking Ground–dove (C. cruziana) is exceptional in that the male is blue gray whereas the female is brown. Males of the New World genus Claravis are generally blue–gray in overall color whereas the females are mostly brown (Howell & Webb 1995). The Lemon Dove (Columba larvata) is unique among pigeons in that some populations are sexually dimorphic in color and others are not (Fry et al. 1985).
Flightlessness. Different breeds of pigeons have been selected for varying abilities to maintain sustained flight over long periods. Highfliers and Tipplers have been selected for endurance, and may stay in the air for many hours (Levi 1965). Some others may be poor fliers and have morphological anatomical peculiarities associated with this atrophied behavior. Darwin (1868:186) noted shortening in the length of the sternum, reduction in the prominence of its crest, and reduction in length of scapulae and furcula associated with poor flight in some pigeon breeds. Similar anatomical modifications have been described for the poor–flying Pheasant Pigeon (Otidiphaps nobilis) by Glenny & Amadon (1955).
The breed known as the Parlour Roller is completely flightless (Entriken & Erway 1972). Bird species on islands often become flightless over time as a result of lack of selection pressure from predators (Diamond 1981). At least two cases of flightlessness in columbiforms from islands have occurred in history, namely the Dodo (Raphus cucullatus) of Mauritius and the Solitaire (Pezophaps solitaria) of Rodrigues Island (Greenway 1958). Both species, now extinct, were closely related to the Nicobar Pigeon (Caloenas nicobarica; Shapiro et al. 2002).
VOCALIZATIONS
Darwin (1868, 1872) also called attention to voice breeds selected for in domestic pigeons, namely the Trumpeters and Laughers. The Rock Dove's courtship song consists of roughly three components: "a coo" followed by a stuttering "croo" followed by two notes of short duration which may be rendered as "wok wok". The full courtship song may be rendered as "coo–croowok–wok" lasting about 1.5 seconds.
In the voice breeds, humans have selected for a coda consisting of hypertrophy and repetition of these various components so that the songs may be extended to as long as 60 seconds (Baptista & Abs 1983). Some 13 breeds of Trumpeters may be recognized (Levi 1965). In the English Trumpeter, one of the breeds studied by Darwin, it is mostly the coo portion that is repeated in the coda. In the Laugher, another race mentioned by Darwin, it is mostly the "wok wok" that is repeated. In the Altenberger Trumpeter, a breed not available to Darwin, it is the stuttering "croo" portion that is repeated. The voice breeds also differ in rate of delivery of notes in the coda (Baptista & Abs 1983).
Parallels may be found in both the seed–eating and fruit–eating pigeons. The Namaqua Dove has a short (1.09 seconds) courtship song. Members of the closely related genus Turtur have a long stuttering song lasting some 8.74 seconds, reminiscent of what has occurred in the Trumpeter pigeon breeds (Baptista & Abs 1983, Baptista 1996). Among fruit doves of the genus Ptilinopus we find species such as Pink–headed Fruit–dove (Ptilinopus porphyreus) and P. cinctus with short advertising songs, whereas species such as the P. iozonus or P. pulchellus have longer stuttering songs. Long songs are heritable in crosses between voice breeds of domestic pigeons and between the genera Turtur and Oena (Baptista 1996).
Various Zebra Dove (Geopelia striata) populations sing songs differing in duration and number of elements (C.J.O. Harrison 1969). Humans have selected for voice breeds with songs of long duration, and cross–fostering experiments have demonstrated that songs develop independent of learning experience (Layton 1991).
FLIGHT DISPLAYS
Many dove species perform aerial displays as courtship or aggressive signals. The male Rock Dove (Columba livia; Darwin's pigeons precursor) introduces this display by launching off a building or cliff face with exaggeratedly slow, deep wingbeats. This is followed by 2 to 3 loud wing–claps which precede a gliding phase during which the tail is spread and the wings held over the back in a dihedral or "V". The glide is followed by another clapping bout, gliding and clapping repeated a number of times before he descends to a perch, still holding his wings in a dihedral and often rocking from side to side.
Goodwin (1983) noted that during the glide phase domestic pigeons hold their wings higher up the back than the ancestral rock dove. In the Swing Pouter breed, males clap up to as many as 30 times rather than 2 to 3 times as in the rock dove. During the glide phase the wings are held steeply up over the back and he traces a "u" shaped path rather than the straight path of the ancestor before the next clap bout (Nicolai 1976). In the Rhine Ringbeater, and in the now extinct breed known as the Finnikin, the male flies in circular paths over the female (Nicolai 1976).
Thus, during the course of domestication humans have selected for the shape of the flight path, the number of claps and the angle at which the wings are held during the glide phase. These same components vary between distantly related extant pigeon species (Johnson & Clayton 2000a, Pereira et al. 2007). White–winged (Zenaida asiatica), Namaqua (Oena capensis) and Brown Cuckoo–doves (Macropygia phasianella) begin their display with a steep ascent and loud clapping wings followed by a descent with wings spread sideways or slightly below the horizontal. The Band–tailed Pigeon (Columba fasciata) traces a circular path during his display flight (Peeters 1962) and is thus reminiscent of Rhine Ringbeaters. The ancient domestic pigeon breed known as the Syrian Dewlap has been selected to fly straight up toward the sky to some 1500 m and circle, and at a signal from its owner to fold its wings and stoop straight down like a hunting Peregrine Falcon (Falco peregrinus) (Bodio 1990). During the courtship flight of the Papuan Mountain Pigeon (Gymnophaps albertisii) the male flies almost straight up tracing a spiraling path to a height up to 2530 m above the canopy. It then partly or entirely folds its wings and plummets straight down like a falling rock (Coates 1985) and is thus reminiscent of the Syrian Dewlap.
The number of claps during the flight display of extant pigeon species also varies: Ring–necked doves (Streptopelia capicola) and Topknot Pigeons clap only once, Ruddy Ground Doves (Columbina talpacoti) clap once or twice and White–tipped Doves (Leptotila verreauxi) clap two or three times. The Orange–bellied Fruit–dove (Ptilinopus iozonus) produces a long series of claps as it ascends and then glides down with wings slightly below the horizontal.
DISCUSSION
As noted earlier, pigeons are one of the most successful of bird families, occupying a variety of habitats including some of the harshest on earth. They have also successfully colonized continental and oceanic islands throughout the world (Baptista et al. 1997). R.F. Johnston (1990, 1992) demonstrated that within 4 centuries after their introduction into the New World, female Rock Doves have evolved clines in size correlated with latitude. This indicates that directional and then stabilizing selection on descendants of dispersing pioneers may occur in relatively few generations. If such rapid evolution is typical of the family then it is no wonder that columbiforms have been so successful as colonizers in so many diverse habitats. High mutation rate and the winnowing effects of natural selection would enable descendants of colonizers to quickly adapt to new situations.
Adaptive value of variations. Available evidence strongly suggests that nature has selected for differences in body size, proportions of appendages in relation to body size, various ornamentations (e.g. ceres, crests), colors of soft parts, sexual dichromatism, flight abilities, flight displays and elaborate vocalizations in columbiform species that parallel analogous traits in the original Darwin's pigeons and other domestic breeds of the Rock Rove (Table 1).
Differences in body size often reflect dietary preferences in pigeons. For example, Diamond (1973, 1975) studied fruit–doves and found that small species (Ptilinopus spp.) preferred feeding on small fruit, medium–sized doves on medium–sized fruit and large doves (notably the Ducula spp.) tended to feed on large fruit. Variation in bill length also probably reflects adaptation for specialized modes of foraging (Goodwin 1983). The bill of the Galapagos Dove (Zenaida galapagoensis) is not only elongated but slightly down curved, reminiscent of the bill of the Impeyan Pheasant (Lophura impeyanus). Both species use their bill as a digging tool (Grant & Grant 1979).
Ornaments such as hypertrophied eye–ceres, nose–ceres, bright orbit–skin colors, bright irises are probably the results of selection for species recognition. Some doves may voluntarily contract their irises to accentuate eye colors when sexually aroused (H. M. Horblit pers. obs.). These then function as striking visual signals, especially if eye–color contrasts with color of orbital skin. In species where males are more elaborately adorned than females, ornaments may also be results of pressures from sexual selection. For example nose–ceres in Nicobar Pigeons (Caloenas nicobarica) and eye–ceres in Diamond Doves (Geopelia cuneata) tend to be larger in males than in females.
Goodwin (1983) has suggested that elongated tails may be adaptations for specific modes of flight (see also Hummel 1990). In some species, notably various Australian doves, elongated tails are ornamented and are raised and spread during their bowcoo displays (Frith 1982). Tails then often function as visual signals: selection may once again be for species isolating characters.
Vocalizations along with visual displays may function as species recognition characters. Species characters are coded in rhythm, pitch and pitch relationships of notes, and in tonal quality (Baptista 1996). Playback studies have revealed that pigeons respond to songs with conspecific characters and ignore allospecific songs without those characters (Gürtler 1973, Blockstein & Hardy 1989).
Pigeons imprint sexually on parents or foster parents with specific colors or color–patterns (Baptista et al. 1983, Goodwin 1983). Colors and color–patterns may have been selected as species isolating characters. Ali & Ripley (1969) noted that Snow Pigeons are difficult to distinguish from their background, their black–and–white coloration rendering them cryptic. Thus in some cases, colors in pigeons may be the result of selection for camouflage.
Flightlessness in birds including various pigeons often develops on islands. Absence of predators renders frequent flight unnecessary. Since development and maintenance of muscle complexes associated with flight are energetically costly, Diamond (1981) postulated that atrophied flight muscles associated with flight were selected for as energy saving devices.
Goodwin (1960) reviewed the subject of sexual dichromatism in columbiforms and noted that species graded from color monomorphism between the sexes (as in P. cinctus), to birds that are slightly color dimorphic (as in Z. macroura) to species that are markedly color dimorphic between the sexes (e.g. P. melanospila). He noted that distinct color or color patterns are usually confined to head, neck or breast regions in males of highly sexually dimorphic species. This he pointed out is possibly the result of sexual selection for characters used in close range sexual and hostile encounters.
Processes in artificial selection. Darwin (1868) noted two different processes in artificial selection. Fanciers could select for "excessively slight individual differences" (p. 234) and over a number of generations arrive at an exaggerated new character state through a series of intermediate steps. He gave as examples the elongated bills of some domestic pigeon breeds and the exaggerated number of tail feathers and erect carriage of the tail in Fantails. The Java Fantail, he noted, is the link between the Rock Dove and the Fantail, having an intermediate number of tail feathers and having the tail carried slightly above the body. The elaborate differences in markings and color–patterns between the sexes in columbiforms have most likely arisen through a number of intermediate steps mediated by accumulation of micromutations.
Fanciers could also select for "greater differences" or "sports" (Darwin 1868, p. 234). Selection for extreme color dimorphism between the sexes in various domestic pigeon breeds are an example of selection for sports, the result of one mutational event. Dimorphism on overall body coloration between the sexes as in P. victor (the male is bright orange and the female green), Ruddy Quail–Dove (Geotrygon montana) (males are purplish chestnut and females olive brown) or the various Claravis species (males are mostly blue and females brown) could be the result of selection after similar single mutational events in nature.
Adaptive value of colors and patterns. Both sexes participate in incubation in columbiforms. Males usually incubate from mid–morning to mid–afternoon, whereas females incubate from mid–afternoon through the night into the next morning (Skutch 1964). Goodwin (1960) has proposed a mechanism whereby predation may have brought about this pattern of differences in incubation schedule between the sexes. He suggested that some nest predators hunting by sight would tend to do so in the early morning or evening and would thus spot the sitting parent. A bright colored incubating male (e.g. as in P. victor) is more likely to attract attention than a dull colored female. Thus concealing coloration in females would be selected for as the brighter colored males incubating at these time are eliminated.
It should be pointed out however that the negative value of bright colors as cryptic characters cannot be assumed but must be tested through observation in the field. For example in the Eclectus Parrot (Eclectus roratus) males are mostly bright green and females are mostly bright red in body coloration. Females are more obvious than males in the open. However, from an observation of a female of this species flying into a tree in New Guinea one can learn that its red bright color is not necessarily disadvantageous. It remained very still and despite her bright color it took several minutes to locate her. By remaining just below the leaves she looked like one of the many cast shadows falling on the tree's branches. It would have been impossible to find her if her point of entry into the tree was not known (L. F. Baptista pers.obs.).
Field observations indicate that the bright orange of the male P. victor resting in a tree blends in and resembles the many dead leaves hanging on surrounding branches. Thus his gaudy colors may be just as cryptic as the female's green under the appropriate circumstances (Orenstein & Bruce 1976). Under certain circumstances even bright colors function in promoting crypticity. Sexual dimorphism in doves may have arisen mostly through sexual selection with little pressure of differential selection by predators on brighter individuals.
Mechanisms of inheritance. Darwin (1868; also this study) called attention to similarity in plumage between the Snow Pigeon of the Himalayas and various domestic pigeon breeds. One may also see that colors and color patterns in Chalcophaps stephani, Turtur tympanistria and Caloenas nicobarica also appear in various domestic breeds (see above). Additionally, Whitman (1919) noted that the checker wing pattern of some domestic breeds appears repeatedly in several unrelated lines of columbiforms. Whereas Darwin (1868) considered the blue–bar of the extant Rock Dove the older character state and the checker of some conspecific individuals the derived state, Whitman (1919) argued that in fact checker probably occurred in the ancestral columbiform and that the expression of this pattern is somehow masked in most species during evolution. The checker pattern appears in various extant species as adults, or in some cases only in juveniles [e.g. in the Eared Dove (Zenaida auriculata), L. F. Baptista pers. obs.] and is lost during the post juvenile molt into adult plumage.
Gould (1991) took exception to Whitman's (1919) views arguing that neither the checker nor the two–barred morphs represents the primitive state in domestic pigeons. In his view the ancestor of domestic pigeon breeds was probably a population represented by a continuum between checkered and two–barred forms. Whitman (1919) noted that in extant Rock Dove populations checker does appear as a rare mutation whereas the two–bar is the rule. Checker is dominant to two–bar and is inherited in simple Mendelian fashion (Levi 1965, R.F. Johnston & Janiga 1995). Thus, the two morphs are not a continuum. Whitman may very well be correct as illustrated by hybridizing experiments with other avian taxa (see below).
J.M. Harrison (1953, 1959) noted that in crosses between various ducks [e.g. Wigeon (Anas penelope) and Northern Shoveler (A. clypeata)], F1 hybrids often have the bridled facial pattern and distinct green head coloration of a third species, the Baikal Teal (A. formosa). Crosses between various estrildid finches with clear breasts and bellies often produce hybrids with scaled plumage patterns typical of the Spice Finch (Lonchura punctulata), not included in the crossings (Steiner 1959, 1966; C.J.O. Harrison 1962) For example, crosses between Chestnut–breasted Mannikins (Lonchura castaneothorax) with a clear chestnut breast and Diamond Sparrows (Emblema guttata) with a black breast produce hybrids that are completely scalloped in breast and sides as in the Spice Finch (L. F. Baptista, pers. obs.). A cross between a White–rumped Munia (Lonchura striata) and a Java Sparrow (Padda [Lonchura] oryzivora) in the collection of the California Academy of Sciences possesses a brown breast bordered by a black band as in Padda [Lonchura] fuscata of Timor. This distinct breast color and pattern in the F1 are absent in the parental species.
These data confirm that oftentimes character states not present in parental species, but present in a third related species, will appear in their F1 hybrids. Similar observations have been made with regard to plumages of hybrid carduelid finches (Fehrer 1993) and components of courtship displays of duck hybrids (Kaltenhäuser 1971). Thus one or perhaps both parental forms appear to possess in their genome the gene or genes controlling the expression of these character states but that somehow these genes are being prevented from expressing themselves. By making a hybrid, genomes are altered permitting expression of these characteristics. Two possible events may have occurred in the F1 (Baptista & Horblit 1990): 1). Perhaps blocking or controller genes are removed or turned off in the F1 hybrid permitting the expression of a rarely expressed allele or gene complex or 2). Perhaps each parental form possessed only a portion of the gene complex required to control expression of the particular character states and by making a hybrid the necessary genes are once more reunited in the reconstructed genomes.
The facts that in wild Rock Doves checker and two–bar are (inherited as) two discrete characters, and that in other taxa construction of hybrids produces character states present in related taxa, lend support to Whitman's (1919) suggestion that checker is an old character state in Columba livia and columbiforms in general. Possibly the genes controlling many of the color patterns, visual displays and vocal attributes shared by domestic pigeons and various extant columbiform species were present in the proto–columbiform, and due to mutation and natural selection appear as complete complexes, not de novo, but as a result of suppression and expression of single regulatory genes.
150 years after the Origin of Species. Darwin used different breeds of the Rock Dove known at his time to support his argument for selection, an engine leading to descent with modification. He was able to capitalize on this study system because he had a profound knowledge of their natural history. More importantly, by breeding his own birds he gained first–hand knowledge on the efficiency of artificial selection to favor desired morphological traits.
Throughout this essay, we discussed several observations on the natural history of Darwin's pigeons, new breeds not available at Darwin's time, and several species of extant pigeon and doves. By carefully looking at morphological and behavioral traits, we offered some thoughts for future research on this group for both, domestic breeds and pigeons in the wild. It is noteworthy that about 200 breeds of the domestic pigeons were not known to Darwin (See Darwin 1868 and Levi 1965). These breeds are the result of artificial selection in the course of two or three human generations (e.g. Levi 1965, Hollander & Mangile 1994). This makes domestic breeds excellent candidates to study underlying genetic mechanisms and the response to selection of several traits covered in this essay.
Moreover, recent studies on the phylogenetic relationships of the columbiformes allow mapping of morphological and behavioral traits to let us know how often these traits have appeared in the family. More importantly, the fact that most wild pigeon and dove species can be kept and bred in captivity opens the possibility to employ quantitative trait loci techniques to finally elucidate if the traits observed in wild species share a common origin with those observed in Darwin's pigeons and other domestic breeds.
ACKNOWLEDGMENTS
Derek Goodwin, Sylvia Hope, Richard Johnston, Ernst Mayr, David McElvey, Kevin Padian, Kenneth Parkes, Jonathan Losos and Kevin Jonhson read previous versions of this paper and offered helpful comments. The authors thank sincerely Kathleen Berge, who typed and assisted in editing previous versions of this manuscript and John Dumbacher for preserving Luis Baptista's legacy at the California Academy or Sciences.
ABOUT THE AUTHORS
LUIS FELIPE BAPTISTA (1941–2000) was a world renowned ornithologist in the field of bioacous–tics. His studies on song dialects of the white–crowned sparrow (Zonotrichia leucophrys) are fundamental in the field. Among his major publications are "The Life of Birds" together with the late Carl Welty and the section on the family Columbidae in the prestigious series "Handbook of the Birds of the World" by Lynx Editions where Luis was the leading author. Luis was also an accomplished bird breeder, especially of estrildid fiches and pigeons: this skill led him to the creation of the breeding and repatriation program for the Socorro Dove of the Revillagigedo Archipelago, Mexico. He was recognized as an authoritative figure in the field of ornithology. Shortly after Luis' untimely death, Ernst Mayr wrote "His knowledge of the living bird and its habits was more comprehensive and sound than that of any other person."
JUAN ESTEBAN MARTÍNEZ GÓMEZ is a postdoctoral fellow at the Instituto de Ecología, A.C.; he conducted his doctoral research under the direction of Robert Ricklefs and Luis Baptista using estrildid finches as a model. Since 1995 Juan collaborated with Luis Baptista in several conservation programs in the Revillagigedo Archipelago. After Luis Baptista's death Juan is carrying out the recovery program of the Socorro Dove.
HELEN MARY HORBLIT was a long term collaborator of Luis Baptista. She is a coauthor with Luis of the chapter on the family Columbidae in the "Handbook of the Birds of the World" as well as several other papers. Luis and Helen are the founders of the Island Endemics Foundation, an organization whose primary objective is the conservation of the endemic flora and fauna of the Revillagigedo Islands in Mexico.
LITERATURE CITED
Ali, S., & S. D. Ripley. 1969. Handbook of the birds of India and Pakistan. Vol. 3. Oxford Univ. Press, Bombay. [ Links ]
Bannerman, D. A., & W. M. Bannerman. 1966. Birds of the Atlantic Islands. Vol. 3. Oliver and Boyd, London. [ Links ]
Baptista, L. F. 1996. Nature and its nurturing in avian vocal development, pp. 39–60. In: D. E. Kroodsma and E. H. Miller, (eds.). Ecology and evolution of acoustic communication in birds. Cornell Univ. Press, Ithaca, NY. [ Links ]
Baptista, L. F., & M. Abs. 1983. Vocalizations, Pp. 309–325. In: M. Abs, (ed.). Behavior and physiology of the pigeon. Academic Press, New York, NY. [ Links ]
Baptista, L. F., W. I. Boarman, & P. Kandianidis. 1983. Behavior and taxonomic status of Gray son's Dove. Auk, 100:907–919. [ Links ]
Baptista, L. F., & H. M. Horblit. 1990. Inheritance and loss of the straw display in Estrildid Finches. Avicultural Magazine, 96:141–152. [ Links ]
Baptista, L. F., & P. W. Trail. 1992. The role of song in the evolution of Passerine diversity. Systematic Biology, 41:242–247. [ Links ]
Baptista, L. F., P. W. Trail, & H. M. Horblit. 1997. Columbidae, Pp. 60–231. In: J. del Hoyo, A. Elliott, & J. Sargatal, eds. Handbook of the birds of the world. Vol. 4. Lynx Edicions, Barcelona. [ Links ]
Beehler, B. M., T. K. Pratt, & D. A. Zimmerman. 1986. Birds of New Guinea. Princeton Univ. Press, Princeton, NJ. [ Links ]
Berthold, P. 1967. Zur creme–farbung von Ducula bicolor (Scopoli). Journal of Field Ornithology, 108:491–493. [ Links ]
Blockstein, D. E., & J. W. Hardy. 1989. The Grenada Dove Leptotila wellsi is a distinct species. Auk, 106:339–340. [ Links ]
Bodio, S. J. 1990. Aloft. Lyons and Burford, New York. [ Links ]
Coates, B. J. 1985. The birds of Papua New Guinea, non–passerines. Vol. 1. Dove Publications, Alderley, Queensland, Australia. [ Links ]
Darwin, C. 1859. On the origin of species by means of natural selection or the preservation of favored races in the struggle for life. John Murray, London. [ Links ]
Darwin, C. 1868. The variation of animals and plants under domestication. John Murray, London. [ Links ]
Darwin, C. 1871. The descent of man and selection in relation to sex. John Murray, London. [ Links ]
Darwin, C. 1872. The expression of the emotions in man and the animals. John Murray, London. [ Links ]
Delacour, J. 1980. Wild pigeons and doves. T.F.H. Publications, Inc., Neptune, NJ. [ Links ]
Diamond, J. 1973. Distributional ecology of New Guinea birds. Science, 179:759–769. [ Links ]
Diamond, J. 1975. Assembly of species communities, Pp. 342–444. In: M. L. Cody & J. M. Diamond, eds. Ecology and evolution of communities. Harvard Univ. Press, London. [ Links ]
Diamond, J. M. 1981. Flightlessness and fear of flying in island species. Nature, Lond. 293:507–508. [ Links ]
Dunning Jr., J. B. 1992. CRC Handbook of Avian Body Masses. CRC Press, London, Tokyo. [ Links ]
Eiselen, G. 1939. Untersuchungen über den Bau und die Entstehung von Schmalzkielen bei Tauben. Z. Wiss. Zool., 152:409–438. [ Links ]
Entriken, R. K., & L. C. Erway. 1972. A genetic investigation of Roller and Tumbler Pigeons. Journal Heredity, 63:351–354. [ Links ]
Fjeldsä, J., & N. Krabbe. 1990. Birds of the High Andes. Zoological Museum, Univ. of Copenhagen and Apollo Books, Copenhagen and Svenborg. [ Links ]
Frith, H. J. 1982. Pigeons and doves of Australia. Rigby Publishers, Sydney, Adelaide, Australia. [ Links ]
Fry, C. H., S. Keith, & E. K. Urban. 1985. Evolutionary expositions from 'The birds of Africa': Halcyon song phylogeny; Cuckoo host partitioning; systematics of Aplopelia and Bostrychia, Pp. 163–180. In: K. L. Schuchmann, ed. Proc. Int. Symp. Afr. Vert., (Bonn 1984). [ Links ]
Gibbs, D. & E. Barnes. 2000. Pigeons and Doves: A Guide to Pigeons and Doves of the World. Yale University Press. New Haven, Connecticut [ Links ]
Glenny, F. H., & D. Amadon. 1955. Remarks on the Pigeon Otidiphaps nobilis. Auk, 72:199–203. [ Links ]
Goodwin, D. 1960. Sexual dimorphism in pigeons. Bull. B.O.U., 5:45–52. [ Links ]
Goodwin, D. 1983. Pigeons and doves of the world. 3rd ed. Cornell Univ. Press, Ithaca, NY. [ Links ]
Gould, S. J. 1991. What the immaculate pigeon teaches the burdened mind (views of C. O. Whitman on orthogenesis vs. natural selection theory). Natural History, 100:12–21. [ Links ]
Grant, P. R., & K. T. Grant. 1979. Breeding and feeding ecology of the Galapagos Dove. Condor, 81:397–403. [ Links ]
Greenway Jr., J. C. (1958): Extinct and Vanishing Birds of the World. Special Publication No. 13. Amer. Committee for International Wild Life Protection. New York, NY. [ Links ]
Gürtler, W. 1973. Artisolierende Parameter des Revierrufs der Türkentaube (Streptopelia decaocto). Journal für Ornithologie, 114:305–316. [ Links ]
Harrison, C. J. O. 1962. A silverbill x Bengalese finch hybrid. Avicultural Magazine, 68:30–33. [ Links ]
Harrison, C. J. O.1969. Some comparative notes on the Peaceful and Zebra Doves (Geopelia striata spp.) with reference to their taxonomic status. Emu, 69:66–71. [ Links ]
Harrison, J. M. 1953. On the significance of variation of pattern in birds. Bull. B.O.U., 73:37–40. [ Links ]
Harrison, J. M. 1959. Comments on a wigeon x Northern shoveler hybrid. Bull. B.O.U., 79:142–151. [ Links ]
Hartman, F. A. 1961. Locomotor mechanisms of birds. Vol. 143. Smithsonian Miscellaneous Collections, Washington, DC. [ Links ]
Hollander, W. F. & R. J. Mangile. 1994. Three new recessive behavior mutants in the domestic pigeon. Behavior Genetics 24: 181–186. [ Links ]
Howell, S. N. G., & S. Webb. 1995. A guide to the birds of Mexico and Northern Central America. Oxford Univ. Press Inc., New York, NY. [ Links ]
Hummel, D. 1990. On the aerodynamics of the tail in birds, Pp. 730–747 in Acta XX Congressus Internationalis Ornithologici. [ Links ]
Jacob, J., & V. Ziswiler. 1982. The Uropygial Gland, Pp. 199–324. In: D. S. Farner, J. R. King, and K. C. Parkes, eds. Avian Biology. Vol. VI. Academic Press, New York, NY. [ Links ]
Jehl Jr., J. R., & K. C. Parkes. 1983. 'Replacements' of land bird species on Socorro Island, Mexico. Auk, 100:551–559. [ Links ]
Johansson, J. 1927. Studies on inheritance in pigeons. VI. Number of tail–feathers and uropygial glands. Genetics, 12:93–107. [ Links ]
Johnson, K. P. & D. H. Clayton. 2000a. A molecular phylogeny of the Dove Genus Zenaida: mitochondrial and nuclear DNA sequences. Condor, 102: 864–870. [ Links ]
Johnson, K. P. & D. H. Clayton. 2000b. Nuclear and mitochondrial genes contain similar phylogenetic signal for pigeons and doves (Aves: Columbiformes). Molecular Phylogenetics and Evolution, 14:141–151. [ Links ]
Johnson, K. P., S. De Kort, K. Dinwoodey, A. C. Mateman, C. ten Cate, C. M. Lessells, and D. H. Clayton. 2001. A molecular phylogeny of the dove genera Streptopelia and Columba. Auk, 118(4): 874–887. [ Links ]
Johnston, D. W. 1988. A morphological atlas of the avian uropygial gland. Bulletin of the British Museum (Natural History), Zoology, 54:199–259. [ Links ]
Johnston, R. F. 1990. Variation in size and shape in pigeons, Columba livia. Wilson Bulletin, 102:213–255. [ Links ]
Johnston, R. F. 1992. Geographic size variation in rock pigeons, Columba livia. Italian Journal of Zoology, 59: 111–116. [ Links ]
Johnston, R. F., & M. Janiga. 1995. Feral pigeons. Oxford Univ. Press, London and New York. [ Links ]
Kaltenhäuser, D. 1971. Über Evolutionsvorgänge in der Schwimmentenbalz. Z. Tierpsychol., 29:481–540. [ Links ]
Kingdon, J. 1990. Island Africa, the Evolution of Africa's rare Animals and Plants. Collins, London. [ Links ]
Layton, L. 1991. Songbirds in Singapore, the growth of a pastime. Oxford Univ. Press, New York. [ Links ]
Levi, W. M. 1965. Encyclopedia of pigeon breeds. T.F.H. Publications, Inc., Jersey City, NJ. [ Links ]
Mayr, E. 1992. Darwin's principle of divergence. Journal of the History of Biology, 25:343–359. [ Links ]
Menon, G. K. 1984. Glandular functions of avian integument: an overview. Journal of the Yamashina Institute for Ornithology, 16:1–12. [ Links ]
Nicolai, J. 976. Evolutive Neuerungen in der Balz von Haustaubenrassen (Columba livia var. domestica) als Ergebnis menschlicher Zuchtwahl. Zeitschrift fur Tierpsychologie, 40:225–243. [ Links ]
Orenstein, R. I., & M. D. Bruce. 1976. Comments on the nesting and plumage of the Orange Dove Ptilinopus victor. Bull. B.O.C., 96:2–4. [ Links ]
Peeters, H. J. 1962. Nuptial behaviour of the Band–tailed Pigeon in the San Francisco Bay Area. Condor, 64:445–470. [ Links ]
Pereira, S. L., K. P. Johnson, D. H. Clayton, & A. J. Baker. 2007. Mitochondrial and Nuclear DNA Sequences Support a Cretaceous Origin of Columbiformes and a Dispersal–Driven Radiation in the Paleogene. Systematic Biology, 56: 656–672. [ Links ]
Pratt, H. D., P. L. Bruner, & D. G. Berrett. 1987. The birds of Hawaii and the Tropical Pacific. Princeton Univ. Press, Princeton, New Jersey. [ Links ]
Shapiro, B., D. Sibthorpe, A. Rambaut, J. Austin, G. M. Wragg, O. R. P. Bininda–Emonds, P. L. M. Lee, & A. Cooper. 2002. Flight of the Dodo. Sience, 295: 1683–1683. [ Links ]
Skutch, A. F. 1964. Life histories of Central American pigeons. Wilson Bulletin, 76:211–247. [ Links ]
Steiner, H. 959. Kreuzungsversuche zur Vererbung artspezifischer Merkmale. Archiv der Julius Klaus–Stiftung für Vererbungsforschung, 34:220–228. [ Links ]
Steiner, H.1966. Atavismen bei Artbastarden und ihre Bedeutung zur Feststellung von Verwandts–chaftsbeziehungen. Review Suisse de Zoologie, 73:321–337. [ Links ]
Weathers, W. W. 1983. Birds of Southern California's Deep Canyon. Univ. Calif. Press [ Links ]
Whitman, C. O. 1919. Orthogenetic evolution of Pigeons. Posthumous works of C. O. Whitman. Vol.3. Carnegie Inst., Washington, DC. [ Links ]
Williams, J. B., D. Bradshaw & L. Schmidt. 1995. Field metabolism and water requirements of spinifex pigeons Geophaps plumifera in Western Australia. Australian Journal of Zoology, 43:1–15. [ Links ]














