Servicios Personalizados
Revista
Articulo
Indicadores
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex vol.25 no.3 Xalapa dic. 2009
Artículos originales
Temporal changes in a comunity of dung beetles (Insecta: Coleoptera: Scarabaeinae) resulting from the modification and fragmentation of tropical rain forest
Cambios a través del tiempo en una comunidad de escarabajos copronecrófagos (Insecta: Coleoptera: Scarabaeinae) como consecuencia de la modificación y fragmentación de un bosque tropical lluvioso
Ingrid QUINTERO1 and Gonzalo HALFFTER2 *
1 Biological Dynamics of Forest Fragments (BDFFP) and Smithsonian Tropical Research Institute, Av. Andre Araujo 1753 – Aleixo Cep 69060–001 C.P. 478, Manaus –AM, BRAZIL. ingridquin@gmail.com
2 Instituto de Ecología, A.C., A.P. 63, 91000 Xalapa, Veracruz, MEXICO.
* Autor for correspondence:
gonzalo.halffter@inecol.edu.mx
Recibido: 12/03/2009
Aceptado: 27/08/2009
ABSTRACT
In order to determine the changes in biological diversity over time in different habitats of a fragmented tropical rain forest in Manaus, Brazil, we compared capture data from two windows in time: 1986 and 2000. We used beetles of the subfamily Scarabaeinae as an indicator group. Both sets of samples were collected from the same sites and following the same methodology. The only difference was that in 2000 most of the pastures that had been created as isolation barriers had been replaced by secondary vegetation in different stages of development. Beetles were collected from the following habitats: pasture, secondary vegetation, 1 ha, and 10 ha fragments of forest, and continuous rainforest. The main results follow. 1) After the dramatic decrease in Scarabaeinae species richness that followed the creation of the pastures and the isolation of the fragments there was a notable recovery of biodiversity. We associate this with the enormous tract of continuous rainforest that surrounds the study area since the sites were recolonized by rainforest species. 2) The high number of tourist species recorded for the pastures is evidence of the ease with which Scarabaeinae can overcome the physical barriers imposed by fragmentation. Over time, many of the tourist species coming from the intact forest can become colonizers. 3) Even when there is no human intervention, there is a high degree of heterogeneity in the spatial and temporal distributions of the Scarabaeinae in the rainforest. 4) For coprophagous beetles, the effects of forest fragmentation are a function of both forest fragment size and the nature of the matrix in which the fragments occur. In our study the development of secondary vegetation favored the connection between fragments and the continuous forest.
Key words: Diversity, matrix effect, forest fragmentation, long–term effects, secondary forest.
RESUMEN
Con el propósito de determinar los cambios en la diversidad biológica a través del tiempo en distintos hábitats de una selva lluviosa tropical fragmentada (Manaus, Brasil), comparamos dos ventanas: la primera correspondiente a las capturas realizadas en 1986, y la segunda en 2000. Como grupo indicador usamos los escarabajos de la subfamilia Scarabaeinae. En las dos ventanas, las colectas se realizaron en los mismos sitios y siguiendo la misma metodología. La única diferencia fue que para el 2000, la mayor parte de los pastizales establecidos como barreras de aislamiento habían sido remplazados por vegetación secundaria con distintos grados de desarrollo. Se colectó en los siguientes hábitats: pastizal, vegetación secundaria, fragmentos de bosque de 1 ha, y 10 ha, bosque original continuo. Los principales resultados fueron: 1) Después de la reducción drástica en la riqueza de especies de Scarabaeinae que siguió a la creación de los pastizales y al aislamiento de los fragmentos, se presentó una importante recuperación de la biodiversidad. Este fenómeno lo asociamos a la enorme extensión de selva continua que rodea al área de estudio, ya que la recolonización ocurrió con especies de selva. 2) El alto número de especies turistas encontradas en los pastizales es evidencia de la facilidad con que los Scarabaeinae "saltan" las barreras físicas impuestas por la fragmentación. Con el tiempo, al repetirse los casos, muchas especies turistas que proceden del bosque continuo pueden convertirse en colonizadoras. 3) Aún sin ninguna intervención humana, existe un alto grado de heterogeneidad en las distribuciones espacial y temporal de los Scarabaeinae en la selva tropical lluviosa. 4) En lo que respecta a los efectos de la fragmentación del bosque sobre los escarabajos coprófagos, además de la extensión de los fragmentos, es importante la naturaleza de la matriz en que éstos quedan incluidos. En nuestro estudio el desarrollo de la vegetación secundaria favoreció la interconexión entre los fragmentos y el bosque continuo.
Palabras clave: diversidad, efecto de la matriz, fragmentación de la selva lluviosa, efectos a largo plazo, bosque secundario.
INTRODUCTION
Fragmentation and habitat loss are likely the main threats to the local and regional biodiversity in the tropics (Noss 1983, Wilcox & Murphy 1985). The effects range from the loss of some species (Powell & Powell 1987, Bierregaard et al. 1992, Brühl et al. 2003, Lehtinen et al. 2003) to changes in community structure and composition (Lovejoy et al. 1986, Laurance 1990, Didham et al. 1996, Alcala et al. 2004, Hill & Currian 2005). Ecosystem function and high order interactions are also affected (Aizen & Feinsinger 1994, Benítez–Malvido et al. 1999, Malo et al. 2001, Bruna & Kress 2002, Tscharntke & Brandl 2004).
Disturbances do not usually occur simultaneously over large areas, and so the result at the landscape level is frequently a complex mosaic of pastures, croplands, areas with secondary vegetation and remnants of the original forest (Nestap et al. 1991, Turner 2005). Under these conditions, the attributes of the matrix that surrounds the fragments are of great importance for maintaining species richness within the fragments. Complex matrices that have abundant arboreal cover can mitigate the loss of the species that are characteristic of the forest (Perfecto & Vandermeer 2002, Pineda et al. 2005), facilitate the movement of individuals between fragments (Aberg et al. 1995, Renjifo 2001) and reduce the severity of edge effects on forest remnants (Gascon et al. 1999, Borges & Stouffer 1999). On the other hand, although the modification and fragmentation of the landscape causes changes in the diversity and abundance of animal species (Lovejoy et al. 1986, Wolff et al. 1994, Robinson et al. 1995, Hagan et al. 1996, Powell & Powell 1987, Didham et al. 1996, Davies & Margules 1998, Debinsky & Holt 2000), there is a tendency to think that these are definitive, rather than taking into account that with the development of secondary vegetation or other changes that occur over time, communities can begin to recover (Dunn 2004, Quintero & Roslin 2005). Furthermore, there have been few studies that examine these effects in the long term (Debinsky & Holt 2000, McGarigal & Cushman 2002) owing to the practical and logistical difficulties of repeat sampling in the same place using the same methodology.
The Biological Dynamics of Forest Fragments Project is a large–scale effort in central Amazonia to evaluate the effects of fragmentation of tropical rainforest on the diversity of different groups of organisms, and is being done in an ideal setting for studying what happens over time. It has forest fragments of different sizes and for each fragment there is a detailed record of its age and isolation processes (Bierregaard et al. 1992).
The copronecrophagous beetles of the subfamily Scarabaeinae were chosen as the indicator group for this study because they have been widely used in biodiversity studies in fragmented rainforest, they are sensitive to anthropogenic change and are of functional importance in tropical ecosystems (Halffter & Matthews 1966, Favila & Halffter 1997, Estrada et al. 1998, Andresen & Feer 2005). This group also lends itself to regular and controlled sampling.
The main objective of this study was to compare the alpha, beta and gamma diversity (Whittaker 1972, Halffter 1998), the community composition and Scarabaeinae guild structure among continuous forest, fragments of different sizes, pastures and the entire landscape at two points in time, between which important changes had occurred on the landscape.
Given that matrix quality can influence the dynamics of the populations and communities in fragmented landscapes, we expected: 1) a significant increase in the alpha diversity of the fragments and the gamma diversity of the landscape once the pastures bordering the fragments had been replaced by secondary vegetation; and 2) greater similarity in the composition and structure of Scarabaeinae communities in the matrices, fragments and continuous forests as the landscape became more complex and connectivity increased.
MATERIAL AND METHODS
Study Area. The study was done in the reserves of the Biological Dynamics of Forest Fragments Project (BDFFP) SI/INPA, located 80 km north of Manaus, Brazil (2°24'26"–2°25'31" S, 59°43'40"–59°45'50" W). The original vegetation of this landscape is classified as Terra Firme forest (Pires & Prance 1985). For the BDFF project forest fragments with areas of 1, 10, 100 and 1000 ha were separated by cattle pastures. The strips of pasture are 70 to 600 m wide and isolate the fragments from the continuous forest that extends for kilometers in all directions. The creation and isolation of the fragments started in 1980 and continued until 1991 (Bierregaard et al. 1992).
The first sample (T1) was obtained during the dry season (May–June) of 1986 by Bert Klein (Klein 1989). The second sample (T2) was also collected during the dry season (June–July) in 2000 by Ingrid Quintero, in order to obtain comparable data. Andresen (2002) observed no significant differences in the richness and abundance of Scarabaeinae on comparing collections from the dry and rainy seasons. The two sets of samples were collected from three reserves: Dimona (located in Fazenda Dimona), Colosso and Cidade Powell (located in Fazenda Esteio) (see Antongiovanny & Metzger 2005 for a general map of the area and the location of the reserves). The same four habitats were sampled on both occasions: continuous forest, 1 ha and 10 ha fragments, and the matrix between the fragments (pastures and secondary vegetation).
There were important changes to the landscape over the time. At T1, six years after selective fragmentation had occurred; the fragments were isolated by pastures. In one of these pastures (Cidade Powell) there were small patches of vegetation (scattered small trees) that were starting a fast process of secondary regeneration. Approximately 4 or 5 years later the majority of the pastures were abandoned owing to the lack of incentives for the cattle farmers and low production (see Bierregaard et al. 1992, Bierregaard & Stouffer 1997). The pastures were then cut or burned to a greater or lesser degree, resulting in the formation of mosaics of secondary vegetation in different stages and states of succession, and these now surround the fragments. The younger secondary vegetation that regenerated in the pastures of Colosso was cut and burned regularly and is dominated by species of Vismia. In Cidade Powell, the secondary vegetation originating from cutting the forest and later abandoning the pasture is dominated by species of Cecropia (see Borges & Stouffer 1999, Mesquita et al. 2001, Nascimento et al. 2006) (Fig. 1).
The sizes of the fragments have remained constant over time with the exception of the continuous rainforest located in Dimona which was transformed into a 100 ha fragment (see Antongiovanny & Metzger 2005). This fragment was sampled at T2, as was a new area of continuous forest located 2.5 km to the west of the fragment in order to have a reference site for the continuous forest. Given the replacement of pastures by secondary vegetation, new pasture sites (Dimona and Colosso) were sampled for their inclusion in the comparison and in order to have, for T2, data for all the habitats that had been sampled in 1986.
Beetle sampling and comparison of the species collected in 1986 and 2000.
Collection methodology and site selection in 2000 were based on Klein's proposal (1989) for his 1986 collection. To capture the beetles we used pitfall traps made from a collecting jar 14 cm in diameter and 5 cm deep. This was half filled with a solution of chloral hydrate 25% (T1) and 5% (T2), inside which we placed a smaller jar (4 cm diameter x 5 cm deep) containing the bait. The trap was protected with a plastic plate to prevent water from getting in. We set up a line of six traps, 17 m apart at each sampling point. These were alternately baited with human excrement and decomposing meat. The traps were left in place for four days and were checked every 12 h at dawn (approx. 0600 h) and dusk (approx. 1800 h). The bait was changed every 24 h. A total of 266 traps/day were used at T1 and at T2, plus the traps placed in the new pastures for T2. The traps in the rainforest were placed 350 m away from the edge. For the other sampling points we applied the same criteria used by Klein (1989).
The specimens captured during T2 were identified using taxonomic keys, checked against collections and by consulting specialists. It was not possible to identify some specimens to the species level given the current knowledge of their genera. All of the material that had been collected at T1 and deposited in the Bureau of Entomology (USDA) in Gainesville, Florida was examined, with the exception of a few examples that had been sent to Antonio Martínez (Argentina) for identification; i.e. species that appeared to be unique to Klein's collection (1989) (R. E. Woodruff personal communication). Another exception in the reconstruction of the material from Klein's (1989) collection concerns the data from the continuous forest in Cidade Powell, which did not exist in 2000. However, we verified that the material collected in the continuous forest located in Dimona represented twice the sampling effort of the other habitats. Therefore, although there was only data for the two continuous forests that had been sampled (Dimona and Colosso) the sampling effort was the same in all habitats. Hence, the data that we include in T1 are not identical to those of Klein (1989) as they represent a reconstruction of collection data and the verification of identifications based on collection material.
Data Analysis. To evaluate the representativeness of the samples from T1 and T2, and determine the expected richness for each site, we used species accumulation curves compared with abundance dependent non–parametric richness estimators (ACE, Chao1, Jackknife 1) (Colwell & Coddington 1994, Boulinier et al. 1998, available in EstimateS v. 7.0 software Colwell 2004). Randomizing the sample addition sequence 100 times smoothed the curves out and revealed the asymptote. Total abundance per species in 1986 was added to the program as independent samples given that we did not have the abundance data per trap.
Changes in species composition over time: Alpha, Beta and Gamma diversity. To quantify the contribution of local richness (alpha diversity) –mainly that of the community that was richest in species– and the heterogeneity of sites (beta diversity) to regional richness (gamma diversity) (Whittaker 1972, Halffter 1998) for each sample (T1 and T2) we did the following analysis: At each sampling site, we calculated the value of local alpha diversity, defined as the total number of species collected, and from these we calculated mean alpha diversity per habitat. To determine species turnover between the sites of a given habitat and along the continuum or disturbance gradient, we used Whittaker's beta index: Bw = S/ · –1; where S is the number of species recorded in a set of samples and · is the mean number of species (Whittaker 1960, Koleff et al. 2003). To calculate gamma diversity we used the formula proposed by Lande (1996): Y = α + β; where α is mean alpha diversity for the landscape and β, is the mean diversity between the communities studied.
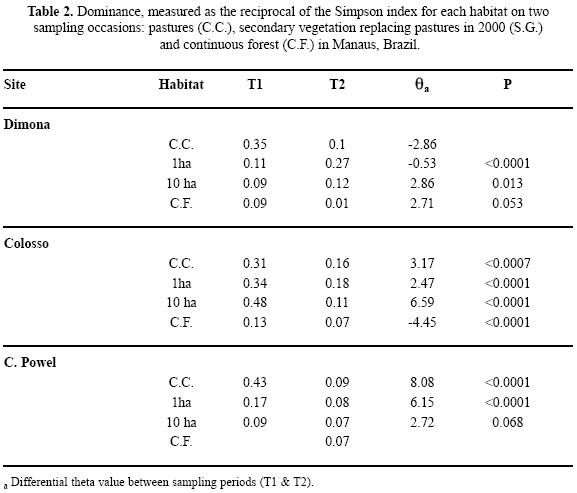
Changes in the Scarabaeinae community structures over time. To identify the nature of the changes in the beetle community over time caused by fragmentation and the modifications to the vegetation, we did the following analyses. To observe changes in the hierarchy of species at each site over time we plotted Whittaker curves (Feinsinger 2001), and coded the most abundant species. Additionally, some species that were rare but important for the purpose of comparison were also coded. To ensure that these abundance distributions were not the product of random variation we used two methods, both related to the availability of abundance data per trap at each site. For T1, we generated a new distribution based on the sum of the frequencies for the species recorded in each site and we tested the fit with a Kolmogorov–Smirnov test. With this new distribution (fit to a Poisson distribution) we generated resamplings of the mean and obtained the probability values for rejecting the null hypothesis. For T2 we calculated mean abundance for each species and generated a new matrix fit to a Poisson distribution and then modeled the quality and quantity of species that added up to 70% of all the individuals on randomizing 10000 times. These simulations were done with R Project software (R Development Core Team 2005). Changes in dominance between sites over time were measured with the reciprocal of Simpson's index (1/D) which we selected for its sensitivity to common species and the wide use it has been given (Magurran 1988). This returned a differential value (θ) that determines the statistical significance with 1000 randomizations (statistical package: Species, Diversity & Richness 3.0, www.irchouse.demon.co.uk).
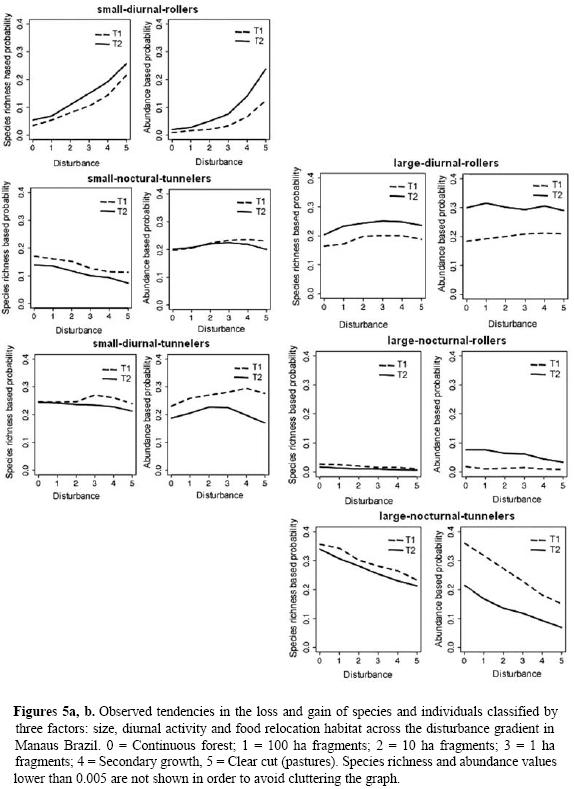
To determine the spatial and temporal distribution of resource use, we classified the Scarabaeinae along three dimensions: 1) size: small and large (Cambefort 1991); 2) temporal segregation: diurnal and nocturnal (Halffter et al. 1992); and 3) food relocation habit: tunnelers and rollers (Eurysternus is considered a roller) (Bornemissza 1969). Species were classified according to these criteria and we obtained a matrix of possible combinations in each of the sites sampled for both T1 and T2. To estimate the proportion represented by each of the combinations and observe tendencies in species richness and abundance for both T1 and T2, as well as their relationship with disturbance intensity, we used a multinomial test based on the Monte Carlo series from the R statistical software package (R Development Core Team 2005) with the MNP library (Imai & Dyk 2005). The calculations were done as a function of combinations of the relative species richness and abundance data, and we ran three series of 64000 events from which we estimated the probability based on the last 1000 events. For those sites that had only been sampled during T2, such as secondary vegetation and the 100 ha fragment in Dimona, the simulation was extrapolated from T1 to evaluate tendencies over time.
RESULTS
Sample Representativeness. The species accumulation curves obtained from our samples did not reach asymptotes (Fig. 2). However, total mean representativeness values were close to 80%. As a qualitative observation, the majority of the sites that had low representativeness values was also highly disturbed and were characterized by a high number of species that were locally rare but regionally abundant.
Diversity
Alpha, Beta and Gamma. For T1 total richness was 53 species; for three of these there was only one specimen and two were not reported by Klein (1989). For T2 we recorded 61 species (Table 1). Of the abundant species, Onthophagus haematopus was abundant in both T1 and T2. The abundance of Dichotomius aff. boreus and Dichotomius sp. (grp. lucasi) decreased between T1 and T2 while Ateuchus murrayi and Uroxys sp. increased in relative abundance. Canthon aff. acutiformis, another of the most abundant species, was exclusive to pastures with the same relative percentage in T1 and T2. Taking both samples into account 15 genera were recorded, 14 from continuous forest and fragments, and one restricted to pastures. In T2 eight new species were recorded, and a new genus (Trichillum). Dichotomius imitator was the only species that was present in T1 but not recorded in T2 (Dichotomius sp. 5 for Klein 1989) (Appendix 1).
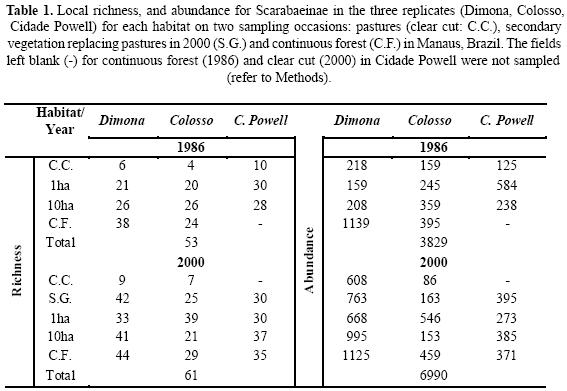
For T1 the continuous forest contributed the greatest alpha value to the landscape diversity. Diversity values decreased as the degree of disturbance increased. Comparing T1 and T2, there is a significant increase in diversity (36% more new species) even for fragments that are small and surrounded by pastures. The secondary vegetation that replaced the pastures has alpha values similar to those of the fragments. The new pastures that we sampled qualitatively have the same mean alpha richness as the pastures that were sampled in T1 (Table 1).
For beta diversity, our results suggest that for the two periods sampled there is a high degree of dissimilarity in species composition between the sites of the same habitat. For the fragments compared, over time there is a decrease in species turnover, but this does not happen when pastures are compared with small fragments or with secondary vegetation (data for the latter comparison not presented). Species turnover values decrease when the larger fragments are compared with continuous forest (Fig. 3).
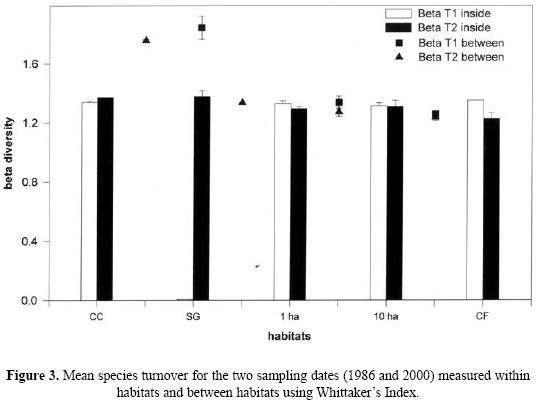
The three diversity values are: 53 species (γ) = 21.33 (α) + 31.66 (β) for T1, and 61 species (γ) = 28.66 (α) + 32.34 (β) for T2.
Structure
Species hierarchy and the dimensions of the Scarabaeinae guild. The analyses based on simulations for T1 show that the distributions for the frequency of observed species were not random (Kolmogorov–Smirnov P>0.80). The dominance–diversity curves clearly show changes between habitats and times, both in the slope of the curves and in species hierarchy and dominance. For the pastures sampled in T1, the distribution was simple, represented by four species with relatively uniform abundances. When the pastures were replaced by secondary vegetation in T2 there was a change in dominance to forest species with distributions similar to those observed in the forest. The curves for fragments in T2 are smoother and with less dominance compared to those for T1 (Fig. 4), and the differential value (0) was significant (Table 2). The simulation test done on the T2 sites shows that in the majority of sites, the species that reach 70% of the abundance within the 95% confidence intervals are responsible for the dominance (Table 2). In general there were changes in species hierarchy, especially for the 10 ha fragments and continuous forest (Fig. 4).
Based on the classification of Scarabaeinae by size, temporal segregation and food relocation habit there were seven combinations of these properties. In species richness and especially in abundance the best represented group was small, nocturnal tunnelers; though the pastures are only able to support the group of small, diurnal rollers (with the accidental collection of a few individual species classified as other combinations). For T2, and as the vegetation developed, all the groups of beetles recovered. In T1 a smaller number of large species were recorded in small fragments, especially nocturnal rollers. For T2 there is a recovery of large species on the landscape and an apparent similarity between all the communities with canopy cover (Fig. 5). For both large and small species there is a tendency to a greater number of diurnal rollers in more disturbed habitats.
DISCUSSION
Although this study and that of Quintero and Roslin (2005) are complementary, the analyses are based on different data. Quintero and Roslin (2005) used T1 data from Klein (1989) where abundance was summarized by treatment (C.C., fragments of 1 ha, 10 ha and C.F.), and not by site. Our T2 data includes the entire June to August 2000 collection effort, representing three times Klein's sample in 1986 (page 3305 and the Ecological Archives E086–181–A1 in Quintero & Roslin 2005). For the present study, T1 data were rebuilt from Bureau of Entomology of USDA Gainesville FL material. T2 Data includes the same sampling effort used by Klein, i.e. 288 traps/day + 24 traps/day in a fragment of 100 ha at Dimona.
Sample representativeness. Species accumulation curves did not reach asymptotes in either T1 or T2, but the reasons are likely different in the different habitats. In the pastures and small fragments there were many species that only appeared once but that were common on the scale of the landscape. Some heliophile forest species can cross small distances of pasture, and species typically found in this environment can invade small remnants of forest that have a pronounced edge effect (Ås 1999). These tourist species do not establish viable populations (Schmida & Wilson 1985), but can inflate expected richness values in each habitat. On the other hand, in large fragments and continuous forest there was a significant number of rare species and therefore the accumulation curves did not reach their asymptote. Novotny & Basset (2000) mention the difficulty in reaching asymptotic values in inventories of insects in tropical forests. However, observed richness values for each site do fall within the expected values according to the estimators used and therefore do support our interpretation of the results.
Changes in Scarabaeinae composition over time. At T1, mean richness was lower in the fragments than in the continuous forests (Klein 1989, see Andresen 2003 who collected over the same area) and there were notable differences in composition and diversity between fragments of the same size. Recently isolated fragments that are surrounded by pastures can host small species. These can become locally extinct, gradually decreasing the diversity of the fragments and, owing to stochastic events, there could be wide variations in composition and richness among recently created fragment populations (see Ewers & Didham 2006). However, as demonstrated for T2, with the growth of secondary vegetation there was an increase in the connectivity between fragments and with the continuous forest, and the fragments recovered their diversity (Tocher et al. 1997, Malcolm 1997, Bierregaard & Stouffer 1997, Gascon et al. 1999). The quality of the matrix surrounding the fragments is a determining factor in both the local and regional permanence of species (Vandermeer & Perfecto 1997, Fahrig 2001, Ricketts 2001). In the secondary vegetation dominated by Vismia the Scarabaeinae fauna was less diverse than it was in the secondary vegetation dominated by Cecropia, and diversity in the latter was similar to that of the continuous forest (Quintero & Roslin 2005). This has also been observed for other groups of fauna (Borges & Stouffer 1999; Vasconcelos 1999; Antongiovanni & Metzger 2005). The high species richness for Scarabaeinae hosted by secondary vegetation–even in its early successional stages–is similar to the richness of other tropical forests (Shahabunddin et al. 2005, Avendaño–Méndoza et al. 2005), when we take into account the specific and historical characteristics of each group of animals.
The greater diversity recorded at T2 could be the result of the regeneration of the vegetation which, in turn, resulted in the formation of more niches for the mammals that the beetles depend on. For example, Uroxys besti and Canthon quadrigattatus were not recorded at T1 but are strongly associated with sloths and howler monkeys (Halffter & Matthews 1966, Ratcliffe 1980), and have specific nesting and feeding patterns (Ratcliffe 1980, Vaz–de– Mello & Louzada 1997). The fact that these two mammals are folivores and depend on Cecropia allow U. besti and C. quadrigattatus to survive in disturbed landscapes (Chiarello 2000, Gilbert & Setz 2001, Lopes & Ferrari 2001).
Beta diversity made the strongest contribution to landscape diversity at 59.74% (T1) and 53% (T2). This is not just a result of the anthropogenic changes to the landscape. Our study provides evidence of the high within–site and between–site heterogeneity of tropical rain forest (as recently observed in dung beetle communities by Gardner et al. 2008 and Navarrete & Halffter 2008). Regarding the temporal dynamics, spatial heterogeneity–quite obvious in tropical forests–is characteristic of natural communities (Wolda 1983, Condit et al. 2002), and should be included as a variable when evaluating the effects of fragmentation (McGarigal & Cushman 2002).
Klein (1989) said that in small forest fragments diversity would decrease and there would be colonization by pasture species. In light of our results, the development of secondary vegetation favored recolonization of the fragments by shade–loving forest species to the point where only 11% of all the species on the landscape were restricted to the continuous forest. So, although Klein's hypothesis is valid for a recently fragmented landscape, it is less representative of the situation fourteen years later. The tendency to extrapolate results observed at a specific time and space to larger scales can overstate the consequences of anthropogenic changes to biological diversity in tropical forests (see Haila 2002, Fahrig 2003) if the fact that the matrix in many landscapes is also changing over time is not also taken into account (Jules & Shahani 2003).
From our results we can deduce that the permanence of species richness in partially fragmented tropical forests not only depends on the fragment size, but also on the nature of the matrix and the time that has elapsed since human intervention; that is, the scales of space and time over which the transformation has occurred. It is evident that the matrix can change in only a few years and that these changes can have a decisive effect on species diversity. On the other hand, the new conditions created by human activities favor some species, and affect total landscape diversity. For this landscape in Manaus, Brazil the introduction of pastures favored edge or heliophilic species such as Canthon aff. acutiformis, Canthon aff. acuticularis, Canthon lituratus and Pseudocanthon aff. xantorum all of which are strongly associated with natural gaps in mature forest (F. Z. Vaz–de–Mello, personal communication) and were commonly collected in the pastures (see also Scheffler 2005).
Observed changes in Scarabaeinae guilds. What makes large species–especially nocturnal tunnelers–more likely to disappear when the forest is disturbed? This phenomenon is particularly evident in 1986 before the mitigating effects of the secondary forest took hold (2000). In a savannah–forest system on the Ivory Coast, Krell et al. (2003) observed that roller species were "better competitors" than the tunnelers in the savannah (where higher levels of competition were observed), and the tunnelers, in turn, were better than the resident species. However the rollers which exploit the resource more quickly require specific environmental conditions, such as higher dung and soil temperature. In the gallery forests which are shadier and cooler than the savannah, the resident species were more abundant, although there was more competition; lower energetic expenditure made them ecologically more tolerant. This hypothesis might be partially applicable to our study system. In the forest, large, nocturnal tunnelers dominate the resource but once the landscape changes (i.e. when fragments of forest are created), the contrast between the environmental differences in the forest relative to the matrix becomes drastic (Bierregaard et al. 1992), with the result that these species become confined to the forest. In contrast, heliophile species common to the edges and forest gaps such as C. aff. acuticularis, C. lituratus and P. aff. xantorum would be better adapted to the new environmental conditions of the pastures. As occurs in savannahs, these species (small, diurnal rollers) dominate in the pastures (Halffter & Matthews 1966). With the changes in the vegetation of the edges that had occurred by T2, birds and mammals had recolonized the rain forest fragments (Bierregaard & Stouffer 1997, Malcolm 1997, Gilbert 2003, M. Santamaría–Goméz personal communication), thus restoring the food supply. There is not much difference in temperature inside the fragments and at their edges (E. Bruna, personal communication). Under these conditions large, nocturnal species were more homogeneously distributed in T2.
Our results on the proportion of functional guilds in the forests and pasture cannot be generalized to the entire Brazilian Amazon. In the eastern Amazon, Scheffler (2005) found that in pastures small diurnal rollers represented only 5% of total abundance while tunnelers dominated at 88% of the population. In the Ducke Reserve, approximately 100 km away from our study sites, Vulinec (2002) found that diurnal rollers were more limited to forests and the nocturnal tunnelers were more widespread in disturbed areas. There is no clear reason for this contradiction, but this is partly a result of the lack of information on the biogeography of the Scarabaeinae in the Amazon and the Neotropics. Studies on this topic are a priority if we are to understand the relationship between the historical and evolutionary processes acting on these species and the scales on which fragmentation and anthropogenic disturbances occur.
New contributions of this study that complement to Quintero & Roslin (2005). The proposed methodological analysis of this work, which included data reconstruction per site for T1 and from our own data collection, allowed us to make a comparison over time (1986 and 2000) using the same sampling effort. This approach allowed us to apply a new approach not included in Quintero and Roslin (2005). We were able to observe high heterogeneity, both spatial (by comparing fragments of the same area and continuous forest that are relatively close to each other) and temporal (by comparing each site at two different times). We also observed that in continuous forest, which has not been altered, there are important changes to species hierarchy over time, and this reflects its dynamism under apparently stable conditions.
The known richness and abundance for each site allowed us to include a behavioral analysis of the guild of Scarabaeinae in relation to the disturbance gradient over a significant time span. This analysis clearly shows that for small fragments there is no significant change in parameters such as abundance or diversity when compared to continuous forest (under the circumstances in the PDFBB areas). But, before fragmentation occurs, when environment conditions are stable, there is apparently a prolonged effect or trend in the structure of these communities. Some authors point out that changes in the structure of functional groups in Scarabaeinae can result in changes in the functionality of its ecosystems. Further study will reveal whether this effect over a much longer period of time is reflected in functional changes in the ecosystem that we have studied.
General conclusions
1. The effects of fragmentation and particularly of disturbed areas change depending on the matrix. In this study, the landscape is immersed in a vast expanse of continuous forest and this played a role in the recovery of the vegetation and mammalian fauna over a relatively short period of time, with the consequent beneficial effect for the Scarabaeinae. The high number of tourist species detected in this study is evidence of the ease with which the Scarabaeinae can overcome the physical barriers imposed by fragmentation. Over time, many tourist species that mainly come from the continuous forest can become colonizers (Avendaño–Mendoza et al. 2005 and bibliography therein).
2. The meta–analysis performed by Nichols et al. (2007) for some tropical landscapes (Vulinec 2002, Avendaño–Mendoza et al. 2005, Quintero & Roslin 2005, Shahabuddin et al. 2006 and unpublished data, included on the review from Nichols et al. 2007) along with the results of our research, suggest that complex matrices composed of secondary vegetation could be important for conserving some dung beetle species from the original pool that are present in the forest remnants. However, as mentioned by Gardner et al. (2008) in some landscapes, the importance of secondary forest to maintaining a typical and significant forest dung beetle fauna is less certain. The caution given by Gardner et al. (2008) about the importance of this landscape element should be evaluated according to the specific conditions for each landscape and real possibilities of conservation for the dung beetle fauna.
3. Our study highlights the fact that in the absence of human intervention heterogeneity is high (beta diversity) in the spatial distribution of the Scarabaeinae in tropical forests, and this has been confirmed to be true over time. This heterogeneity is not often taken into account when the effects of human activities on diversity are analyzed.
4. Under the conditions of our study the areas of secondary vegetation were recolonized by Scarabaeinae in a relatively short period of time and by almost all the rainforest species. This process was also influenced by the time elapsed after the disturbance and land use history. Secondary forests should be taken into consideration in effective management plans for the conservation of Amazonian forests exposed to human intervention (see Dunn 2004).
5. The conversion of rainforest into pastures causes changes in the structure of the Scarabaeinae guilds, resulting in a greater proportion of small, diurnal species particularly of the roller habit. These changes appear to be associated with the higher light levels in the pastures.
ACKNOWLEDGEMENTS
We are sincerely grateful to the BDFFP team, José Camilo Hurtado–Guerrero, C.R.V. Fonseca, and to INPA for logistical and technical support. We thank Alejandro Castro–Luna, Carlos Flechtmann and Federico Escobar for their invaluable comments on earlier versions of this manuscript. Thanks also to Robert E. Woodruff, Bruce Gill and Fernando Vaz–de–Mello for help with specimen identification. Roger Guevara assisted with the statistics. Bianca Delfosse translated the manuscript from the original in Spanish. Financial support was provided by CNPq and BDFFP (Brazil), Alexander von Humboldt Institute (Colombia) and CONACyT (Mexico). This is a publication in the BDFFP technical series (#532).
LITERATURE CITED
Aizen, M. & P. Feinsinger. 1994. Forest fragmentation, pollination, and plant reproduction in a Chaco dry forest, Argentina. Ecology, 75:320–341. [ Links ]
Alcala, E. L., A. C. Alcala & C. N. Dolino. 2004. Amphibians and reptiles in tropical rainforest fragments on Negros Island, the Philippines. Environmental Conservation, 31:254–261. [ Links ]
Andresen, E. 2002. Dung beetles in a central Amazonian rain forest and their ecological role as secondary seed dispersers. Ecological Entomology, 27:257–270. [ Links ]
Andresen, E. 2003. Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography, 26:87–97. [ Links ]
Andresen, E. & F. Feer. 2005. The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. Pp. 331–349. In: P.M. Forget, J.E. Lambert, P.E. Hulme and S.B. Vander Wall (Eds.). Seed fate: predation, dispersal and seedling establishment. CABI International, Oxon, UK. [ Links ]
Antongiovanni, M. & J. P. Metzger. 2005. Influence of matrix habitats on the occurrence of insectivorous bird species in Amazonian forest fragments. Biological Conservation, 122:441–451. [ Links ]
Ås, S. 1999. Invasion of matrix species in small habitat patches. Conservation Ecology. Available from http://www.consecol.org/vol3/iss1/art1 (accessed March 2007). [ Links ]
Avendaño–Mendoza, C., A. Morón–Ríos, E. B. Cano & J. León–Cortés. 2005. Dung beetle community (Coleoptera: Scarabaeidae: Scarabaeinae) in a tropical landscape at the Lachua Region, Guatemala. Biodiversity and Conservation, 14:801–822. [ Links ]
Benítez–Malvido, J., G. García–Guzmán & I. D. Kossmann–Ferraz. 1999. Leaf–fungal incidence and herbivory on tree seedlings in tropical rain forest fragments: an experimental study. Biological Conservation, 91:143–150. [ Links ]
Bierregaard Jr., R. O., T. Lovejoy, V. Kapos, A. A. dos Santos & R. Hutchings. 1992. The biological dynamics of tropical rain forest fragments. Bioscience, 42:859–866. [ Links ]
Bierregaard Jr., R. O. & P. C. Stouffer. 1997. Understory birds and dynamic habitat mosaics in Amazonian rainforest. Pp. 138–155. In: W. F. Laurance and R. O. Bierregaard Jr. (Eds.). Tropical remnants: Ecology, management and conservation of fragmented communities. University of Chicago Press, Chicago. [ Links ]
Borges, S. H. & P. C. Stouffer. 1999. Bird communities in two types of anthropogenic successional vegetation in Central Amazonia. Condor, 101:529–536. [ Links ]
Bornemissza, G. F. 1969. A new type of brood care observed in the dung beetle Oniticellus cinctus (Scarabaeidae). Pedobiologia, 9:223–225. [ Links ]
Boulinier, T., J. Nichols, J. Sauer, J. Hines & K. Pollock. 1998. Estimating species richness: the importance of heterogeneity in species detectability. Ecology, 79:1018–1028. [ Links ]
Brühl, C. A., T. Eltz & K. E. Linsenmair. 2003. Size does matter – effects of tropical fragmentation on the leaf ant community in Sabah, Malaysia. Biodiversity and Conservation, 12:1371–1389. [ Links ]
Bruna, E. M. & W. J. Kress. 2002. Habitat fragmentation and the demographic structure of an Amazonian understory herb (Heliconia acuminata). Conservation Biology, 16:1256–1266. [ Links ]
Cambefort, Y. 1991. Biogeography and evolution. Pp. 51–67. In: I. Hanski and Y. Cambefort (Eds.). Dung beetle ecology. Princeton University Press, Princeton, New Jersey. [ Links ]
Chiarello, A. G. 2000. Density and population size of mammals in remnants of Brazilian Atlantic forest. Conservation Biology 14:1649–1657. [ Links ]
Colwell, R. 2004. EstimateS: Statistical estimation of species richness and shared species from samples. Version 7.0. Available from http://viceroy.eeb.uconn.edu/estimates (accessed March 2005). [ Links ]
Colwell, R. & J. Coddington. 1994. Estimating terrestrial biodiversity through extrapolation. Philosophical Transactions of the Royal Society of London Series B, 345:101–118. [ Links ]
Condit, R., N. Pitman, E. G. J. Leigh, J. Chave, J. Terborgh, R. B. Foster, P. V. Nuñez, S. Aguilar, R. Valencia, G. Villa, H. C. Muller–Landau, E. Losos & S. P. Hubbell. 2002. Beta–diversity in tropical forest trees. Science, 295:666–669. [ Links ]
Davies, K. F., and C. R. Margules. 1998. Effects of habitat fragmentation on carabid beetles: experimental evidence. Journal of Animal Ecology, 67:460–471. [ Links ]
Debinski, D. M. & R. D. Holt. 2000. A survey and overview of habitat fragmentation experiments. Conservation Biology, 14:342–355. [ Links ]
Didham, R. K., J. Ghazoul, N. E. Stork & A. J. Davis. 1996. Insects in fragment forest: a functional approach. Trends in Ecology and Evolution, 11:255–260. [ Links ]
Dunn, R. R. 2004. Recovery of faunal communities during tropical forest regeneration. Conservation Biology, 18:302–309. [ Links ]
Estrada, A., R. Coates–Estrada, A. Anzures & P. Cammarano. 1998. Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. Journal of Tropical Ecology, 14:557–593. [ Links ]
Ewers, R. M. & R. K. Didham. 2006. Confounding factors in the detection of species responses to habitat fragmentation. Biological Review, 81:117–142. [ Links ]
Fahrig, L. 2001. How much habitat is enough? Biological Conservation, 100:65–74. [ Links ]
Fahrig, L. 2003. Effects of habitat fragmentation on biodiversity. Annual Review of Ecology, Evolution and Systematics, 34:487–515. [ Links ]
Favila, M. E. & G. Halffter. 1997. The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zoológica Mexicana, 72:1–25. [ Links ]
Feinsinger, T. 2001. Designing field studies for biodiversity conservation. Island Press, Washington, DC. [ Links ]
Gardner, T. A., M. L M. Hernández, J. Barlow & C. A. Peres. 2008. Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forest for neotropical dung beetles. Journal of Applied Ecology, 45:883–893. [ Links ]
Gascon, C., T. Lovejoy, R. O. Bierregaard Jr., J. R. Malcolm, P. C. Stouffer, H. L. Vasconcelos, W. F. Laurance, B. Zimmerman, M. Tocher & S. H. Borges. 1999. Matrix habitat and species richness in tropical remnants. Biological Conservation, 91:223–230. [ Links ]
Gilbert, K. A. 2003. Primates and the fragmentation of the Amazon forest. Pp. 145–157. In: L. K. Marsh (ed.) Primates in fragments. Kluwer, New York. [ Links ]
Gilbert, K. A. & E. Z. F. Setz. 2001. Primates in a fragmented landscape: six species in central Amazonia. Pp. 262–270. In: R. O. Bierregaard Jr., C. Gascon, T. Lovejoy and R. C. G. Mesquita (Eds). Lessons from Amazonia. Yale University Press, New Haven. [ Links ]
Hagan, J. M., W. M. Vander–Haegen & P. S. McKinley. 1996. The early development of forest fragmentation effects on birds. Conservation Biology, 10:188–202. [ Links ]
Haila, Y. 2002. A conceptual genealogy of fragmentation research: from island biogeography to landscape ecology. Ecological Applications, 12:321–334. [ Links ]
Halffter, G. 1998. A strategy for measuring landscape biodiversity. Biology International, 36:3–17. [ Links ]
Halffter, G., M. E. Favila & V. Halffter. 1992. A comparative study of structure of scarab guild in Mexican tropical rain forest and derived ecosystems. Folia Entomológica Mexicana, 84:131–156. [ Links ]
Halffter, G. & E. G. Matthews. 1966. The natural history of dung beetles of subfamily Scarabaeinae (Coleoptera: Scarabaeidae). Folia Entomológica Mexicana, 12–14:1–312. [ Links ]
Hill, J. L., & P. J. Curran. 2005. Fragment shape and tree species composition in tropical forest: a landscape level investigation. African Journal of Ecology, 43:35–43. [ Links ]
Imai, K. & D. Dyk. 2005. MNP: R package for fitting the multinomial probit models. Journal of Statistical Software, 14:1–32. [ Links ]
Jules, E. S. & P. Shahani. 2003. A broader ecological context to habitat fragmentation: why matrix habitat is more important than we thought. Journal of Vegetation Science, 14:459–464. [ Links ]
Klein, B. 1989. Effects of forest fragmentation on dung and carrion beetles communities in Central Amazonia. Ecology, 70:1715–1725. [ Links ]
Koleff, P., K. J. Gaston & J. J. Lennon. 2003. Measuring beta diversity for presence–absence data. Journal of Animal Ecology, 72:367–382. [ Links ]
Krell, F. T, S. Krell–Westerwalbesloh, I. WeiB, P. Eggleton & K. E. Linsenmair. 2003. Spatial separation of Afrotropical dung beetle guilds: a trade–off between competitive superiority and energetic constraints (Coleoptera: Scarabaeidae). Ecography, 25:210–222. [ Links ]
Lande, R. 1996. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos, 76:5–13. [ Links ]
Laurance, W. F. 1990. Comparative responses of five arboreal marsupials to tropical forest fragmentation. Journal of Mammalogy, 71:641–653. [ Links ]
Lehtinen, R. M., J. B. Ramanamanjato & J. G. Raveloarison. 2003. Edge effects and extinction proneness in a herpetofauna from Madagascar. Biodiversity and Conservation, 12:1357–1370. [ Links ]
Lopes, M. A. & S. F. Ferrari. 2000. Effects of human colonization on abundance and diversity of mammals in eastern Brazilian Amazonia. Conservation Biology, 14:1658–1665. [ Links ]
Lovejoy, T., R. O. Bierregaard Jr., A. Rylands, J. R. Malcolm, C. Quintela, L. Harper, K. S. Brown, A. Powell, G. V. Powell, H. O. Shubart & M. B. Hays. 1986. Edge and other effects of isolation on Amazon forest fragments. Pp. 257–285. In: M. E. Soulé (Ed.). The science of scarcity and diversity. Sinauer Associates, Sunderland. MA. [ Links ]
Malcolm, J. R. 1997 Biomass and diversity of small mammals in Amazonian forest fragments. Pp: 207–221. In: W. F. Laurance and R. O. Bierregaard Jr. (Eds.). Tropical remnants: Ecology, management and conservation of fragmented communities. University of Chicago Press, Chicago. [ Links ]
Malo, J. E., J. Leirana–Alcocer & V. Parra–Tabla. 2001. Population fragmentation, florivory, and effect of flower morphology alterations on the pollination success of Myrmephila tibicinis (Orchidaceae). Biotropica, 33:529–534. [ Links ]
Magurran, A.E. 1988. Ecological Diversity and its measurement. Croom Helm, London. [ Links ]
McGarigal, K. & S. Cushman. 2002. Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecological Applications, 12:335–345. [ Links ]
Mesquita, R. C. G., K. Ickes, G. Granade & G. B. Williamson. 2001. Alternative successional pathways following deforestation in the Amazon Basin. Journal of Ecology, 89:528–537. [ Links ]
Nascimento H. E. M., A. C. S. Andrade, J. L. C. Camargo, W. F. Laurance, S. G. Laurance & J. E. L. Ribeiro. 2006. Effects of the surrounding matrix on tree recruitment in Amazonian forest fragments. Conservation Biology, 20:853–860. [ Links ]
Navarrete, D. & G. Halffter. 2008. Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodiversity and Conservation, 17:2869–2898. [ Links ]
Nestap, N. D., C. Uhl & E. A. S. Serrao. 1991. Recuperation of a degraded Amazonian landscape: forest recovery and agricultural restoration. Ambio, 20:248–255. [ Links ]
Nichols, E., T. B. Larsen, S. Spector, A. L. V. Davis, F. Escobar, M. E. Favila, K. Vulinec & The Scarabaeinae Research Network. 2007. Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta–analysis. Biological Conservation, 137: 1–19. [ Links ]
Noss, R. F. 1983. A regional landscape approach to maintain diversity. BioScience, 33:700–706. [ Links ]
Novotny, V. & Y. Basset. 2000. Rare species in communities of tropical insect herbivores: pondering the mystery of singletons. Oikos, 89:564–572. [ Links ]
Perfecto, I. & J. Vandermeer. 2002. Quality of agroecological matrix in a tropical montane landscape: ants in coffee plantations in southern Mexico. Conservation Biology, 16:174–182. [ Links ]
Pineda, E., C. Moreno, F. Escobar & G. Halffter. 2005. Frog, bat, and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conservation Biology, 19:400–410. [ Links ]
Pires, J. M. & G. T. Prance. 1985. The vegetation types of the Brazilian Amazon. Pp. 109–145. In: G. T. Prance and T. Lovejoy (Eds). Key environments: Amazonia. Pergamon Press, New York. [ Links ]
Powell, A. H. & G. V. Powell. 1987. Population dynamics of male Euglossine bees in Amazonian forest fragments. Biotropica, 19:176–179. [ Links ]
Quintero, I. & T. Roslin. 2005. Rapid recovery of dung beetle communities following habitat fragmentation in Central Amazonia. Ecology, 86:3303–3315. [ Links ]
R Development Core Team. 2005. R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna. ISBN 3–900051–07–0, URL. Available from http://www.r-project.org. (accessed March 2007). [ Links ]
Ratcliffe, B. 1980. New species of Coprini (Coleoptera: Scarabaeinae) taken from the pelage of three toed sloths (Bradypus tridactylus L.) (Edentata: Bradypodidae) in Central Amazonia with a brief commentary of scarab–sloth relationships. The Coleopterists Bulletin, 34:337–350. [ Links ]
Renjifo, L. M. 2001. Effect of natural and anthropogenic landscape matrices on the abundance of subandean bird species. Ecological Applications, 11:14–31. [ Links ]
Ricketts T. H. 2001. The matrix matters: effective isolation in fragmented landscapes. The American Naturalist, 158:87–99. [ Links ]
Robinson, G. R., J. F. Quintin & M. L. Stanton. 1995. Invasibility of experimental habitat islands in a California winter annual grassland. Ecology, 76:786–794. [ Links ]
Scheffler, P. 2005. Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. Journal of Tropical Ecology, 21:9–19. [ Links ]
Schmida, A. & M. Wilson. 1985. Biological determinants of species diversity. Journal of Biogeography, 12:1–20. [ Links ]
Shahabuddin, C., H. Schulze & T. Tscharntke. 2005. Changes of dung beetle communities from rain forest towards agroforestry systems and annual cultures in Sulawesi (Indonesia). Biodiversity and Conservation, 14:863–877. [ Links ]
Tocher, M., C. Gascon & B. Zimmerman. 1997. Fragment effects on a central Amazonian frog community: a ten–year study. Pp. 124–137. In: W. F. Laurance and R. O. Bierregaard Jr. (Eds.). Tropical remnants: ecology, management and conservation of fragmented communities. University of Chicago Press, Chicago. [ Links ]
Tscharntke, T. & R. Brandl. 2004. Plant–insect interactions in fragmented landscapes. Annual Review of Entomology, 49:405–430. [ Links ]
Turner, M. G. 2005. Landscape ecology: what is the state of the science? Annual Review of Ecology, Evolution and Systematics, 36:319–344. [ Links ]
Vandermeer, J. & I. Perfecto. 1997. The agroecosystem: a need for the conservation biologist's lens. Conservation Biology, 11:1–13. [ Links ]
Vasconcelos, H. L. 1999. Effects of forest disturbance on the structure of ground–foraging ant communities in central Amazonia. Biodiversity and Conservation, 8:409–420. [ Links ]
Vaz–de–Mello, F. Z. & J. N. C. Louzada. 1997. Considerações sobre forrageio arbóreo por Scarabaeidae (Coleoptera: Scarabaeoidea), e dados sobre sua ocorrência em floresta tropical do Brasil. Acta Zoológica Mexicana, 72:55–61. [ Links ]
Vulinec, K. 2002. Dung beetle communities and seed dispersal in primary forest and disturbed land in Amazonia. Biotropica, 34:297–309. [ Links ]
Whittaker, R. H. 1960. Vegetation of the Siskiyou mountains, Oregon and California. Ecological Monographs, 30:279–338. [ Links ]
Whittaker, R. H. 1972. Evolution and measurement of species diversity. Taxon, 21:213–251. [ Links ]
Wilcox, B. A. & D. D. Murphy. 1985. Conservation strategy: the effects of fragmentation on extinction. American Naturalist, 125:879–887. [ Links ]
Wolda, H. 1983. Diversity, diversity indices and tropical cockroaches. Oecologia, 58(3): 290–298. [ Links ]
Wolff, J., E. M. Schauber & W. D. Edge. 1997. Effects of habitat loss and fragmentation on the behavior and demography of gray–tailed voles. Conservation Biology, 11:945–956. [ Links ]














