Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Acta zoológica mexicana
versão On-line ISSN 2448-8445versão impressa ISSN 0065-1737
Acta Zool. Mex vol.24 no.1 Xalapa Abr. 2008
Artículos originales
Bionomics of a novel species of Argyrotaenia (Lepidoptera: Tortricidae) presents in Mexican avocado orchards
Ninfa M. ROSAS-GARCÍA & Jesús M. VILLEGAS-MENDOZA
Laboratorio de Biotecnología Ambiental, Centro de Biotecnología Genómica-Instituto Politécnico Nacional. Blvd. del Maestro s/n, Col. Narciso Mendoza, Reynosa, Tamp. CP.88710. MÉXICO. nrosas@ipn.mx
Recibido: 8 de enero de 2007
Aceptado: 20 de septiembre de 2007
ABSTRACT
A novel species of Argyrotaenia (Lepidoptera: Tortricidae) Stephens, was found in some avocado orchards in the Pacific coast from the Mexican states of Nayarit and Michoacan. Individuals obtained from the field were reared under laboratory conditions to determine their life cycle and biology. An artificial diet designed for lepidopterans, as well as a temperature of 26 ± 1ºC and a relative humidity of 65 ± 5% allowed the development of one generation per month and successful insect development. Female pupae showed an increase in weight and size through five generations, while weight and size in male pupae remained stable. The complete life cycle of this insect is around 32-36 days under laboratory conditions, and developmental stages using this diet under laboratory conditions mentioned above are as follows: egg, 5-6 days, larva, 18–20 days, pupa, 6-7 days, and adult, 15-20 days. Argyrotaenia has five larval instars in the laboratory. This study has clarified confusion surrounding this novel species of Argyrotaenia with some members of the genus Amorbia (Lepidoptera: Tortricidae), due to similarity in morphology and habits in both insects.
Key words: Life cycle, artificial diet, developmental stages, larval instars, head-capsules, avocado orchards.
RESUMEN
Una nueva especie, no descrita de Argyrotaenia (Lepidoptera: Tortricidae) Stephens, fue encontrada en algunos huertos de aguacate, ubicados en la costa del Pacífico en los estados de Nayarit y Michoacán, México. Algunos individuos de este insecto se recolectaron en campo y se criaron bajo condiciones de laboratorio para determinar su ciclo biológico y su biología. Para este propósito se utilizó una dieta artificial especialmente diseñada para insectos lepidópteros, así mismo se utilizó una temperatura de 26 ± 1ºC y una humedad relativa del 65 ± 5%. Estas condiciones permitieron obtener una generación por mes y un desarrollo adecuado del insecto. Las pupas hembra mostraron un incremento en peso a través de 5 generaciones, mientras que el peso y el tamaño de las pupas macho permanecieron constantes. El ciclo de vida completo de este insecto bajo condiciones de laboratorio es de aproximadamente 32-36 días, la duración de las etapas de desarrollo son las siguientes: huevecillo, de 5 a 6 días, larva, de 18 a 20 días, pupa de 6 a 7 días y el adulto de 15 a 20 días y la larva sufre 5 ínstares larvarios. Este estudio aclara la confusión existente entre esta nueva especie y algunos miembros del género Amorbia (Lepidoptera: Tortricidae) debido a que la morfología y los hábitos son similares en ambos insectos.
Palabras clave: Ciclo de vida, dieta artificial, etapas de desarrollo, ínstares larvarios, cápsulas cefálicas, aguacate.
INTRODUCTION
The genus Argyrotaenia Stephens 1852 encompasses approximately 88 species distributed in the Western Hemisphere from Canada to Argentina (Brown & Cramer 1999), and many species of Argyrotaenia are pests on a variety of economically important crops.
The most important and representative species of this genus, A. velutinana Walker (redbanded leafroller), and A. citrana Fernald (orange tortrix), are found in North America. Other important species such as A. ljungiana Thunberg and A. sphaleropa Meyrick are found in Europe and in South America respectively (Trematerra & Brown 2004).
According to Brown & Cramer (1999), field studies have revealed a surprisingly large number of undescribed species in the New World. A good example of this are the several new species of this genus that have been reported in Mexico restricted to the higher elevation states of Mexico, Morelos, Puebla and, Veracruz where A. spinacallis, A. unda, A. octavana, A. coconinana, and A. bialbistriata have been found (Brown & Cramer 1999). Further in Argentina, two new species have been reported, A. pomililiana, and A. tucumana, (Trematerra & Brown 2004).
The members of this genus are polyphagous, however, they have preferred host plants such as apple or orange trees. Nevertheless, avocado crops, among others, have been attacked by this insect. Since 1949 A. citrana (orange tortrix) was found damaging to avocados in California as a minor pest, the larvae feed on the bark of green twigs, on terminal buds, and foliage, after webbing the leaves together, and the fruit resulted damaged if a fruit and a leaf came together (Ebeling & Pence 1957).
With the exception of A. velutinana and A. citrana, very little information has been published on the biology and life cycle of other species of Argyrotaenia. This could be due to that the study of this insect is particularly difficult because larvae spend their life hidden in the leaves and covered by webs, moreover, the similar morphology and feeding habits of this insect to other avocado pest, Amorbia, has caused confusion among growers and wrong taxonomic identification of this quarantine pest.
Other Argyrotaenia species have been reared under laboratory conditions (Glass & Hervey 1962, Roelofs, 1967) in order to obtain a large insect supply for various studies (Vanderzant 1974). As no information is available for this new species of Argyrotaenia the aim of this work was to determine its life cycle and biology under laboratory conditions using an artificial diet designed for lepidopterans.
MATERIAL AND METHODS
Larvae of Argyrotaenia sp. were collected from Hass avocado tree branches with visible infestation such as rolled leaves and webs, and from damaged fruits. Avocado orchards were located at the Pacific coast from the Mexican states of Nayarit and Michoacan, and collections were done from early August to late October 2004 and 2005. The larvae were carefully withdrawn from the leaves with a camel's hair brush and were allowed to complete their larval development in plastic jars (4 L capacity) fed with fresh avocado foliage. Mature leaves were washed before presentation to larvae to remove any contaminant. To corroborate taxonomic identification 14 adult moths were sent to Systematic Entomology Laboratory, USDA, and these specimens were kept for the U.S. National Collection.
The artificial diet was prepared using the ingredients shown in Table 1, agar was dissolved in boiling water, after this, all solid materials were added, and the mixture was blended in a Hamilton Beach blender for 10 min, after that, all liquid ingredients were added and blended for 5 more min. Five ml of the diet was poured into 30 mlplastic cups immediately, and allowed to dry for 2 h (Rosas-García 2002). The diet should be prepared and use immediately; however unused cups can be refrigerated for several days.
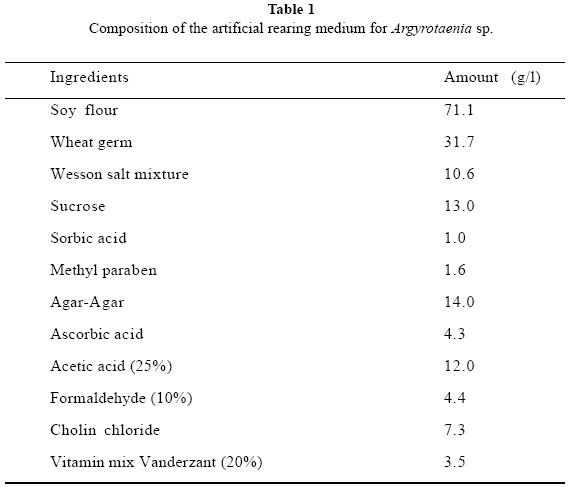
The first generation of hatched larvae in laboratory, which was obtained after females oviposited on the leaves surface from an avocado branch placed inside a wooden cage (30 cm x 30 cm x 30 cm), was treated as follows: a group of 10 larvae was placed in a 50 ml-plastic cup containing 30ml of artificial diet, each cup was closed with a cardboard lid and placed under controlled conditions, temperature 26 ± 1ºC, 65 ± 5% relative humidity and a photoperiod of 14:10 h (light: dark). Fifteen days later, larvae were transferred to fresh diet to allow completion of development to pupae.
Pupae were placed in adult mating chambers consisting of plastic jars (4 L capacity) covered with a piece of cheese cloth (30 cm x 30 cm) held in place with a rubber band. Adults laid eggs on a high density polyethylene lining the inner cylindrical surface of the jar. Two 30 ml-plastic cups, each plugged with a small cotton ball, were filled with a 15% sucrose solution for feeding adults. Egg collection was made daily by cutting out the area where the eggs were laid, sometimes if possible, eggs were lifted from polyethylene with a scalpel. Egg masses were placed into a hatching chamber consisting of a rounded plastic base (3.0 cm diameter x 0.7cm deep) placed on the surface of artificial diet contained in a 50 ml-plastic cup. The chambers were closed with a plastic lid for 5 days, after that time the plastic lid was removed and replaced with a cardboard lid until egg hatch occurred. (Rosas-García 2002). Ten couples (male and female) were chosen and placed individually in adult mating chambers in order to determine egg number per mated female.
Insect development in the laboratory was determined by investigating the following parameters: duration of eggs, larvae, pupae and adults. Pupae were sexed based on examination of the 8th and 9th sterno-abdominal segments (Trudel et al. 1995) by using a stereoscopic microscope (40X) (Zeiss Stemi DV4). Weight was determined for each individual using an analytical balance and size was measured using a dial caliper. These parameters were measured through five generations. Obtained data were analyzed with ANOVA and mean separation was done with Tukey's Test (P ≤ 0.05), (SPSS, version 10.0).
Larval instars were determined by analyzing head-capsule widths of 50 larvae during each larval stage. Under microscopic observation (10X) head-capsules were placed on a glass slide in a drop of transparent nail enamel. The facial area of the head-capsules was placed parallel to the surface of the slide to make sure that width and length planes were perpendicular to the vision axis of the microscope. Headcapsules were observed with a phase contrast microscope (Olympus BX51) and photographed with a digital camera (Hitachi KP-D50) using the program Image Pro-Express 4.0. To measure length and width of each head-capsule, images were analyzed using the program UTHSCSA Image Tool version 3.0 (Wilcox et al. 2002). The program was calibrated using a line of known length in micrometers.
Obtained data were analyzed with ANOVA and mean separation tests were made using Duncan Test (P ≤ 0.05), (SPSS, version 10.0).
RESULTS
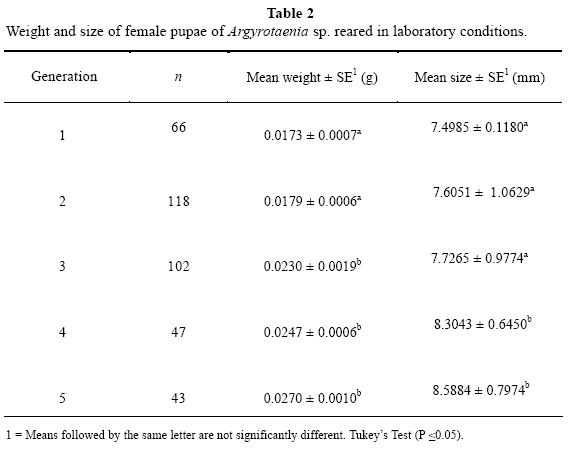
The duration of developmental stages using artificial diet under laboratory conditions were: egg, 5-6 days, larva, 18-20 days, pupa, 6-7 days, and adult, 15-20 days. The complete cycle is around 32-36 days. Female adults begin fertile ovipositions the third day after emergence; however, the number of eggs per female could not be determined as females in isolated couples did not lay eggs. The weight of female pupae showed a high significant difference (F = 8.536, d,f. = 4, P < 0.001), across generations, because a weight increment was observed from the third to the fifth generation (Table 2). However, in the third generation weight increased but size remained the same as in the previous two generations. The last two generations exhibited an increase in recorded measurements with respect to the first two generations. Female pupae exhibited an average weight of 0.0211 ± 0.0006 g, with a lower limit of 0.0158 g, and an upper limit of 0.0223 g. Pupal size also showed a highly significant difference (F = 13.765, d.f. = 4, P < 0.001) across generations. The average size was 7.8191 ± 0.0521 mm, and size increased from the fourth generation. The lower size limit was 7.2628 mm, and the upper was 9.9217 mm.
There was no statistical difference in the weight and size of male pupae among first, third, fourth, and fifth generations; however the weight and size of the second generation showed a significant difference (F=10.776, d.f. = 4, P < 0.001) (Table 3). Weight of male pupae averaged 0.0188 ± 0.0003 g, with a lower limit of 0.0161 g and an upper limit of 0.0195 g. Second generation male pupae size also showed a significant difference (F = 10.606, d.f. = 4, P < 0.001) compared to the others. The size was around of 6.919 ± 0.0419 mm, with a lower limit of 7.2342 mm and an upper limit of 7.7744 mm.

Widths of the larval head-capsules during larval development showed a significant difference (F = 337.531, d.f. = 4, P < 0.001), in the same way the length of the head-capsules showed a high significant difference (F = 357.179, d.f. = 4, P < 0.001). Each instar could be defined according to the head-capsule measurements (Table 4) indicating that larvae underwent five instars in the laboratory on artificial diet.

DISCUSSION
Pest rearing under laboratory conditions is required when insects must be studied to determine their life cycle and biology in addition to obtaining several specimens for identification and deposition in museum collections. An artificial diet intended for lepidopterans was successfully utilized only after the first generation because the newly hatched larvae had not previously been in contact with any natural food source and artificial diet was readily accepted. No attempt was made to determine the nutritional requirements of this insect via manipulations of diet ingredients as the diet used successfully produced large healthy adult moths.
In general terms, the results of this study suggest that the nutritional requirements of this insect are similar to those of other lepidopterans and are capable of adapting to established environmental conditions in laboratory. However, eggs per female could no be counted because adult couples did not lay eggs while isolated; such behavior should be further studied.
The pupal weight was determined as a life cycle parameter, and it is considered as an indicator of the efficiency of the rearing conditions, this parameter is frequently taken as a surrogate of adult body weight, and is also an accurate expression of the total weight gain achieved by the larvae (García-Barros 2006).
In this study we observed an increment of weight and size of female pupae, which were the best adapted to laboratory conditions, although male pupae did not show an increment in weight or size; these measurements were constant from the first to the fifth generation with the exception of the second generation where individuals were bigger than the others. This could be possible because of adaptive changes, or probably male larvae require some other ingredient which was not included in the diet, decreasing the insect development potential.
Larval head capsule width or length is a reliable tool for identifying instars in this lepidopteran. As no overlapping was observed, larval instars could be easily determined. This insect undergoes 5 larval instars, which is similar to the number of instars reported for Argyrotaenia velutinana (Glass 1963).
The behavior of this insect, the damage caused to the plant and the resemblance in larval morphology make this pest confused by growers with species of Amorbia. This means that Argyrotaenia has been present in avocado orchards as a minor pest, and has been confused with other species such as Amorbia emigratella. The mistaken identification of this insect is a fact that could impact avocado exports, since Amorbia is a quarantine pest. Moreover, the type of control applied against this pest is not the most suitable, and it runs the risk of being unintentionally spread to other areas with exported avocados. This paper contributes to the knowledge of this new pest not previously reported in avocado crops in Mexico, and to elucidate the confusion among avocado growers related to this insect pest identification.
ACKNOWLEDGEMENTS
This research received financial support from National Council for Science and Technology and Nayarit State Government, Project No. 9452. Authors wish to thank Dr. J. Brown from Systematic Entomology Laboratory, Agriculture Research Service, U. S. Department of Agriculture for insect identification, and Dr. José Luis Hernandez for his technical advice.
REFERENCES
Brown, J.W. & A. Cramer, 1999. Five new species of Argyrotaenia (Tortricidae: Archipini) from Mexico and the Southwestern United States. J. Lepidopterists' Soc.. 53:114-125. [ Links ]
Ebeling. W. & R.J. Pence. 1957. Orange Tortrix on Avocados. Pest becoming of increasing economic importance on certain varieties of avocado in some orchards in the coastal areas. Calif. Agric. 11:13-14. [ Links ]
García-Barros, E. 2006. Number of larval instars and sex-specific plasticity in the development of the small heath butterfly, Coenonympha pamphilus (Lepidoptera: Nymphalidae). Eur. J. Entomol. 103:47-53. [ Links ]
Glass, E.H. 1963. A pre-diapause arrested development period in the red-banded leaf roller, Argyrotaenia velutinana. J. Econ. Entomol. 56(5):634-635. [ Links ]
Glass, E.H. & G.E.R. Hervey. 1962. Continuous rearing of the red-banded leaf roller, Argyrotaenia velutinana. J. Econ. Entomol. 55(3):336-340. [ Links ]
Roelofs, W.L. 1967. Agarless medium for mass rearing the red-banded leaf roller. J. Econ. Entomol. 60(5):1477-1478. [ Links ]
Rosas-García, N.M. 2002. Elaboración de formulados de Bacillus thuringiensis var. kurstaki y determinación de la actividad tóxica contra larvas de Diatraea saccharalis (Fabricius) (Lepidoptera: Pyralidae) en laboratorio y campo. Tesis de Doctorado en Ciencias con especialidad en Biotecnología. F.C.B. División de Estudios de Postgrado. U.A.N.L. Monterrey, N.L. México. [ Links ]
Trematerra, P. & J.W. Brown. 2004. Argentine Argyrotaenia (Lepidoptera: Tortricidae): Synopsis and descriptions of two new species. Zootaxa 574:1-12. [ Links ]
Trudel, R., E. Bauce, J. Cabana & C. Guertin. 1995. Rearing technique for Dioryctria abietivorella (Lepidoptera: Pyralidae). J. Econ. Entomol. 88(3):640-643. [ Links ]
Vanderzant, E.S. 1974. Development, significance, and application of artificial diets for insects. Annu. Rev. Entomol. 19:139-160. [ Links ]
Wilcox, C.D., S. Brent, W. Dove, D. McDavid & D. B. Greer. 2002. Image Tool version 3.0. University of Texas Health Science Center, San Antonio, Texas. [ Links ]














