Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex no.89 Xalapa ago. 2003
Artículo
Ecological patterns in necrophilous Staphylinidae (Insecta: Coleoptera) from Tlayacapan, Morelos, México
Juan Márquez
Centro de Investigaciones Biológicas, U. A. E. H. Apartado postal 1-69, Plaza Juárez, Pachuca, Hidalgo, 42001, MÉXICO. jmarquez@uaeh.reduaeh.mx
Recibido: 11 de junio 2002
Aceptado: 21 de noviembre 2002
Resumen
Se estudiaron los Staphylinidae necrófilos en cinco sitios de Tlayacapan, Morelos, México. Se colectaron 5,192 ejemplares de 76 especies, utilizando necrotrampas modelo NTP-80. Los sitios 1 (bosque de pino-encino), 3 (bosque mesófilo de montaña) y 4 (bosque tropical caducifolio) presentaron menor grado de perturbación, poca variación de temperatura y alta humedad; en contraste, los sitios 2 (bosque de pino) y 5 (ecotono entre cultivos y bosque tropical caducifolio) presentaron mayor perturbación, alta variación de temperatura y baja humedad. La mayor riqueza de especies, abundancia y diversidad se registró en los primeros sitios (1, 3 y 4) posiblemente debido a la mayor abundancia de alimento, refugios y a las condiciones abióticas más estables y favorables. La mayor riqueza de especies y abundancia ocurrió durante la época de lluvias en todas los sitios, excepto en el sitio 4, donde el mayor pico de abundancia fue en la época seca debido a que los recursos se concentran en la parte más húmeda de este lugar. Los resultados sugieren que los estafilínidos necrófilos se distribuyen preferentemente en sitios poco perturbados y muy húmedos. Los niveles de humedad durante la época seca pueden ser un factor limitante en la distribución y/o actividad de algunas especies.
Palabras clave: Staphylinidae, necrófilos, patrones ecológicos, Tlapacoyan, México.
Abstract
Necrophilous Staphylinidae were studied using carrion traps model NTP-80 at five sites in Tlayacapan, Morelos state, México. A total of 5,192 specimens belonging to 76 species were collected. Three sites, 1 (pine-oak forest), 3 (cloud forest), and 4 (tropical deciduous forest) had little temperature variation, high humidity and relatively undisturbed conditions; in contrast, sites 2 (pine forest) and 5 (cultivated area and tropical deciduous forest mixture) had greater temperature variation, lower humidity and more disturbance. Highest species richness and abundance were recorded in sites 1, 3 and 4, possibly because of more abundant food resources, microhabitats and stable favorable abiotic conditions. Maximum species richness and abundance occurred during the rainy season except at site 4, which had dry season peaks; many staphylinids are concentrated in the most humid part of this site, seeking resources available only there during the dry season. The faunas of sites 3 and 4 were most similar, that of site 2 least similar to the others. The richer faunas of sites 1, 3, and 4 suggest that most necrophilous staphylinids prefer less disturbed and moister sites. Dry season moisture levels may be an important factor limiting the distribution and/or activity of some species.
Key Words: Staphylinidae, necrophilous, ecological patterns, Tlapacoyan, Mexico.
Introduction
Rove beetles (Staphylinidae) make up a large but poorly known element of biodiversity in most terrestrial ecosystems: over 47,000 species have been described worldwide, placed in more than 3,300 genera (Newton et al. 2000 and unpubl. data). In even only moderately rich habitats (e.g., oak woodland in northern Illinois, USA) there can be some 200 species coexisting (Thayer, unpubl. data) and higher diversity have been found in tropical forests (Chung et al. 2000, ca. 350; Hammond 1990, ca. 750). Quantitative studies in several forest habitats have found it to be the most abundant and speciose family of beetles (Farrell & Erwin 1988, Peru, tropical rainforest canopy, most diverse non-phytophagous group; Davies & Margules 2000, temperate Australia, Eucalyptus forest pitfall trapping; Chung et al. 2000, Malaysia, tropical forests and plantations) or even the second most speciose family of arthropods (Basset 1991, tropical Australia, rainforest canopy).
México is an important part of the Meso-American hotspot of biodiversity (Myers et al. 2000), with 1456 species of rove beetles recorded so far (Navarrete-Heredia et al. in press), it is likely that there are 5,000 or more (Navarrete-Heredia & Newton 1996). Mexican rove beetles are in general poorly known, as is the case in most areas outside western Europe. Members of this highly diverse group exploit several resources and have a variety of feeding habits, but many, perhaps most, are predators. Evidence from other parts of the world suggests that many have specific habitat and/or microhabitat requirements, but the composition and dynamics of this important component of Mexican forest ecosystems has only just begun (reviewed by Navarrete-Heredia et al. in press, and references below).
Carrion is an effective bait to collect many rove beetles and other insects. These species can be considered necrophilous in a broad sense. Some staphylinids may come to the carrion to feed directly on it, whereas others are predators of different arthropods or other invertebrates. It is not well known which species are specifically "necrophagous" and which are "necrophilous"; for the latter, their degree of preference for carrion is not well known. Carrion-baited traps can be useful to study staphylinids, because they can collect a large number of species and specimens, and their accurate sampling (Hanski & Hammond 1986) allows meaningful comparisons across space, habitat types, and time.
Tlayacapan is an interesting country because within a relatively small area there are five different vegetation types and it is enclosed in a conservation area, the "Corredor Biológico Chichinautzin" (Contreras & Urbina 1995). Several publications and some thesis have been produced previously on particular groups of staphylinids of Tlayacapan (Márquez & Navarrete-Heredia 1995; Navarrete-Heredia & Márquez 1995, 1998; Navarrete-Heredia 1995, 1996, 1998), and irregular collections have been made during the last seven years in addition to the systematic sampling described herein. The integration of this information will contribute further to the knowledge of the Tlayacapan staphylinid diversity.
Taxonomic information (keys and comments), degree of association with carrion, and distributional records resulting from this study have been presented elsewhere (Márquez 2001), but ecological considerations are the goal of the present work. In particular, species richness, abundance, evenness, and phenology are analyzed with reference to environmental parameters for each site. Also similarity among sites is analyzed. Species belonging to the subfamily Aleocharinae were excluded, because of the great difficulties in identifying them (Seevers 1978).
Study site. Tlayacapan, the principal town of the municipality with the same name, is located in the northern part of the state of Morelos (18°57'12" N and 98°59'00" W) at an elevation of 1,630 m. For more information, including a map of the collecting site locations, see Contreras & Urbina (1995), Márquez (2001), Márquez & Navarrete Heredia (1995), and Navarrete-Heredia (1995).
Elevation was measured with an altimeter at the sampling sites, which are west and northwest of the town of Tlayacapan. Many classification systems have been proposed for the complex vegetation of México. The English-language names used here for vegetation types are those of Leopold (1950), with their equivalent Spanish-language terms given in parentheses below (following Rzedowski 1978 and as used by Márquez 2001, with the exception noted below). The sites were as follows:
Site 1, pine-oak forest (bosque de pino-encino) at 1,874 m elevation, has dense vegetation and is little disturbed compared to sites 2 and 5. It is closest to site 2 (which is at a higher elevation and to the northeast), then to site 3 (at a slightly lower elevation and to the west), and more distant from sites 5 and 4 (to the southeast and south, respectively).
Site 2, pine forest (bosque de pino) at 1,930 m elevation, has only scattered trees, probably because of the rocky soil and human activities. It is close only to site 1 (at a lower elevation to the southwest). It is less disturbed than site 5, but more disturbed than sites 1, 3 and 4.
Site 3, cloud forest (bosque mesófilo de montaña) at 1,783 m elevation, has dense vegetation and is little disturbed; the specific sampling site was at the bottom of a canyon that has a seasonal river. It is closest to sites 1 and 2 (at higher elevations to the east) and is separated by the canyon from site 4 by an area of pine-oak forest at a higher elevation that includes site 1.
Site 4, tropical deciduous forest (bosque tropical caducifolio [=selva baja caducifolia of Márquez 2001]) at 1,534 m elevation, has similar conditions to site 3, but is in a separate canyon with a small permanent river. It is closest to site 5 (at a slightly higher elevation to the northeast), and more distant from sites 1-3 (at higher elevations to the north).
Site 5, mixture of cultivated area and tropical deciduous forest (ecotono entre cultivo de temporal y bosque tropical caducifolio [=selva baja caducifolia of Márquez 2001]) at 1,634 m elevation, has a few trees and a predominance of shrubs. The forest is very close to the cultivated areas (separated by only approximately ten meters) at the same elevation; the site is about the same distance from the site 4 (at a slightly lower elevation to the southwest) and sites 1-3 (at higher elevations approximately to the northwest). This site was selected to contrast a highly disturbed site with the other little-disturbed ones.
Methods
One carrion trap model NTP-80 (Morón & Terrón 1984) baited with squid was installed at each site. Sampling was done monthly, with bait replacement, during one year (July, 1995 to June, 1996; 60 samples). Air temperature and relative humidity were measured monthly at each sampling site with a thermometer and hygrometer, respectively. Irregular sampling done over the last seven years complements these standardized data on the staphylinid diversity of the area. Specimens from this study are deposited in the Museo de Zoología, Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D. F. México (MZFC).
The Shannon diversity index (H') and evenness index (E) were used (Magurran 1988). The SPDIVERS computing program (Ludwig & Reynolds 1988) was used to calculate the diversity and evenness. A "t" test was applied to known whether the diversity measures are significantly different among sites (Magurran 1988). The Sorensen index (quantitative version, CN, Magurran 1988) was used to measure faunal similarity between sites.
Results and discussion
A total of 5,192 specimens were collected, belonging to 11 subfamilies, 39 genera and 76 species. Forty-two taxa were identified to species, nine as near to species, 24 to genus and morphospecies, and one to subtribe. The numbers of specimens collected, by species and month in each site, should be consulted in Márquez (2001).
Environmental temperature variation
There are no meteorological stations at the studied sites to provide detailed environmental records, such as rainfall, humidity or temperature, that would allow thorough investigation of staphylinid preferences with regard to such abiotic factors. It is reasonable to assume that the records taken in the field are not really sufficient to assess these factors, but may provide a general indication of preferences. Each of the five sites had variable temperatures in the dry season (late November to May), in contrast with the rainy season temperatures, which were relatively homogeneous (Table 1). The highest temperatures were measured at sites 5 (cultivated area-tropical deciduous forest mixture) and 2 (pine forest), followed by sites 4 (tropical deciduous forest), 1 (pine-oak forest), and 3 (cloud forest). The highest temperature variations (17°C) were also at sites 5 (17° to 34°C) and 2 (16° to 33°C); the smallest temperature variation (10°C) was at site 3 (17° to 27°C), with variation between sites (11°C), 1 and 4 strongly different from that (both 16° to 27°C) (Table 1).

Environmental humidity variation
Variation in humidity were similar at all sites, the rainy season (June to early November) having higher humidity and the dry season (late November to May) lower (Table 1). The lowest humidity levels were from February to April, late in the dry season. Sites 2 and 5 had the lowest average humidity and greatest variation, and sites 3, 4 and 1 had higher and slightly less variable average humidities (Table 1). Differences among sites were more pronounced in the dry season than the rainy season.
Species richness
Seventy-six species of Staphylinidae were collected. Staphylininae was the subfamily with highest species number (40), followed by Tachyporinae (10), Paederinae (7), Oxytelinae (4), Pselaphinae (4), Scaphidiinae (4), Proteininae (2) and Omaliinae (2). Only one species each of Osoriinae, Pseudopsinae and Steninae were collected.
In similar studies in other Mexican localities, Staphylininae have also been the subfamily with the highest species number (Huacuja 1982, Hidalgo; Ruíz-Lizárraga 1993, Guerrero; Santiago 1999, Veracruz; Jiménez-Sánchez et al. 2000, Estado de México, 2001, Guerrero), and similar results have been obtained for staphylinids associated with debris of the leaf-cutting ants Atta mexicana (Márquez & Navarrete Heredia 1995) and with mushrooms (Navarrete-Heredia 1996) from the Tlayacapan area. In addition, many Staphylininae species have been observed in debris or soil and under rocks in the study area. This large species number contrasts with the other subfamilies collected, some species of which are found mainly in specific habitats, such as mushrooms, rocks along rivers, dung, etc. The subfamily composition of hand-collecting might, however, be influenced somewhat by the larger average body size of Staphylininae.
The highest number of species was collected in site 3 (44), followed closely by site 4 (43) and site 1 (41), then site 5 (27), with site 2 extremely depauperate (14) (Table 1). It is likely that the less disturbed conditions and the temperature and humidity of sites 1, 3 and 4 provide more resources and microhabitats for staphylinids; in contrast, sites 2 and 5, which are very disturbed (especially 5), have higher temperature variation and lower humidity, resulting in adverse physical conditions and probably poorer development of microhabitats. Another interesting result is the higher species richness in the very disturbed site 5, in contrast to site 2. Site 5 presented nearly twice as many species as the less disturbed site 2, in spite of the similar temperature and humidity conditions. This could indicate a greater degree of tolerance by some staphylinid species for these conditions, and the ability of these species to use various resources derived from human activities (such as domestic animal carcasses, mouse carcasses among the crops, dung, and garbage), or might reflect the higher habitat heterogeneity compared to the other sites.
Abundance
Of the total specimens, 34.53% were collected in site 3 (1,793 specimens), 29.64% in site 1 (1,539 specimens), 26.35% in site 4 (1,368 specimens), 5.82% in site 5 (302 specimens) and 3.66% in site 2 (190 specimens) (Table 1). The abundance in site 3 ranked it highest in this respect, as for species richness, but the relative abundance ranks of sites 1 and 4 (which differed only slightly in species richness) were reversed. Some species collected in both of the latter two sites had different abundances, e.g., Anotylus aff. fragilis (Sharp) (667 specimens in site 1, 5 in site 4) and Belonuchus rufipennis (Fabricius) (110 specimens in site 1,687 in site 4). Other species shared by these sites also have different densities, as suggested by the low Sorenson (quantitative) similarity between the two (see faunal similarity). The abundance ranks of sites 2 and 5 were the same as their respective species richness ranks. Again, there were differences in the abundance of shared species: Belonuchus apiciventris (Sharp) and B. rufipennis were the two most abundant species in site 2 (89 and 50, respectively), but only the second was dominant in site 5 (14 and 177, respectively, with B. apiciventris outranked by three other species).
Belonuchus rufipennis was the most abundant species overall, with 1,827 specimens, followed by Anotylus aff. fragilis (903), Phloeonomus centralis Blackwelder (365), Belonuchus apiciventris (317), Philonthus sericans (Gravenhorst) (265), Chroaptomus flagrans (Erichson) (197), Belonuchus oxyporinus (Sharp) (174), Belonuchus sp. nov. 2 (162), Proteinus sp. (108) and Styngetus adrianae Navarrete-Heredia (100). The remaining species were represented by fewer than 100 specimens each (nearly all by fewer than 50), 21 of them by only one specimen.
Abundance in carrion traps can be an indicator of affinity for carrion, but some species are also very abundant in other resources and apparently do not have a strong preference for carrion, or, on the contrary, a species may have a preference for carrion but a low abundance because of actual rarity or seasonal or habitat factors.
The abundance is not necessarily direct evidence of the importance of a species in some ecological processes, such as predation or carrion scavenging. Some abundant species are small, such as Anotylus aff. fragilis, Phloeonomus centralis, and Proteinus sp., and 100 specimens of them may have a weight similar to that of ten Platydracus specimens. Species of Belonuchus, Platydracus, Philonthus, plus Chroaptomus flagrans and Styngetus adrianae are large insects, very abundant in carrion, and are important as predatory species in this habitat. Their role as carrion scavengers is not clearly known, but probably they are not as important as other beetles (silphids or scarabs). Indeed, to the extent that they prey upon Diptera larvae or other carrion-consuming insects, these large staphylinids might actually slow the disappearance of carrion if they have a major impact on dipteran numbers. Others, such as Anotylus spp., Phloeonomus spp., and Proteinus sp., might be involved in consuming carrion, but their food habits are not well understood.
Belonuchus rufipennis, the most abundant species in this study, was collected in all sites and all the year, as found also by Jímenez-Sánchez et al. (2001) in Guerrero; it is apparently very tolerant of disturbed conditions, and may be preying on some pest insects in site 5, where it made up over half the individuals captured.
Diversity
The Shannon diversity index (H') takes both species richness and abundance into account, and usually has been found to fall between 1.5 to 3.5, rarely exceeding 4.5 (Margalef 1972, cited by Magurran 1988). Evenness (E) expresses the distribution of abundances or degree of dominance and takes values from 0 to 1, with the maximum value indicating that all species are equally abundant (Magurran 1988). The highest faunistic diversity was in site 1, followed by sites 3, 4, 5 and 2 (Table 2), with the latter being near the minimum. The highest evenness was also in site 1, followed by sites 2, 3, 5 and 4 (Table 2).

The comparison of diversity values between each pair of sites indicate that this is significantly different, with the exception of site 1 versus site 3, and site 2 versus site 5 (Table 3). Significant differences in diversity can result from differences in species richness, in evenness, or in both.
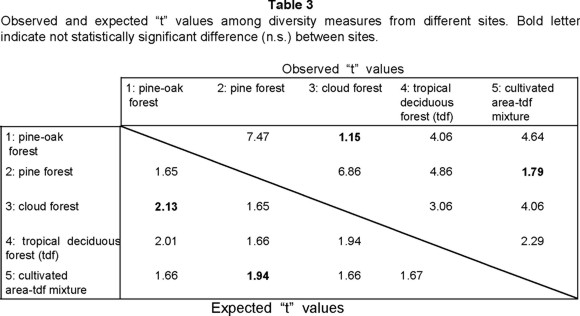
Similar diversity in sites 1 and 3 can be expected due to their similar abiotic conditions, conservation degree, geographic proximity, species richness and abundance. In contrast, sites 2 and 5 have different disturbance degree, species richness and abundance, with only the abiotic conditions somewhat similar, but the more homogeneous distribution of specimens number by species (high evenness) in site 2 is the principal fact that caused not significant diversity differences. In contrast, sites 3 and 4 have significant differences, in spite of to have only one species of difference. The result was apparently caused by higher evenness and abundance in site 3 compared with site 4. The same appears to be the case for sites 1 and 4.
Species distribution in the study area and biological comments
Some species were collected exclusively at each site (Table 2), except for the site 2. These results do not necessarily indicate that the species are truly exclusive to the site where they were collected, but may result from low trap efficiency or unattracted to carrion, such that those species are not consistently collected in carrion traps. Some species might have a preference for (but not necessarily a restriction to) the vegetation type characteristic of the site where they were collected. These include: in site 1 Platydracus sp. nov. 26 and Platydracus sp. nov. 36; in site 3 Megarthrus aff. altivagans Bernhauer and Belonuchus viridipennis Baudi; in site 4 Gabrius sp., Paederomimus gentilis Sharp, Quedius sp. and Platydracus biseriatus (Sharp); in site 5 probably Oxytelus laqueatus (Marsham), Anotylus aff. insignitus (Gravenhorst) and Heterothops boops Bernhauer. Although most of these collections were represented by singletons and the species may simply be rare. The inference of habitat preference for some species is supported by previous contributions (Márquez & Navarrete-Heredia 1995, Navarrete-Heredia 1996), by the records of species occurring in similar vegetation types (Márquez 2001), and by the observations from the irregular sampling in the last seven years at the study area.
Some species were collected only in undisturbed sites and others only in disturbed ones, and it is possible that such species might be indicators of degree of disturbance. Sites 3, 4 and 1 are relatively undisturbed, and sites 2 and 5 very disturbed. Proteinus sp., Pseudopsis sulcata complex, Belonuchus sp. nov. 1, Belonuchus sp. nov. 2, and Paederomimus angularius (Erichson) were collected exclusively in the undisturbed sites and Phloeonomus centralis, Belonuchus oxyporinus, and Chroaptomus flagrans had distinctly higher abundances there. In contrast, Platydracus mendicus (Sharp) was collected with much higher abundance in disturbed than undisturbed sites. Jíménez-Sánchez et al. (2001) similarly found this species to be more abundant in pasture than in tropical deciduous forest, but they found it even less abundant in a more disturbed cultivated site. Only a few species, such as Belonuchus rufipennis, B. apiciventris, and B. basiventris (Sharp), were commonly collected in both disturbed and natural undisturbed sites. These associations with different disturbance level could be misleading because of the relatively small sample sizes and the limited sampling methods used. More field work in the study area and in adjacent zones is required.
Phenology
The highest species and specimen numbers were collected during the rainy season months at four of the five sites; the exception was site 4 (tropical deciduous forest). There the maximum species richness and abundance was observed in the dry season, although its second highest total was in the rainy season. At site 3, the second highest monthly number of specimens was collected in May -- the very end of the dry season --and the humidity had already increased to rainy season levels (Table 1).
The exceptional phenology observed in site 4, and to a lesser degree in site 3, may be explained by the particular conditions of these sites: although they have strong seasonal variation with temperature and humidity, very similar to site 1, both are in canyons. In the dry season, the moisture is reduced, but less so in the canyon bottom, where the water remains all year, and many insects are limited to this area. The traps were installed in these deep sites, where staphylinids were present in great numbers, especially in the dry season. Site 1, despite its generally similar temperature and humidity, was flat and more open and lacked this late dry season primary or secondary peak of staphylinid abundance.
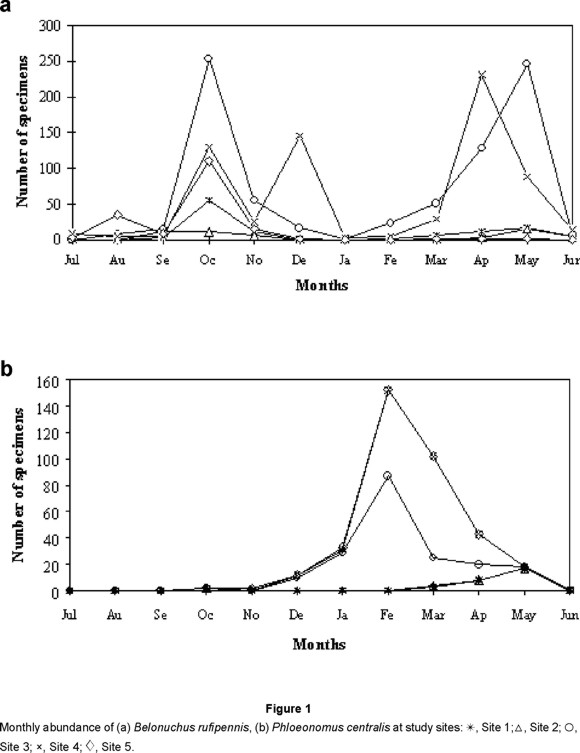
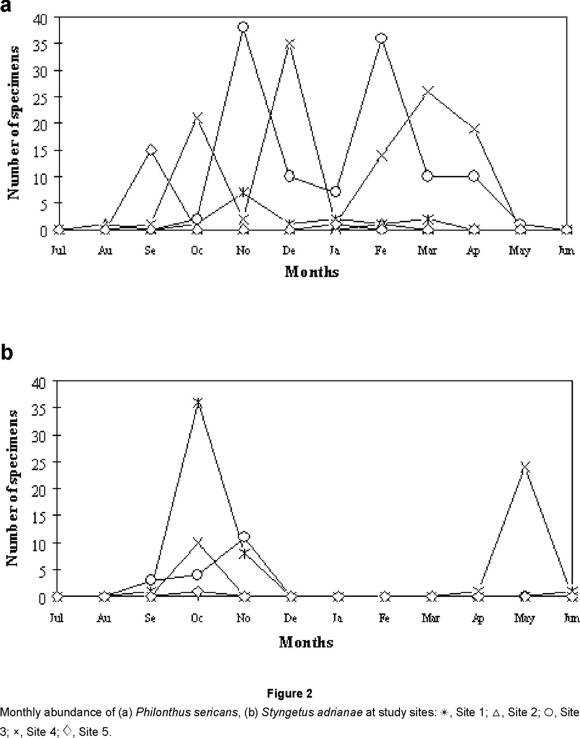
Six of the ten most abundant species (Belonuchus apiciventris, B. oxyporinus, Belonuchus sp. nov. 2, Chroaptomus flagrans, Proteinus sp. and Anotylus aff. fragilis) were more common in the rainy season. Belonuchus rufipennis had one principal peak of abundance in each season at sites 3 and 4 (the latter with an additional early dry-season peak), but only one peak (rainy season) at sites 1 and 5 (Fig. 1a); it may be a bivoltine species. Phloeonomus centralis was abundant only in the dry season (Fig. 1b). Philonthus sericans was collected in all sites almost year-round, with staggered peaks of abundance at different sites and almost no collections in the late dry to early rainy seasons (Fig. 2a). Styngetus adrianae seems to be present mainly at the time of transition between seasons (Fig. 2b), although the late dry-season peak at site 4 may reflect concentration of the population from a larger than usual area in the relatively moist area near the trap. Jiménez-Sánchez et al. (2001) also found two peaks for Belonuchus rufipennis in Guerrero, although at slightly different times, and collected Phloeonomus centralis only in the dry season.
Other species with interesting phenology were Belonuchus basiventris, which was collected in sites 1, 3 and 4 with bimodal abundance like that of B. rufipennis; B. xanthomelas Solsky was mainly collected in the dry season. Phloeonomus sp. nov. is partly sympatric and partly synchronous with P. centralis; Belonuchus sp. nov. 1 with B. apiciventris; and Belonuchus sp. nov. 2 with B. oxyporinus. Several species of Philonthus and Platydracus were abundant in the rainy season, and Gastrisus newtonorum Navarrete & Márquez was collected only then. With regard to Tachyporinae species, only Coproporus hepaticus (Erichson) was somewhat abundant, mainly in the dry season. It is not possible to comment on the phenology of other species represented by only one or a few specimens. For species collected in adequate numbers, these patterns are also generally similar to those found by Jiménez-Sánchez et al. (2001).
Faunal similarity
Using the Sorenson quantitative index, CN, the most similar sites were 3 and 4, followed rather distantly by site pairs 3 and 1, 4 and 5, 2 and 5, and then all others (Table 4). These results are initially surprising since sites 3 and 4 are different forest types, differ in elevation by 200 m, and are separated by higher-elevation oak-pine forest and pine forest. Their high faunal similarity, like their high species richness, may result from their both being situated in moist canyons with very similar seasonal temperature and humidity profiles that provide relatively stable and highly suitable habitats for Staphylinidae. Although a high similarity between sites 1 and 3 might be expected from their vegetation types, elevations, and adjacency, their difference in dry-season humidity (and thus perhaps precipitation) is greater than for sites 3 and 4; this, together with the canyon location of site 3, may account for their lower measured faunal similarity. Overall, the similarities between undisturbed sites (3 pairs, mean CN = 44.44%) and between disturbed sites (1 pair, CN = 37.21%) are greater than the similarities between mixed pairs of disturbed and undisturbed sites (6 pairs, mean CN = 20.30%). The average similarity of each site to all other sites shows a ranking of 3>4>5>1>2.
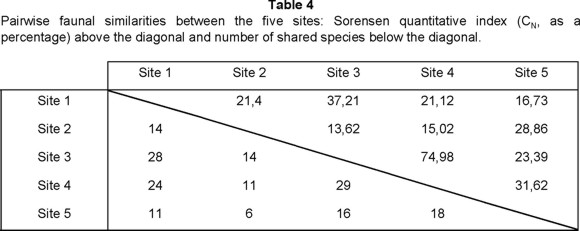
Site 1 was most similar to site 3, and next most similar to sites 2 and 4 equally. One might expect greater similarity between sites 1 and 2 than 1 and 4, because of their more similar vegetation types, elevation, and proximity, but the seasonal temperature and humidity profiles of sites 1 and 4 are more alike than those of 1 and 2, which may affect their faunal similarities. The small species number at site 2 (14) could also contribute to its low similarities to all other sites, since it reduces the potential shared species number, while the higher species number of site 4 permits a higher maximum possible similarity with the other richer sites. Site 2 was most similar to 5, the other disturbed site, and the next most species-poor one. Site 5 was most similar to 4, however, presumably as a result of the much higher number of shared species, which could result from the more similar vegetation in 4 and the forested part of 5.
Navarrete-Heredia (1996) studied the beetles associated with mushrooms near San José de los Laureles, Tlayacapan. His study area was partially situated in sites 1 and 3 of this study. He collected 46 species of staphylinids, 21 of which are shared with the 57 carrion species collected at sites 1 and/or 3 of the present study. Presently only a qualitative comparison can be made between the two studies because of differences in sampling. The Sorensen qualitative similarity index (CS, Magurran 1988, not directly comparable to the figures above) resulting from comparison of these two studies is 40.78%, indicating that numerous species are presumably not restricted to the resources upon wich they were found.
Conclusion
Carrion baited traps allowed the collection of good samples of species and specimens of staphylinid beetles from Tlayacapan. The highest records of species richness, abundance, and diversity in sites 1, 3 and 4 suggest that most necrophilous staphylinids are distributed preferentially in less disturbed and moister native forests. A similar result (although with smaller differences between habitat types) was obtained by Jiménez-Sánchez et al. (2001). Some species, however, were collected preferentially in the most disturbed sites, e. g. site 5; this is an interesting demonstration of the broader ecological tolerance of some species, that apparently are able to use a variety of microhabitats derived from human activities. A better understanding of the environmental factors influencing the distribution and abundance of necrophilous staphylinid species will require studies at a larger number of sites to allow comparisons between sites that differ in fewer (ideally, single) environmental variables.
Acknowledgements
I thank M. K. Thayer (Field Museum of Natural History, Chicago, USA) for her strong contribution to this work and the identification of Omaliinae species. I extend my thanks to A. F. Newton Jr. (Field Museum of Natural History, Chicago, USA) for his helpful comments on the manuscript and for the identification of Platydracus species. I thank the Field Museum of Natural History (Chicago, Illinois, USA) for financial support to visit their collection and identify specimens. I also thank the two anonymous reviewer for their comments.
Literature cited
Basset, Y. 1991. The taxonomic composition of the arthropod fauna associated with an Australian rainforest tree. Austr. Jour. Zool. 39: 171-190. [ Links ]
Contreras, M. T. & F. Urbina. (eds). 1995. Historia Natural del área de protección de Flora y Fauna silvestre Corredor Biológico Chichinautzin. Centro de Investigaciones Biológicas, Universidad Autónoma del Estado de Morelos; Cuernavaca, Morelos, México. 35 pp. [ Links ]
Chung, A. Y. C., P. Eggleton, M. R. Speight, P. M. Hammond & V. K. Chey. 2000. The diversity of beetle assemblages in different habitat types in Sabah, Malaysia. Bull. Entomol. Res. 90: 475-496. [ Links ]
Davies, K. F. & C. R. Margules. 2000. The beetles at Wog Wog: a contribution of Coleoptera systematics to an ecological field experiment. Inver. Tax. 14: 953-956. [ Links ]
Farrell, B. D. & T. L. Erwin. 1988. Leaf-beetle community structure in an Amazonian rainforest canopy. Pp. 73-90. In: Jolivet, P., E. Petitpierre & T. H. Hsiao. (eds.). Biology of Chrysomelidae. Kluwer Academic Publishers. [ Links ]
Hammond, P. M. 1990. Insect abundance and diversity in the Dumoga-Bone National Park, N. Sulawesi, with special reference to the beetle fauna of lowland rain forest in the Toraut region. Pp. 197-254. In: Knight, W. J. & J. D. Holloway. (eds.). Insects and rain forests of South East Asia (Wallacea). Royal Entomological Society, London. [ Links ]
Hanski, I. & P. Hammond. 1986. Assemblages of carrion and dung Staphylinidae in tropical rain forest in Sarawak, Borneo. Ann. Entomol. Fennici 52: 1-19. [ Links ]
Huacuja, A. H. 1982. Análisis de la fauna de coleópteros Staphylinidae saprófilos de Zacualtipán, Hidalgo. Tesis Profesional, Fac. de Ciencias, UNAM. México, D. F. 147 pp. [ Links ]
Jiménez-Sánchez, E., J. L. Navarrete-Heredia & J. Padilla-Ramírez. 2000. Estafilínidos (Coleoptera: Staphylinidae) necrófilos de la Sierra de Nanchititla, Estado de México, México. Folia Entomol. Mex. 108: 53-78. [ Links ]
Jiménez-Sánchez, E., J. Padilla-Ramírez, S. Stanford-Camargo & R. Quezada García. 2001. Staphylinidae (Insecta: Coleoptera) necrófilos de "El Salto de las Granadas", Guerrero, México. Pp.55-68. In: Navarrete-Heredia, J. L., H. E. Fierros-López & A. Burgos-Solorio (eds.). Tópicos sobre Coleoptera de México. Universidad de Guadalajara y Universidad Autónoma del Estado de Morelos, Guadalajara, México. [ Links ]
Leopold, A. S. 1950. Vegetation zones of Mexico. Ecology 31: 507-518. [ Links ]
Ludwig, A. J. & J. F. Reynolds. 1988. Statistical Ecology. A primer on methods and computing. John Wiley & Sons. New York, U.S.A 337 pp. [ Links ]
Magurran, A. E. 1988. Ecological Diversity and its measurement. Croom Helm, London, Great Britain. 179 pp. [ Links ]
Márquez, L. J. 2001. Especies necrófilas de Staphylinidae (Insecta: Coleoptera) del municipio de Tlayacapan, Morelos, México. Folia Entomol. Mex. 40(1): 93-131. [ Links ]
Márquez, L. J. & J. L. Navarrete Heredia. 1995. Especies de Staphylinidae (Insecta: Coleoptera) asociadas a detritos de Atta mexicana (F. Smith) (Hymenoptera: Formicidae) en dos localidades de Morelos, México. Folia Entomol. Mex. 91: 31-46. [1994] [ Links ]
Morón, M. A. & R. Terrón. 1984. Distribución altitudinal y estacional de los insectos necrófilos en la Sierra Norte de Hidalgo, México. Acta Zool. Mex. (n. s.) 3: 1-47. [ Links ]
Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. Da Fonseca & J. Kent. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-858. [ Links ]
Navarrete-Heredia, J. L. 1995. Aspectos biológicos de Philonthus apiciventris y P. oxyporinus (Coleoptera: Staphylinidae: Staphylininae) en una zona de Morelos, México, con una lista de las especies mexicanas de Philonthus. Anal. Inst. Biol., Univ. Nac. Aut. Méx., Ser. Zool. 66(1): 81-106. [ Links ]
----------. 1996. Coleópteros micetócolos de Basidiomycetes de San José de los Laureles, Morelos, México. Tesis Profesional (Maestría en Ciencias), Fac. de Ciencias, UNAM, México, D. F. 179 pp. [ Links ]
----------. 1998. Descripción de Styngetus adrianae sp. nov., incluyendo nuevos datos de distribución para las especies de Styngetus de México (Coleoptera: Staphylinidae). Folia Entomol. Mex. 101: 59-71. [1997] [ Links ]
Navarrete-Heredia J. L. & J. Márquez. 1995. Rediscovery of Oxyporus flohri (Coleoptera: Staphylinidae) from Mexico and new distributional records of two other Mexican Oxyporus. Ent. News 106(1): 39-43. [ Links ]
----------. 1998. A new Mexican species of Gastrisus (Coleoptera: Staphylinidae). Ent. News 109(4): 225-232. [ Links ]
Navarrete-Heredia, J. L. & A. F. Newton, Jr. 1996. Staphylinidae (Coleoptera). Pp. 369-380. In: Llorente Bousquets, J., A. N. García Aldrete & E. González Soriano. (eds). Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento. Instituto de Biología, UNAM, CONABIO, Fac. de Ciencias, UNAM, México. [ Links ]
Navarrete-Heredia, J. L., A. F. Newton, M. K. Thayer, J. S. Ashe & D. S. Chandler. [2002]. Guía ilustrada de los Staphylinidae (Coleoptera) de México. CONABIO y Universidad de Guadalajara, México D. F. y Guadalajara, México. (In press) [ Links ]
Newton, A. F., M. K. Thayer, J. S. Ashe & D. S. Chandler. 2000. Family 22. Staphylinidae Latreille, 1802. Pp. 272-418. In: Arnett, R. H., Jr. & M. C. Thomas (eds.). American Beetles, Volume 1, Archostemata, Myxophaga, Adephaga, Polyphaga: Staphyliniformia. CRC Press LLC, Boca Raton, FL. xv + 443 pp. [ Links ]
Ruíz-Lizárraga, G. 1993. Contribución al conocimiento de los Staphylinidae (Coleoptera) necrófilos de Acahuizotla Guerrero. Tesis Profesional, Fac. de Ciencias, UNAM. México, D.F. 177 pp. [ Links ]
Rzedowski, J. 1978. Vegetación de México. Edit. Limusa, S. A. México, D. F., México. 432 pp. [ Links ]
Santiago, J. Q. 1999. Los Staphylinidae (Insecta: Coleoptera) necrófilos y coprófilos de un gradiente altitudinal en la región central del Estado de Veracruz. Tesis profesional, Fac. de Biología, Universidad Veracruzana. Xalapa, Veracruz. 126 pp. [ Links ]
Seevers, C. H. 1978. A generic and tribal revision of the North American Aleocharinae (Coleoptera: Staphylinidae). Fieldiana: Zoology, 71: 1-275. [ Links ]














