Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Acta zoológica mexicana
versión On-line ISSN 2448-8445versión impresa ISSN 0065-1737
Acta Zool. Mex no.87 Xalapa dic. 2002
Article
Soil meso-fauna patterns and experiments on leaf litter mite fungivory: preferences, effects on fungal reproduction and decomposition
Roger Guevara, Lorenzo Villedo and Andrea Nájera
Instituto de Ecología, A.C. División de Ecología, Departamento de Biología de Suelos, A. Postal 63, CP 91000, Xalapa, Ver. MÉXICO.
Recibido: 24 de mayo 2001
Aceptado: 11 de abril 2002
Resumen
En la primera parte de este estudio se investigaron los patrones estructurales de la comunidad de mesofauna en un bosque mesófilo de montaña encontrando que la hojarasca y el suelo como tal albergan comunidades estructuralmente diferentes. La hojarasca parece ser un hábitat complejo, heterogéneo, mientras que el suelo parece ser homogéneo. Las principales diferencias entre los dos hábitats están dadas por la abundancia de ácaros y colémbolos y se discuten sus patrones de distribución en relación con aquellos factores que pudiesen estar determinando la actividad de la micobiota en el suelo ya que la fungivoría es un hábito ampliamente distribuido entre ácaros y colémbolos. En la segunda parte de este estudio se realizaron experimentos de microcosmos en los que se observó que la actividad trófica de una especie de ácaro (Oppidae) afecta la producción de esporas de cuatro especies de hongos lo que sugiere un efecto sobre su capacidad competitiva.
Palabras clave: Consumo selectivo, Estructura de la comunidad, Fungivoría, Hongos de la hojarasca, Mesofauna, Oppidae.
Abstract
We investigated patterns of community structure of soil meso-fauna in an tropical cloud forest. The leaf litter habitat and the actual soil below were found to support structurally different communities. Leaf litter appeared to be a complex, heterogeneous, habitat whereas soil was homogeneous. The abundance of mites and collembolans marked the main differences between leaf litter and soil, and given that a large number of species of these taxa are fungivorous their distribution patterns are discussed in relation to those factors that may determine fungal activity. In microcosmos experiments we found that a fungivorous oppid mite (Oppidae) affected the spore production of four species of fungi suggesting a shift in their combative abilities.
Key Words: Community structure, Fungivory, Leaf litter fungi, Oppidae, Selective feeding, Meso-fauna.
Introduction
Decomposition of plant organic matter, the antithesis of photosynthesis, is a major process directly linked to soil fertility. Therefore, a main challenge for soil biologists is to understand the dynamics of the decomposition process and its relations to the origin and maintenance of soil fertility in natural and cultivated systems. In order to do this, it is necessary to understand the biology of decomposer organisms and their interactions with the physical and biotic environments.
Fungi are an important group of decomposer organisms. As a group fungi degrade all kinds of organic matter and man-made products, including even plastic (Cooke & Rayner 1984). Furthermore, fungi are the main decomposer group of the products of primary production, leaf litter, twigs, branches, stems, trunks and roots. Therefore, fungi are central to understanding the process of decomposition in terrestrial ecosystems (c.f., Rodin & Basilevic 1967, 1968). In accordance, fungivory stands up as a key interaction to be study if we aim to understand the dynamics of the decomposition process (c.f., Swift & Boddy 1984) and its implications to soil fertility.
The body of studies of soil fungus-fungivore interactions consists mainly of isolated works, usually focusing only on one aspect of these interactions. Some studies have investigated mechanisms used by fungivores to discriminate among different fungi. For instance, Hedlund et al. (1995) showed that some collembolans differentiate fungal species on the base of aroma compounds. This behavioural capability offers the potential of selective feeding and effects on fungal community structure. Thus, for instance Newell (1984a, 1984b) showed that Onychiurus latus Gisin allowed extended populations of the fungus Mycena galopus [Pers. ex. Fr.] Kummer in the field by selectively feeding on a strong combative species, Marasmius androsaceus L. ex F. The later fungus dominates in collembolan-free systems. Similarly, Parkinson et al. (1979) showed that selective feeding by Onychiurus subtenuis Folsom biased the competitive capabilities of two species of fungi on aspen litter. Furthermore, selective fungivory may also directly influence the genetic structure of fungal populations since mycelium fragmentation (a means of asexual reproduction) may be favoured in some interactions (Bengtsson et al. 1993). In relation to decomposition other studies have shown that fungivory increased the respiration rate of the micro-biota in decomposing leaf litter (Bear et al. 1992, Hedlund et al. 1995, Hedlund & Oehrn, 2000; Kaneko et al. 1998, Siepel & Maaskamp 1994).
In this study we present a general perspective of the interactions between an oppid mite and some common soil fungi. First, we present a brief description of the soil meso-fauna in a remnant of evergreen cloud forest. Then, we set up two experiments to explore three questions. Is the performance of mite colonies affected by different species of fungi used as food resource? Does fungivory by mites affect fungal reproductive success? Does fungivory by mites affect the rate of leaf litter decomposition?
Methods
Study site
This study was carried out in the protected area "Santuario del Bosque de Niebla" (Cloud Forest Sanctuary) located on 19°30' N and 96°57' W' with altitude around 1280 m, near to Xalapa, Veracruz, Mexico. The original plant community in the area was tropical cloud forest that covered the central part of Veracruz state between 1200 and 1600 meters above the sea level (Castillo-Campos 1991). Now, the actual plant community in the protected area (30 ha.) is a mixture of remnants of tropical cloud forest (28%), various successional stages derived from abandoned coffee plantations (20%) and cleared areas (52%) (personal communication of O. Gomez-García).
Soil meso-fauna community
We collected 20 soil cores (20x20x20 cm) and 20 leaf litter samples (20X20 cm) in August 2000. Leaf litter samples were taken from the surface area of each soil core and all samples were collected from randomly selected points. This was done by generating random number between 0 to 1000 which corresponded to distances along the main trail in the study site. All samples were taken 30 m into the forest.
In the laboratory, we placed soil cores and leaf litter samples in Berlese's funnels (c.f., Coleman & Crossley Jr. 1996) with a 2 mm grid and allowed the samples to dry for seven days aided by a 40 W light bulb placed above each sample. All samples were covered with nylon mesh and soil meso-fauna was collected in 70% alcohol. Then, using a stereoscopic microscope, we sorted (at order level and including a category for immature stages) and counted all individuals of soil meso-fauna in each sample.
Experimental design
Because mites were among the most abundant components of the soil meso-fauna and an unidentified fungivorous oppid mite (Oribatida: Oppidae) was especially abundant, we established cultures of this mite (fed on Penicillum sp growing on 2% malt extract at room temperature) for experimental studies.
Decomposition experiment
The first experiment consisted of 80 microcosmos replicates set up in plastic chamber (8 x 8 cm high and diameter) with a 2 cm2 hole in the lid covered with nylon mesh. We added to each container 2 g of wet leaf litter extracted from a bulk amount. Leaf litter was collected at the study site and chopped into 1 cm2 pieces, wet and homogenised. Then, from the bulk chopped leaf litter we weighed 80 samples (2 g each) to a precision of 0.1µg, taking each sample from the centre of the bulk to minimise variation in water content between samples. Then we prepared a suspension of spores of four leaf litter fungi (Doratomyces microsporus (Saccardo) Morton & Smith, Acrogenospora sphaerocephala (Berkeley & Broome) M.B. Ellis, Cylindrocladium heptasporum Castañeda and Menisporopsis novae-zelandiae Hughes & Kendrick) commonly occurring in the leaf litter of the study site. We grew each species in a 5 cm Petri dish (2% malt extract at 30 C) and after 10 days we washed the mycelium surface of each plate with 120 ml of distilled water and mechanically suspended spores and mycelium fragments. These suspensions were mixed and then we added 5 ml to each of the 80 chambers. By artificially increasing the relative dominance (spore number) of these four species, we attempted to minimise potential differences in the composition of the mico-biota between microcosmos. We also eliminated the meso-fauna in all chambers by fumigating them with chlorobenzene crystals (Sigma C7640) for 5 minutes and ventilated them for 30 minutes. Then we added an adult oppid mite and an egg to each of 35 microcosmos whereas, the other 35 chambers were left meso-fauna free. The leaf litter of the remaining 10 chambers was dried and stored as control for initial dry weight. The 70 experimental microcosmos were placed in a greenhouse for 60 days and then dried at 70EC for three days and the leaf litter weighed to a precision of 0.1 µg, together with the dried leaf litter of the 10 control chambers.
Fungal reproductive potential
After weighting the leaf litter as described above we added 10 ml of distilled water to each of the 70 microcosmos and shook them vigorously before taking a 50 µl sample from which we classified and counted all spores under a compound microscope.
Mite performance
In the second experiment we evaluated the performance of the oppid mite on the host fungi Haplographium sp., Cylindrotrichum sp., C. heptasporum and M. novae-zelandiae. We established 30 cultures of each fungus in 5 cm Petri plates (2% malt extract and 2% agar). After one week of incubation we added a surface sterilised egg (washed in 2% formaldehyde and rinsed in distilled water) of the oppid mite to 16 plates of each fungus. All dishes (with and without an sterilised eggs) were stored at room temperature for six weeks. Then, we counted the number of adult mites and eggs in all 120 plates. In this way we assessed the performance of mites in those plates starting with a single egg and separately we analysed the mite colonisation preference by counting the number of adults in dishes that were egg-free at the beginning of the experiment.
Statistical analysis
We used cluster analysis with Ward's method and Euclidean distance (c.f., Digby & Kempton 1987) to explore patterns of soil meso-fauna structure based on absolute and relative abundance. Effects of mites on the rate of leaf litter decomposition and mite performance on different fungal species were evaluated with one way ANOVA. In the decomposition experiment, the final weight of leaf litter was the response variable tested over the fungivory treatment (mites vs. no mites) used as fixed factor. In the mite performance experiment the number of adult mites and laid eggs were used as response variables. The four different species on which mites were fed were used as a fixed factor with four levels. Effects on fungal reproductive potential were explored with MANOVA. The number of spores recovered of each of the four fungus species were used as response variables tested over the fungivory treatment which were used as a fixed factor. We used Duncan's multiple range test to compare observed means in factors that showed significant effects (∝=0.05). All statistical tests were done in the Statistica software (StatSoft-Inc 1999).
Results
Soil meso-fauna community
We used cluster analysis to characterise the structure of soil and leaf-litter meso-fauna. The analysis based on absolute abundance grouped the 40 samples into four distinguishable groups (Fig. 1a). Leaf litter samples were separated in three groups (groups A, C and D) whereas soil samples (group B) were clustered in one group. The main difference between groups was measured by the abundance of mites and collembolans. These taxa showed the highest abundance in all samples. Group B, which included all soil samples except one, was characterised by a low abundance of mites (rounded mean, 41) and collembolans (30) whereas group D had the highest abundance of mites (198) and collembolans (304). Group C presented intermediate abundance values for mites (126) and collembolans (116), whereas group A showed an intermediate abundance value for mites (96) and a low number of collembolans (44). Also, flies, beetles, isopods and juvenile stages presented higher values in group D (22, 12, 5 and 35, respectively) than in groups A (13, 7, 3 and 17, respectively), group B (12, 9, 1 and 21, respectively) and C (15, 6, 2 and 19, respectively). In contrast, ants were more abundant on average in group B (4) than in group A (<1), group C (1) and group D (1).
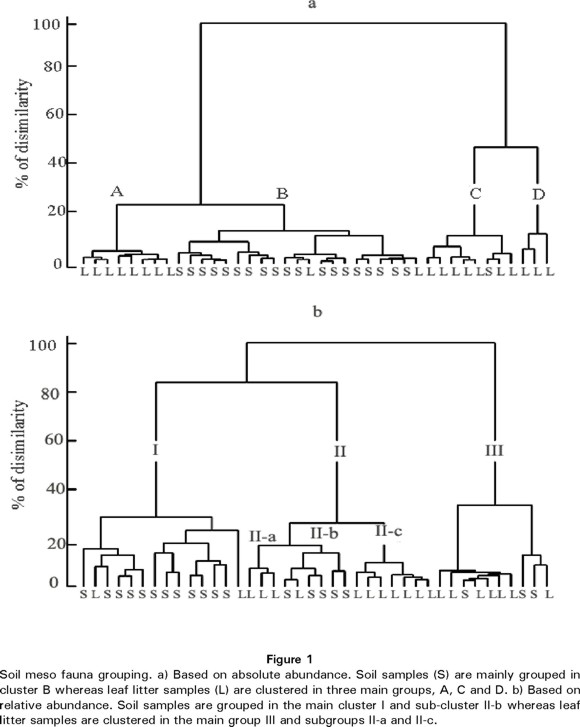
The analysis based on relative abundance showed a complex grouping, but it was still possible to differentiate soil and leaf litter groups (Fig. 1b). At the 50% similarity level there were three groups. Group I was mainly composed of soil samples whereas leaf litter samples were clustered in group III. Group II included soil and leaf litter samples. However, this last group appeared to be composed of three sub-groups at about the 16% similarity level. Sub-groups II-a and II-c consisted solely of leaf litter samples whereas sub-group II-b was largely composed of soil samples. In accordance with the analysis based on absolute abundance, soil clusters (group I and sub-group II-b) were characterised by a low relative abundance of mites and collembolans, whereas the relative abundance of these taxa varied among leaf litter groups. Relative abundance of mites was higher in group II (0.52), especially in sub group II-a (0.60) than in group III (0.30). Also, the relative abundance of ants was higher in soil, group I (0.04), than in leaf litter, groups II (0.01) and III (<0.01). By contrast, collembolans had a higher relative abundance in sub-group II-C (0.49) than in any other group. Opposite from the pattern observed in the absolute abundance analysis, there was a higher relative abundance in the soil (group I) of juveniles (0.17), beetles (0.08), Hymenoptera (0.05) and flies (0.12) than in groups, II (0.01, 0.04, 0.03 and 0.06, respectively) and III (0.05, 0.03.0.03 and 0.04, respectively).
In the study site a morpho-species of oppid mite (Oribatida: Oppidae) was abundant and, based on preliminary feeding tests was fungivorous.
Mite performance
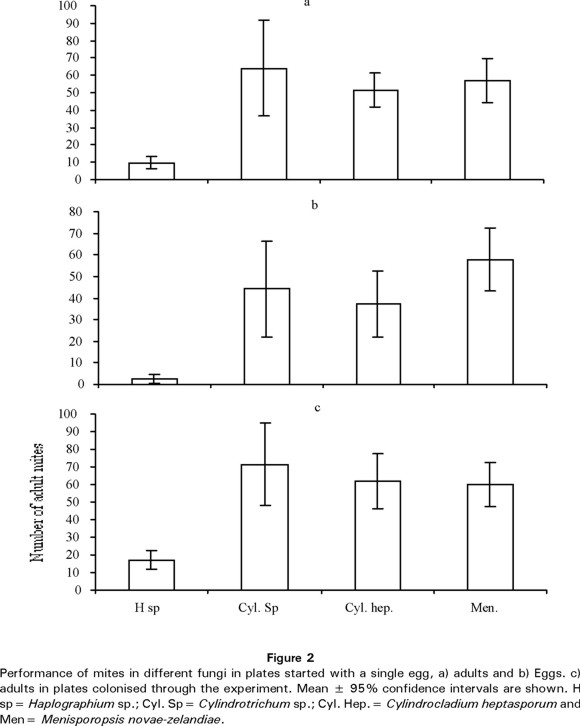
We tested the performance of the oppid mite in an experiment that included four fungi (Haplographium sp, Cylindrotrichum sp. C. heptasporum y M. novae-zelandiae) as food resource. For those plates in which we added an egg at the beginning of the experiment our results showed that there are differences in the quality of the resource (fungi) since we detected significant differences in number of adults (F=10.8, d.f.=3, 51 and P<0.001) and number of eggs (F=10.57, d.f.=3, 51 and P<0.001) among fungi. The number of adult mites (9.81 ± 6.7, mean ± sd) and eggs (2.6 ± 4.5) were significantly lower (P<0.001) in Haplographium sp than in any other fungus (Fig. 2a-b). In accordance, the number of adult mites in those plates that were colonised by the oppid mite (F=7.75, d.f.= 3, 61 and P<0.001) was, also, significantly lower (P>0.001) in Haplographium sp. (17.1 ± 9.9) than in any other fungus (Fig. 2c).
Fungal reproductive potential
We also evaluated the effects of the mite on the reproductive potential of different fungi (D. microsporus, A. sphaerocephala, C. heptasporum and M. novae-zelandiae) in a microcosmos experiment. At the end of the experiment we identified six kinds of spores, based on shape and appearance, and hifa fragments.
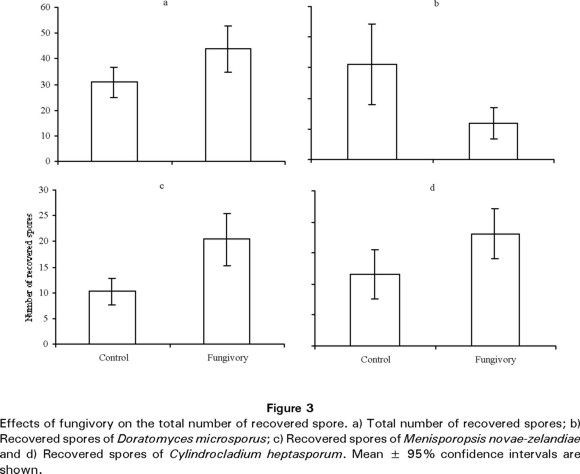
Only four kinds of spores were recovered from over 90% of microcosmos, and these spores corresponded to those species added at the beginning of the experiment. 1) Small spherical spores, D. microsporus. 2) Long and smooth elliptic spores, M. novae-zelandiae. 3) Elliptic spores with septa, C. heptasporum. 4) Dark fig-shaped spores, A. sphaerocephala.
Overall, the MANOVA model was highly significant (U= 0.75, F=4.45, d.f.=5, 64, P<0.01). Post-hoc comparisons showed significant differences in spore numbers between control and microcosmos exposed to fungivory. The overall number of recovered spores (Fig. 3a) was significantly higher (P<0.01) in microcosmos with fungivory (43.6 ± 9.05, mean±95% confidence intervals) than in control microcosmos (30.8 ± 5.93). However, the numbers of different spores varied. Recovery of small spherical spores, D. microsporus (Fig. 3b), was significantly higher (P<0.01) in control microcosmos (3.11 ± 1.30) than in those microcosmos subjected to fungivory (1.2 ± 0.51). By contrast, the number of long and smooth elliptic spores, M. novae-zelandiae (Fig. 3c), and elliptic spores with septa, C. heptasporum (Fig. 4d), was significantly lower (P<0.01 and P<0.05, respectively) in control microcosmos (10.23 ± 2.56 and 11.49 ± 3.96, respectively) than in microcosmos exposed to fungivory (20.4 ± 5.01 and 18.1 ± 4.01, respectively).
Leaf litter decomposition
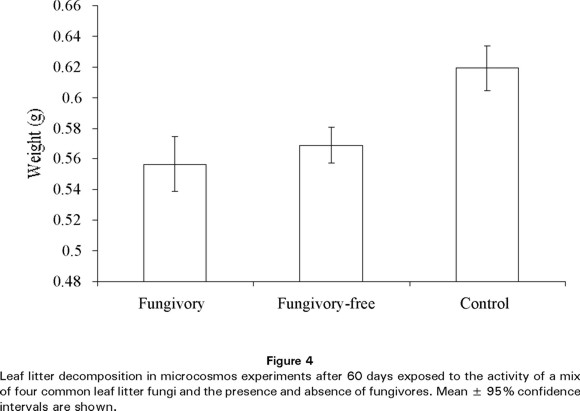
Finally, we compared the weight of leaf litter after 60 days of fungal activity (Fig. 4) in all 80 microcosmos between treatments and the overall control (2 g microcosmos dried at the beginning of the experiment). The overall ANOVA was highly significant (F=7.99; d.f.=22, 77, P<0.01) and the means comparison test showed that the dried leaf litter weight was significantly higher (P<0.001) in the overall control (0.619 g) than in the fungivory (0.557 g) and fungivory-free (0.569 g) treatments; there was no significant difference between treatments.
Discussion
Soil meso-fauna community
The first part of this investigation showed that soil meso-fauna is structurally complex in the study site. Although we found the same orders of arthropods in soil and in leaf litter, their absolute and relative abundance varied between habitats; variation also occurred within leaf litter samples but not soil samples. This reveals that in the study site leaf litter is a heterogeneous habitat which accommodates at least three sub-communities, whereas soil is a homogeneous habitat accommodating only one community. The aim of this research was not to explore those factors related to the heterogeneity of leaf litter as habitat for soil meso-fauna but several factors can be empirically identified: 1) The guild structure of fungi and other decomposers may be affected by the chemical composition of leaf litter and in turn this may affect the distribution of fungivorous meso-fauna. Previous work at the study site (Heredia 1999) showed that leaf litter of Quercus germana Cham and Sch. and Q. xalapensis Humb. sustained a higher diversity of hyphomycetes than leaf litter of Liquidambar styraciflua Oersted. 2) Litter from different tree species decomposes at different rates and this may in itself affect soil meso-fauna distribution. Heredia (1993) found that in an evergreen cloud forest (Biosphere Reserve El Cielo) in north-eastern Mexico, leaf litter of L. styraciflua decomposed faster than that of Quercus sartorii Liemb. and Q. germana, probably due to the preferential activity of Cylindrocladium scoparium Morgan, Verticillium spp. and Fusarium spp. in L. styraciflua leaf litter. 3) In forest ecosystems, even in the range of few square meters, there is, at a single time, a mosaic of micro-environmental conditions in the leaf litter habitat. This mosaic results mainly from the interaction of environmental conditions, thickness of the leaf litter layer, micro-topography and canopy shade distribution. By contrast, the soil is usually covered by the leaf litter layer which may buffer micro-environmental changes making the soil a homogeneous habitat compared to the leaf litter. Other studies have demonstrated that some micro-environmental factors alter the activity of soil meso-fauna (e.g., Worthen et al. 1995).
Mites and collembolans were the taxa with highest abundance and therefore had high weights in the grouping analysis marking clear differences among groups. Detailed analysis of the abundance of these taxa showed that mites and collembolans are more abundant in leaf litter than in soil. Given that a large number of species of these taxa are fungivorous, it is likely that their abundance is correlated to local (micro scale) fungal activity. Predators, a habit also common among mites, may also correlate with this activity as a result of potential prey concentration. Leaf litter micro-environmental heterogeneity may determine largely the actual rate of fungal activity at any given area. Therefore, the leaf litter habitat may be visualise as a dynamic matrix on which different points change from high fungal activity to low activity or even cessation of activity almost in a stochastic (spatially and temporally) fashion. This in turn may be reflected by distribution patterns of soil (fungivorous) meso-fauna. Also, biotic factors (e.g., feedback between fungi and fungivores) are expected to contribute to the dynamics of the leaf litter matrix.
Selective feeding and effects on fungal fitness
Other studies have shown that fungivorous collembolans and mites prey selectively on mycelia of different species of fungi altering the competitive (combative) capabilities of fungi (Kaneko, et al. 1995, Maraun, et al. 1998, McLean, et al. 1996, Newell 1984a, 1984b, Parkinson, et al. 1979). Our results are in accordance to these evidence. We found that Haplographium sp. is a low quality resource for the oppid mite compared to the other three species of fungi. In addition, we recovered a larger number of spores of D. microsporus from control chambers than from those that included mites. Whereas, M. novae-zelandiae and C. heptasporum showed the opposite pattern. From this, we hypothesise that the different species of fungi used in our experiments represent resources of varied qualities to the oppid mite which drives selective feeding. This difference may be on the basis of chemical composition of mycelia [nutrient contents (c.f. Guevara & Dirzo 1999), toxins (Franzolin, et al. 1999, Omoto & McCoy 1998), odours (Bengtsson, et al. 1991, Guevara, et al. 2000, Hedlund, et al. 1995) and flavours] or mechanical properties.
In our experimental design from which we recovered spores we used a single mix of four species of fungi in all microcosmos; therefore, we cannot be precise about the effects of fungivory on the reproductive potential of each of the four species of fungi. However, to our knowledge this is the first study in addressing effects of fungivory on mycelium systems based on a component of fungal reproductive fitness.
Decomposition
We did not detect a significant effect of fungivory on the decomposition of leaf litter in the microcosmos experiment. This result contradicts other studies (Beare, et al. 1992, Hedlund & Oehrn 2000, Rihani, et al. 1995). However, the observed result may be due to the fact that fungi where differentially affected by fungivory. D. microsporus appeared to be more active (high spore production) in fungivore free microcosmos whereas the number of recovered spores of M. novae-zelandiae and C. heptasporum indicates an increase of activity of these fungi in the presence of the fungivore.
Conclusion
Our findings indicate that fungivory affects the activity (spore production) of leaf litter fungi. Also, fungivores performed different on a variety of offered fungi indicating distinct resource (food) qualities. Hence, fungivory by the oppid mite has the potential to affect fungal community structure and the decomposition process although net effects may be masked by differential responses of fungi to the trophic activity of fungivory.
Acknowledgements
We thank G. Heredia, R. M. Arias and M. Reyes for the strains used in this study. Funds for this investigation came from the project CONACYT-I 32791 N and Departamento de Biología de Suelos, INECOL.
Literature cited
Beare, M. H., R. W. Parmelee, P. F. Hendrix, W. X. Cheng, D. C. Coleman & D. A. Crossley. 1992. Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol. Mon. 62: 569-591. [ Links ]
Bengtsson, G., K. Hedlund & S. Rundgren. 1991. Selective odor perception in the soil Collembola Onychiurus armatus. J. Chem. Ecol. 17: 2113-2125. [ Links ]
----------. 1993. Patchiness and compensatory growth in a fungus-Collembola system. Oecologia 93: 296-302. [ Links ]
Castillo-Campos, G. 1991. Vegetación y flora del municipio de Xalapa, Veracruz. Instituto de Ecología, A. C., Xalapa. [ Links ]
Coleman, D. C. & D. A. Crossley Jr. 1996. Fundamentals of soil ecology. Academic Press, San Diego. [ Links ]
Cooke, R. C. & A. D. M. Rayner. 1984. Ecology of soprotrophic fungi. Longman Group Ltd, New York. [ Links ]
Digby, P. G. N. & R. A. Kempton. 1987. Multivariate analysis of ecological communities. Chapman and Hall, London. [ Links ]
Franzolin, M. R., W. Gambale, R. G. Cuero & B. Correa. 1999. Interaction between toxigenic Aspergillus flavus Link and mites (Tyrophagus putrescentiae Schrank) on maize grains: effects on fungal growth and aflatoxin production. J. Stored Prod. Res. 35: 215-224. [ Links ]
Guevara, R. & R. Dirzo. 1999. Consumption of macro-fungi by invertebrates in a Mexican tropical cloud forest: do fruit body characteristics matter? J. Trop. Ecol. 15: 603-617. [ Links ]
Guevara, R., A. D. M. Rayner & S. E. Reynolds. 2000. Orientation of specialist and generalist fungivorous ciid beetles to host and non-host odours. Physiol. Entomol. 25: 288-295. [ Links ]
Hedlund, K., G. Bengtsson & S. Rundgren. 1995. Fungal odour discrimination in two sympatric species of fungivorous collembolans. Func. Ecol. 9: 869-875. [ Links ]
Hedlund, K. & M. S. Oehrn. 2000. Tritrophic interactions in a soil community enhance decomposition rates. Oikos 88: 585-591. [ Links ]
Heredia, G. 1993. Mycoflora associated with green leaves and leaf litter of Quercus germana, Quercus sartorii and Liquidambar styraciflua in a mexican cloud forest. Crypto. Mycol. 14: 171-183. [ Links ]
---------. 1999. Diversidad y sucesión de los hyphomycetes de la superficie de las hojas en descomposición de tres especies arboreas dominantes en un bosque mesófilo de montaña en el centro de Veracruz. Tesis de Doctorado. Universidad Nacional Autónoma de México, Mexico. [ Links ]
Kaneko, N., M. A. McLean & D. Parkinson. 1995. Grazing preference of Onychiurus subtenuis (Collembola) and Oppiella nova (Oribatei) for fungal species inoculated on pine needles. Pedobiol. 39: 538-546. [ Links ]
---------. 1998. Do mites and Collembola affect pine litter fungal biomass and microbial respiration? Appl. Soil Ecol. 9: 209-213. [ Links ]
Maraun, M., S. Migge, M. Schaefer & S. Scheu. 1998. Selection of microfungal food by six oribatid mite species (Oribatidae, Acari) from two different beech forests. Pedobiol. 42: 232-240. [ Links ]
McLean, M. A., N. Kaneko & D. Parkinson. 1996. Does selective grazing by mites and Collembola affect litter fungal community structure? Pedobiol. 40: 97-105. [ Links ]
Newell, K. 1984a. Interactions between two decomposer basidiomycetes and a collembolan under sitka spruce: distribution, abundance and selective grazing. Soil. Biol. Biochem. 16: 227-233. [ Links ]
----------. 1984b. Interactions between two decomposer basidiomycetes and a collembolan under sitka spruce: grazing and its potential effects on fungal distribution and litter decomposition. Soil Biol. Biochem. 16: 235-239. [ Links ]
Omoto, C. & C. W. McCoy. 1998. Toxicity of purified fungal toxin hirsutellin A to the citrus rust mite Phyllocoptruta oleivora (Ash.). J. Invert. Pathol. 72: 319-322. [ Links ]
Parkinson, D., S. Visser & J. B. Whittaker. 1979. Effects of Collembola grazing on fungal colonisation of leaf litter. Soil Biol. Biochem. 11: 529-535. [ Links ]
Rihani, M., J. P. Fonseca & E. Kiffer. 1995. Decomposition of beech leaf litter by microflora and mesofauna. II. Food preferences and action of oribatid mites on different substrates. Rev. Ecol. Biol. Sol 31: 67. [ Links ]
Rodin, L. E. & N. I. Basilevic. 1967. Production and mineral cycling in terrestrial vegetation. Oliver and Boyd, Edinburgh. [ Links ]
----------. 1968. World distribution of plant biomass. In F. E. Eckardt (eds). Functioning of terrestrial ecosystems at the primary production level. United Nations Educational, Scientific and Cultural Organization Press, Paris. pp. 193-202. [ Links ]
Siepel, H. & F. Maaskamp. 1994. Mites of different feeding guilds affect decomposition of organic matter. Soil Biol. Bioche. 26: 1389-1394. [ Links ]
StatSoft-Inc. 1999. Statistica for Windows (computer program manual). StatSoft Inc, Tulsa. [ Links ]
Swift, M. J. & L. Boddy. 1984. Animal-microbial interactions in wood decomposition. In J. M. Anderson, A. D. M. Rayner and D. W. H. Walton (eds). Invertebrate-microbial interactions. Cambridge University Press, Cambridge. pp. 89-131. [ Links ]
Worthen, W. B., B. R. Bloodworth & M. B. Hobbs. 1995. Habitat variability in the effects of predation and microclimate on mycophagous fly communities. Ecography 18: 248-258. [ Links ]














