Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Acta zoológica mexicana
On-line version ISSN 2448-8445Print version ISSN 0065-1737
Acta Zool. Mex n.80 Xalapa Aug. 2000
Article
Reproductive events and social organization in a colony of Anoura geoffroyi (Chiroptera: Phyllostomidae) from a temperate Mexican cave
1 Cristóbal Galindo-Galindo,2 Alondra Castro-Campillo,3 Arturo Salame-Méndez and 2 José Ramírez-Pulido1
1 Universidad Nacional Autónoma de México. Facultad de Estudios Superiores. Unidad Zaragoza. Departamento de Biología. México 09230, D.F. México. E-mail: jrp@xanum.uam.mx
2 Universidad Autónoma Metropolitana. Unidad Iztapalapa, División de C.B.S. Departamentos de Biología. Apdo. Postal 55-535, México 09340, D.F. Mexico.
3 Universidad Autónoma Metropolitana. Unidad Iztapalapa, División de C.B.S. Departamentos de Biología de la Reproducción. Apdo. Postal 55-535, México 09340, D.F. Mexico.
Recibido: 11 de febrero 1999
Aceptado: 21 de octubre 1999
Resumen
Durante dos años se estudiaron los eventos de la reproducción y su relación con la organización social y la conducta en una colonia de Anoura geoffroyi localizada en la cueva "La Mina", San Francisco de las Tablas, Estado de México, 2,670 m. Durante los dos años, los murciélagos mostraron el mismo patrón estacional reproductivo, así como los mismos rearreglos espaciales de la colonia en su refugio. La cópula se efectuó en verano (Junio-Julio) cuando los testículos de los machos alcanzaron su mayor talla y las hembras estaban en estro. Los primeros nacimientos sucedieron a finales de septiembre y los últimos a mediados de noviembre, siendo las hembras monoéstricas y monotocas. La lactancia se inició a fines de septiembre y se prolongó hasta principios de enero. La gestación y la lactancia duraron alrededor de tres meses cada una en la colonia. Las hembras llevaron a cabo los eventos reproductivos en dos grupos secuenciales. En primavera (marzo-mayo) la colonia se organizó en 10 ó 12 grupos de 13 a 15 individuos entre hembras, machos y juveniles. En el verano (junio-agosto) los grupos se redujeron a siete u ocho, pero cada uno constaba de 25 a 30 organismos. Más tarde, el 80% de la población se conglomeró en la colonia de reproducción. En septiembre el grupo constaba de 150 individuos, en su mayoría hembras preñadas. En octubre los machos se fueron y se formó una colonia de maternidad que se rompió en enero con el regreso de los machos. Cuando las hembras salían de la cueva en busca de alimento, dejaban a sus hijos en la colonia al cuidado de un número reducido de individuos, presumiblemente hembras adultas. Los críos al nacimiento estaban desnudos y eran de color rosado. La proporción de sexos fluctuó entre 1:1 y 1.5:1 hembras por machos durante la mayor parte del año, excepto en octubre (3:1), noviembre (8:1)y diciembre (16:0) cuando los machos partieron y se formó la colonia de maternidad.
Palabras clave: Anoura geoffroyi, bosque templado, colonia, comportamiento, México, murciélago de Geoffroy sin cola, Phyllostomidae, reproducción, Cueva templada.
Abstract
Social correlates of reproductive events of a colony of Anoura geoffroyi from the "La Mina" cave at San Francisco de las Tablas, State of Mexico, 2670 m, were studied for two years during which the bats showed consistent seasonal breeding pattern, as well as colony rearrangements. Females are monoestrous and monotocous. Mating took place in the summer (June-July) when males reach maximum testes size and females were in estrus. Births occurred from late September to mid November. Lactation began in late September and extended until early January. Both gestation and lactation lasted three months. Females underwent the reproductive events in two sequential groups. In the spring (March-May), the colony was organized into 10-12 groups of 13-15 individuals, containing females, males and young. In the summer (June-August), the groups were reduced to seven or eight, each containing 25-30 individuals. Later, 80% of the population formed one group, the mating colony. In September, the group was made up of 150 individuals, most of them pregnant females. In October, males left the colony, and a maternity colony was formed, which broke up in January, with the return of the males. When nursing females foraged, they left their offspring in the colony in care of a small number of presumably adult females which roosted in the center of the nursery group. Female-male ratio fluctuated from 1:1 to 1.5:1 during most of the year, except in October (3:1), November (8:1), and December (16:0) when departure of the males and the formation of the maternity colony took place.
Key words: Anoura geoffroyi, temperate forest, behavior, Geoffroy tailless-bat, México, Phyllostomidae, reproduction, temperate cave.
Introduction
Many studies have been conducted on the reproductive patterns of bats, but most of them focus on vespertilionid species (for example, Davis and Hitchckok, 1964; O'Farrell and Studier, 1973, 1975; Tuttle, 1975; Anthony and Kunz, 1977; Gustafson, 1979; Swift, 1980; López Wilchis, 1989, among others) and only a few on phyllostomid bats (Jenness and Studier, 1976; Kleiman and Davis, 1979; Wilson, 1979; Alvarez-Castañeda, 1992; Heideman et al. 1992; Ramírez-Pulido et al. 1993; Heideman and Bronson, 1994).
Anoura geoffroyi is a phyllostomid bat widely distributed in the Neotropical region (Honacki et al. 1982). In Mexico, the species has been reported from the tropical lowlands in the Yucatán Península towards western Durango and Sinaloa, in the Pacific versant, and north to Tamaulipas on the Atlantic versant (Villa-Ramírez, 1967; Hall, 1981).
Most of what is known on the reproduction of this bat has been documented from tropical localities and has been focused in the capture of pregnant or lactating females, as well as on the size of testes (Goodwin and Greenhall, 1961; Schaldach, 1966; Villa-Ramírez, 1967; Tuttle, 1970; Jones et al. 1971; Mares and Wilson, 1971; Wilson, 1979; Alvarez-Castañeda and Alvarez, 1991; Heideman et al. 1992; Heideman and Bronson, 1994; Alvarez and Alvarez-Castañeda, 1996). Abnormal parturition has been described by Alvarez-Castañeda (1992) In fact, there is neither information from temperate localities nor about social and behavioral aspects involved in the reproductive cycle of Anoura geffroyi.
This study describes the relationship between social organization and several events of the reproductive cycle such as recrudescence of testes, estrus, pregnancy, parturition, and lactation as a result of direct observations on a colony of Anoura geoffroyi, with emphasis in behavioral changes of the colony as a whole. Particular attention is paid to females and their young but changes in the volume and position of testes are also described.
Material and methods
Together with the vespertilionid species Myotis velifer and Corynorhinus mexicanus, the studied colony permanently inhabits a cave locally known as "La Mina", 1.5 Km N from the village San Francisco de las Tablas in the municipality of Chapa de Mota, State of Mexico. The cave is located at 2670 m in a well-preserved pine-oak forest (Pinus sp. and Quercus sp.) at 19° 45' N, 99° 30' W.
Cave "La Mina" is located at the Sierra Compleja which belongs to the physiographic Subprovince of the Llanos y Sierras de Hidalgo, within the Mastogeographic Trans-Volcanic Province (Ramírez-Pulido and Castro-Campillo, 1992). The region's bears a subhumid temperate climate (C(w2)(w)(b)ig; García, 1981).
A thorough description of the cave and conditions of the microrefuge will be given elsewhere (Galindo-Galindo et al. unpublished data). The temperature within the refuge remained quite constant (16.5°C) during the two year period; relative humidity was high (86.5%), as is characteristic of the most humid of the subhumid-temperate climates (García, 1981).
"La Mina" was visited three days each month, from January 1990 to December 1991. Additional two-day visits were made from June to October during the second year. This study included a total of 82 field days during the two-year period.
We obtained information about the behavior (i.e., care of young), spatial distribution (colony rearrangements), and size of the colony every month. Data gathering and counting of individuals, which were always made by the same person, started at 9:00 a.m. and usually lasted one hour. We gathered data by directing red light at the colony; care was always taken to avoid, as much as possible, any disturbance. Using this method we recorded the positions and the approximate size of groups resulting from the rearrangements of individuals in the colony along each year, as well as the behavior of individuals.
To determine the time spent by the members of the colony foraging away from the cave, several people took positions at the base of a hole connecting the main body of the cave with a chamber where the species roosted and stayed there until the bats left. Frequent subsequent entries into the cave were made overnight to directly verify the return time of the colony (i.e., when all the bats came back to the cave) and whether the females returned periodically to the chamber during the night to nurse their young.
This also served to count the bats as they interrupted the beam of flash lights directed into the opening of the microrefuge as they left or entered. Total number of bats fluctuated from 170 to 300 according to the reproductive activities of the colony in both years (Galindo-Galindo et al. unpublished data). Detailed information on the fluctuations of the size of the colony and on the refuge will be given elsewhere.
In order to measure, weight and record external physical indicators of the reproductive condition of bats, as well as to approximate the sex ratio, a mist net was placed a short distance from the opening of the cave during the second and third sampling nights. Captured male and female individuals were marked with plastic rings bearing beads of different colors, according to each sampling session. The ring was placed on the right forearms in females, and on the left forearms in males. Except for November and December, each month one to three captured adult males were killed and their testes were dissected and measured with dial calipers to the nearest 0.1 mm. Volume of testes was calculated using the formula for a prolate espheroid [(width)2 X length X 0.523] given by Heideman et al. (1992).
For each individual, the color of the plastic rings; the degree of the ossification of the phalanxes (young, subadults and adults; Davis, 1984); weight (g); and the total length and the length of the forearm (mm) were recorded. In addition, reproductive condition was assessed, that is, whether females were pregnant, as determined by palpation, or lactating, as indicated by relative development of their nipples (small, medium and large). Also, conspicuous changes in the vulva were noted such as coloration, swelling, and secretions. In males, the position of the testes (abdominal or scrotal) was noted, as well as the coloration and appearance of scrotum. Females were considered reproductively inactive when they showed no evidences of estrus, pregnancy, parturition, and lactation. Males were considered inactive when they had abdominal testes.
Results
It was noticed that colony arrangements and sex ratio changed in relation to the reproductive stages, which can be related to specific behavioral and social events. The stages of the reproductive cycle included position and volume of testes in males (Figs. 1, 2, Table 1) and anestrus, estrus, pregnancy, and parturition-lactation in females. The behavioral and social events were mating, formation of maternity colony, care of young, migration of males, weaning, early flights, and break up of the maternity colony (Fig. 2).
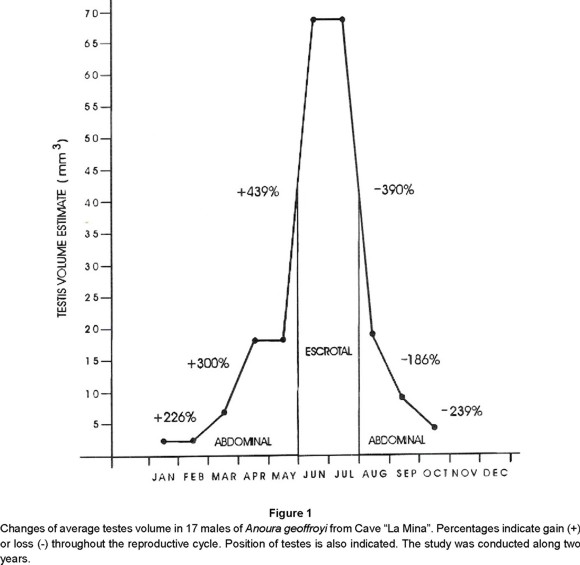
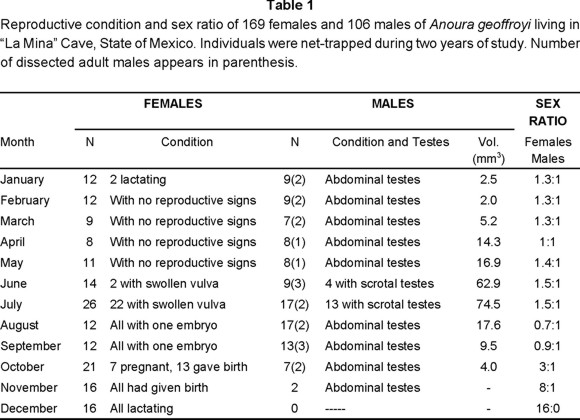
Testes. Increase of volume sensu Heideman et al. (1992) and position of testes, in males, showed a seasonal trend related to the general reproductive pattern of females (Figs. 1, 2, Table 1). Testes remained abdominal from August to May and scrotal in June and July. Changes in the volume of testes were gradual and discontinuous (Fig. 1). Volume increase proceeded during six months and started with a twofold gain of volume from February to March. Then it continued throughout spring (March-May) to reach the maximum volume in summer (June-July), when the females were in estrus (Fig. 2). In these two months, the scrotum was swollen, had lost hair, and showed a yellowish coloration; the volume of testes was 30 times larger than in winter. After July, testes started to lose volume until they reduced to the winter volume. In August, although the scrotum remained swollen, the testes were abdominal and small (Fig. 1, Table 1). Decrease of volume continued for three months during the fall. Average volume of abdominal testes was 9 mm3 (n = 14) with a range of 2.3 (winter) -15.6 mm3 (spring, fall), while average volume of scrotal testes was 68.7 mm3 (n = 5).
Mating. The colony of Anoura geoffroyi at cave "La Mina" showed the same seasonal breeding pattern as well as the same group rearrangements during the two years of the study. Reproductive activities began in the colony of Anoura geoffroyi in the summer (June-July) when mating occurs (Fig. 2). During this process two groups were formed, the first included females that mated from the second half of June until the first half of July, and the second group included females who mated throughout the month of July.
Nearly 40% of captured males exhibited scrotal testes in June, and 76.5% in July (Figs. 1, 2, Table 1). Females showed no conspicuous changes in their genitalia, but by June (14.3%) and, July (84.6%), their vulvas were swollen and reddish with an abundant whitish secretion. These are characteristics that correspond to "heat" within the estrus stage of the reproductive cycle, and are related to female receptivity for copulation.
In early summer (June and early July), the colony formed seven to eight groups of 25 to 30 individuals on the ceiling of the roosting chamber, which were separated from each other by a distance of about 20 cm. As summer progressed, the colony formed one main group with 80% (n = 165) of all individuals at the peak of the ceiling; the remaining 20% (n = 41) of the bats were scattered throughout the lower walls of the chamber in groups of two to three individuals. Female-male ratio of captured individuals at this time was 1.5:1 (Table 1).
During most of the summer (June-July) the mating colony was organized into two groups 20 cm equidistant from each other, which was probably when copulation took place. At the end of August, when the mating period had already ended and gestation had begun, the two groups disintegrated and became six. Once this happened, the groups of two to three individuals tended to merge with the recently formed groups.
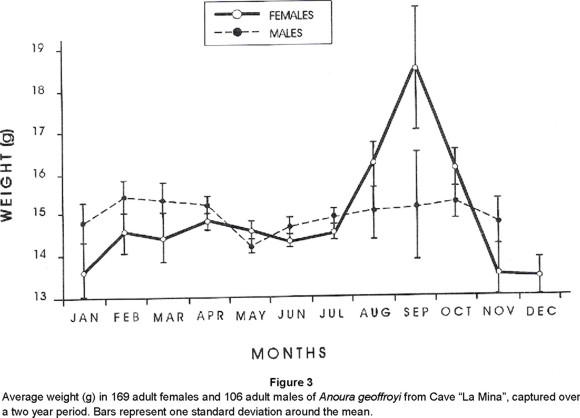
Pregnancy and parturition. Results from both years at "La Mina" cave, show that the colony of Anoura geoffroyi mated and gave birth once a year, a condition which categorizes it as a monoestrus species. Since no more than one young per birth was observed, the species is also monotocous. Gestation lasted no more than three months in the A. geoffroyi colony from "La Mina". Indeed, by the end of July, after most females had shown "heat" characteristics, one female was killed and found to bear an embryo 9 mm long. Also, in August, the weight of all captured females (Fig. 3) had increased markedly and in September it reached a maximum after a 33% gain when the first births occurred. A decrease of weight continued until November, when all females had given birth, towards December and January, when the lowest weights were recorded.
During these two processes the colony changed in sexual composition. While adult males remained within the colony during pregnancy and the sex ratio remained 1.5:1 (Table 1; Figs. 2, 3), by late September they began to abandon the refuge so that by October the sex ratio became 3:1 (Table 1). It was from this time to late October that a maternity colony started to build up when about 180 individuals, mostly pregnant females, formed a compact group at the top of the ceiling. Only two to five groups of either singly or no more than three bats remained separated from the main group on the walls.
As mentioned before, the first females gave birth asynchronously in late September, but most births occurred in the first half of October (Fig. 2). As females entered labor, they went into the center of the group and only the last few females gave birth at the periphery of the colony. At birth, the offspring were completely altricial and pink and the growth of their hair was gradual, but it was usually faster on the dorsal area, which was most exposed to the environment.
Lactation and maternal care. Lactation began in late September and ended in early January (Fig. 2, Table 1) with a duration of about three months in the colony. A portion of the females nursed their offspring from late September to late December while others nursed them from mid October until January. By November, the maternity colony was fully established and contained a majority of females (8:1, Table 1); only two males were captured in this month. In December the females within the chamber were still nursing, males had completely left the colony, and the sex ratio was 16:0 (Table 1).
Figure 3 shows how weight loss in females is clearly related to the metabolic demands undergone by mothers during lactation from October to November; in fact, females regained weight only until February after young have been weaned (Table 1, Fig. 2). Moreover, although mothers spent a lot of time in cleaning their offspring, which remained always attached to their nipples, during the day, it was noticed that they spent less time searching for food at night. That is, while the bats left the microrefuge between two and a half to three hours after sunset and went back between 30 minutes and one hour before sunrise during most of the year, from October throughout January, they left the chamber two hours after sunset and returned two hours before sunrise.
In Anoura geoffroyi, young bats attached to the nipples of their mothers throughout three months until they were capable to obtain their own food (Fig. 2). Indeed, during lactation the excrement of the offspring had a light coloration, which turned dark, as that of adults, after weaning.
Early flights and care of young. Between 15 and 20 days after birth, the largest pups began to move around a little within the nearest 30 cm from their mothers and increased that distance with time. Accordingly, in the beginning, displacements occurred at very short time intervals and the returns were almost always immediate, but later on, the exploration time became longer (Fig. 2).
In November, a few pups started to make short erratic flights inside the chamber, often crashing against the walls, probably as a response to our lights and our presence. In this stage, the offspring did not remain attached to their mothers for long and their movements were more noticeable in the evening (Fig. 2).
Females and, especially, young of Anoura geoffroyi always remained within the same chamber in "La Mina" cave during the two year study. During late September and most of October, the first bats to leave the chamber, in search of food, were lactating females followed by the gestating ones. In no case, the former carried their offspring with them outside the cave nor made any periodic returns to nurse them. In fact, the nursery (group of newborn bats) of A. geoffroyi remained the whole night hanging from the ceiling of the chamber while their mothers searched for food. Nevertheless, it is important to mention that the offspring was not completely abandoned, for there always remained three to five, probably adult females, which roosted in the center of the nursery group, apparently "baby-sitting" them, until the mothers returned (Fig. 2).
Colony rearrangements during the non-reproductive period. The maternity colony broke up when the males returned to the colony in January, which was also the time when presumed immature males, those who had remained either isolated or in small groups in the microrefuge, were reintegrated into the colony and, thus, a compact bisexual group was formed again (Fig. 2). From this time until summer, sex ratio changed from 7:1 to 1.5:1 again when a new reproductive cycle started (Table 1, Fig. 2). The number of individuals in the colony fluctuated from 170 to 300 along the year, depending on the reproductive event, and the leaving of the males (Galindo et al. unpublished data).
During the spring (March-May), the colony contained adult females and males, as well as young recruited from the previous reproductive period, which by then were hardly distinguishable from the adults both in coloration and size (Fig. 2). Bats were arranged into 10 to 12 groups of 13 to 15 individuals each in the higher parts of the chamber. Unfortunately, it was very difficult to identify whether the groups contained only one sex or both sexes but it was obvious that there was a distance of about one meter between each group.
At this time, bats departed quietly from the chamber one hour after sunset in groups of five to eight individuals, every two minutes. However, at the end of spring (May), the number of bats in the departing flight increased from eight to 15 individuals who left the chamber very excitedly every two minutes. The increase of bats in the departing groups resulted in a quicker abandonment of the cave.
Mean body weight of males (Fig. 3) exhibited non significant little variation throughout the year (F = 1.42; df = 10; P < 0.05). Contrary, in females (Fig. 3), weight differences resulted statistically significant (F = 8.75; df = 11; P < 0.05) according to the reproductive event. Females had lowest body weight from November to December during lactation, and the highest from August to October during pregnancy (Figs. 2, 3). In fact, mean body weight between the two sexes showed no differences along the year, but in August (F = 5.6; df = 28; P = 0.025) and September (F = 41; df = 24; P < 0.001), when females start to reach their maximum and parturition starts, respectively (Figs. 2, 3).
Discussion
We document for the first time the general breeding pattern together with some related behavioral and social events in a colony of Anoura geoffroyi, a neotropical phyllostomid species, inhabiting a cave in the temperate climate in the State of Mexico. Reproductive events in this colony are accompanied by a specific social organization perfectly defined which allow us to suggest explanations and to relate such events with other documented behavioral mechanisms. Both reproductive and social events remained constant along the two year of study.
Our results support that Anoura geoffroyi is a seasonal breeding species in any part of its geographical range (i. e., tropical and temperate) as has been proposed by Heideman et al. (1992) and by Heideman and Bronson (1994). In the Mexican temperate cave, "La Mina", seasonal breeding is reflected in both phenotypical changes in the sex organs of males and females, as well as in their reproductive activities.
Heideman et al. (1992) point out that timing of this seasonal breeding pattern seems to be displaced according to the latitudinal location of the populations of Anoura geoffroyi; however, here we found it was constant between some far away localities. At least, changes in the volume of testes of males from "La Mina" cave are completely coincident in time with those documented by Heideman et al. (1992). These authors also found maximum volume of testes in the summer, especially in July (Fig. 3 in Heideman et al. 1992), in males from Trinidad studied in 1990. That is, breeding males of the Mexican cave, located at 19° 45' N, 99° 30' W at 2,670 m are synchronized with breeding males of the Trinidad cave, located at 10° 28' N, 61° 12' E at 240 m. Duration of reproductive events is also highly coincident in the species, since enlargement of testes, pregnancy and lactation last two to three months in this phyllostomid regardless of its distribution (Heideman et al. 1992; results here).
Anoura geoffroyi is monotocous and monoestrous as can be concluded from our results and those of Heideman et al. (1992), who also document only one foetus in each female and one seasonal breeding period. The species follows the general pattern of monoestry described for temperate bat species (Altringham 1998) that is characterized by a synchronous reproductive cycle. At OLa MinaO Cave, this synchronous pattern could be explained by the seasonal fluctuation of food supplies at Sierra Compleja, State of Mexico, which might be related, in turn, with environmental (i.e., photoperiod, temperature, rainy season) and plant phenology changes.
Duration of lactation in phyllostomyid bats has been reported from one to two months, as in Carollia perspicillata (Kleiman and Davis, 1979), and from two to four months, as in neotropical frugivorous species (Fleming et al. 1972; Jenness and Studier, 1976). Therefore, the pattern found for Anoura geoffroyi here corresponds to the second group.
Although, as in this study, Schaldach (1966) and Villa-Ramírez (1967) found lactating females of Anoura geoffroyi in the months of November and December in the States of Oaxaca and Colima, respectively, Alvarez-Castañeda and Alvarez (1991) mention four lactating females from the State of Chiapas in September and Alvarez and Alvarez-Castañeda (1996) found one lactating female in July and two pregnant females in September from the State of México. These differences might be due to either temporal displacement of reproductive events in relation to diverse phenological plant patterns in different latitudinal areas, as has been reported for nectarivorous and pollinivorous bats (Humphrey and Bonaccorso, 1979); or to sampling effects such as failure in capturing females in any reproductive event. In any case, such displacements of lactation, point out the need of in situ studies in selected localities along the geographic range of the species.
One might ask whether group rearrangements precede reproductive events or vice versa. We think that reproductive events within the biological cycle of Anoura geoffroyi are responsible of the colony rearrangements at OLa MinaO Cave. For instance, clustering of females with males is related to estrus, especially in late summer when mating takes place as has been mentioned for pteropodids and other tropical bats (Hill and Smith, 1988). Also, during pregnancy and lactation, only females remain in the refuge within the maternity colony. It is probable that pregnant and lactating females produce a chemical signal (Fleming et al. 1998) which triggers either an agonistic behavior towards males or the response of the latter so that they abandon the cave, thus resulting in the formation of the maternity colony.
Although our data supports sex segregation in this species as had been suggested by Goodwin and Greenhall (1961), and Alvarez and Ramírez-Pulido (1972), sex ratio might vary depending on the location and characteristics of the cave where a colony of Anoura geoffroyi occurs. While at "La Mina" Cave, adult males of A. geoffroyi leave the maternity colony, there is no annual sex ratio variation at Trinidad (Heideman et al. 1992). We ignore where male bats go when they leave "La Mina" Cave; A. geoffroyi just behaves as other temperate-zone bat species of the tropics (Hayward and Cockrum, 1971) where pregnant females come together in unisexual groups until the birth and care of their offspring (Hill and Smith, 1988). Maybe males at Trinidad occupy a different location within the same cave when the maternity colony is formed or maybe females change of place within the cave as in other bat species (Humphrey, 1975; Gustafson, 1979; López Wilchis, 1989).
In both the mating and maternity groups, there are scattered, smaller groups which might involve segregated individuals that do not participate in copulation either because they are: (a) immature or pubertal specimens; (b) aged and no longer sexually active individuals; or (c) they could be females that recently became pregnant.
As to the sex of the segregated individuals, which remain in the maternity colony, they might be immature males. In fact, it has been noticed that in several species of bats (Carter, 1970; Racey, 1982), males reach maturity in the second year of life while females can reproduce in their first year, which could explain why young males segregate from the reproductive group (Twente, 1955). This can also explain why there are two groups of females undergoing the reproductive cycle. That is, one group could be formed by the experienced females and the other by the recently recruited young, but mature, females.
Contrary to other species in which females make periodic returns during the night to nurse their offspring (O'Farrell and Studier, 1973; Anthony and Kunz, 1977; Swift, 1980; Fleming et al. 1998), no female of Anoura geoffroyi returned at night to nurse its pup. Females rather spent one hour less in food searching during lactation, thus having less time for food searching than in nonreproductive periods. Such different behavior suggests that there must be different adaptations, maybe higher-energy content food (i.e., pollen and nectar), to fulfill the function of covering the energy requirements of both the mother and the fetus during pregnancy and to fulfill the function of breast-feeding the offspring.
It must be recalled that although their mothers never came back during the night to nurse them, the young of Anoura geoffroyi never remained alone for there were some adults, presumably females, that stayed with them. Baby-sitting has been documented for insectivorous species (O'Farrell and Studier 1973; 1975), but we found no information about this altruistic behavior on other phyllostomids. Lewis and Pusey (1997) discuss the role of communal defense of the young and "baby-sitting" as a form of communal care, which is perfectly applicable to A. geoffroyi.
Several questions remain unanswered with regards to "baby-sitting" in Anoura geoffroyi. For instance, we do not know whether "baby-sitters", female-individuals that stay behind in care for the offspring during the night, take turns to go out; whether other females take over; whether they are different ones each time, or whether these "baby-sitting" females even nurse the offspring. It would be also important to know the biological condition of these "care takers", as well as whether each female is able to recognize her own offspring upon returning, or rather whether it picks the nearest one newborn.
The presence of baby-sitters in Anoura geoffroyi could be related to the social organization of the colony which, in turn, might involve a twofold cooperative behavior of the colony members. On one hand, it might be related to a previous training for the periodical dispersal of the males, since they leave the colony during most fall and winter. Secondly, it might be related with collaborative care and nursing tasks by other females while the mothers are out (Altringham, 1998). It would be interesting to corroborate both behavioral patterns and relate them to energy savings and adaptation enhancement for the species.
Recognition of her young by females of Anoura geoffroyi might involve vocalizations (Fenton, 1985; Balcombe, 1990; Jones et al. 1991; Balcombe and McCracken, 1992; Scherrer and Wilkison, 1993) together with visual (Fenton, 1985), and olfactory cues (Gustin and McCracken, 1987; Schmidt, 1987; Fleming et al. 1998).
The weight gain-loss patterns of Anoura geoffroyi females show a close relationship with their maternal role. The increase of weight during pregnancy is related to the development of the offspring which represents about 30% of such increase at birth; in fact, young A. geoffroyi are 1/3 of their mother's size. This is the most common pattern among bats (Kurta and Kunz, 1987) and is related to precocious development for flight (Ransome, 1973; 1990; Hughes et al. 1989). Indeed, as seen in other phyllostomids (Kleiman and Davis, 1979), the offspring of A. geoffroyi remain attached to the nipples of their mothers during the day until an advanced age, as can be inferred from their size and appearance. But unlike such species as Dermanura phaeotis (Ramírez-Pulido et al. 1977) or Artibeus lituratus (Tamsitt and Valdivieso, 1965), the young of A. geoffroyi start their first individual efforts for flying at an early age.
Acknowledgments
Many colleagues and friends made possible the fulfillment of this paper. Several eager and enthusiastic students helped in the fieldwork as part of their career courses, we thank especially Adalinda Sánchez, Ramón Quijano, Elsa González, and Claudia Aguilar. Plastic rings and colored bands were kindly provided by Dr. Rodrigo Medellín. Mr. Antonio H. Cabrera translated a first draft of the manuscript. Special thanks to Drs. Carlos Beyer, Scott Creel, Theodore H. Fleming, Gabriela González-Mariscal, Rodrigo A. Medellín, Dr. Vinicio Sosa, and an anonymous reviewer for their valuable comments and criticisms which enriched the manuscript. The same can be said for three anonymous reviewers. Figures were made by Raymundo González Coronel. The National Council of Science and Technology (CONACyT No. 400200-5R29117N) and the General Bureau of Scientific Research and Academic Improvement (SEP 94-01-00-002-247) financed the preparation of this paper.
Literature cited
Altringham, J.D. 1998. Bats. Biology and Behavior. Oxford Univ. Press. Oxford, UK. [ Links ]
Alvarez, T. & S.T. Alvarez-Castañeda. 1996. Aspectos biológicos y ecológicos de los murciélagos de Ixtapan del Oro, Estado de México. Pp .169-182. In: H.H. Genoways and R.J. Baker (eds). Contributions in Mammalogy: A memorial Volume Honoring Dr. J. Knox Jones, Jr. Mus. Texas Tech. Univ. [ Links ]
Alvarez, T. & J. Ramírez-Pulido. 1972. Notas acerca de murciélagos mexicanos. An. Esc. nac. Cien. biol., Mex. 19:167-178. [ Links ]
Alvarez-Castañeda, S.T. 1992. Notas sobre un parto anormal en Anoura geoffroyi (Chiroptera: Mammalia). Southwestern Nat. 37:420-421. [ Links ]
Alvarez-Castañeda, S.T. & T. Alvarez. 1991. Los murciélagos de Chiapas. Esc. nac. Cien. biol., IPN, México. [ Links ]
Anthony, E.L. P., & T.H. Kunz. 1977. Feeding strategies of the little brown bat Myotis lucifugus in the southern New Hampshire. Ecology 58:775-786. [ Links ]
Balcombe, J.P. 1990. Vocal recognition of pups by mother Mexican free-tailed bats, Tadarida brasiliensis mexicana. Anim. Behav. 39:960-966. [ Links ]
Balcombe, J.P. & G.F. McCracken. 1992. Vocal recognition in Mexican free-tailed bats: do pups recognize mothers?. Anim. Behav. 43:79-88. [ Links ]
Carter, D.C. 1970. Chiropteran reproduction. Pp. 233-246. In: B.H. Slaughter y D.W. Walton (eds). About bats: A Chiropteran Symposium. Southern Methodist Univ. Press, Dallas. [ Links ]
Davis, W.H. & H.B. Hitchkock. 1964. Notes on sex ratios of hibernating bats. J. Mamm. 45:475-476. [ Links ]
Fenton, M.B. 1985. Communication in the Chiroptera. Indiana Univ. Press, Bloomington. [ Links ]
Fleming, T.H. 1973. Reproductive cycles of three species of opossums and other mammals in the Panama Canal Zone. J. Mamm. 54:439-455. [ Links ]
Fleming, T.H., E.T. Hooper & D.E. Wilson. 1972. Three Central American bat communities: structure, reproductive cycles and movement patterns. Ecology 53:555-569. [ Links ]
Fleming, T.H., A.A. Nelson & V.M. Dalton. 1998. Roosting behavior of the lesser long-nosed bat, Leptonycteris curasoae. J. Mamm. 79:147-155. [ Links ]
García, E. 1981. Modificaciones al sistema de clasificación climática de Köeppen. Talleres Larios, S.A. México. [ Links ]
Goodwin, G.G. & A.M. Greenhall. 1961. A review of the bats of Trinidad and Tobago. Bull. Amer. Mus. Nat. Hist. 122:187-302. [ Links ]
Gustafson, A.W. 1979. Male reproductive patterns in hibernating bats. J. Reprod. Fertil. 56:317-331. [ Links ]
Gustin, K. & G.F. McCracken. 1987. Scent recognition in the Mexican free-tailed bat, Tadarida brasiliensis mexicana. Anim. Behav. 35:13-19. [ Links ]
Hall, E.R. 1981. The mammals of North America. John Wiley and Sons, New York, vol. 1. XV+600+90. [ Links ]
Hayward, B.J. & E.L. Cockrum. 1971. The natural history of the western Long-nosed bat Leptonycteris sanborni. Western New Mexico Univ., Research Sci. 1:75-123. [ Links ]
Heideman P. D. & F.H. Bronson. 1994. An endogenous circannual rhythm of reproduction in a tropical bat, Anoura geoffroyi, is not entrained by photoperiod. Biol. Reprod. 50:607-614. [ Links ]
Heideman P.D., P. Deoraj & F.H. Bronson. 1992. Seasonal reproduction of a tropical bat, Anoura geoffroyi, in relation to photoperiod. J. Reprod. Fertil. 96:765-773. [ Links ]
Hill, J.E. & J.D. Smith. 1988. Bats: A natural history. British Museum (Nat. Hist.) and the Univ. Texas Press, Austin [ Links ]
Honacki, J.H., K.E. Kinman & J.W. Koeppl (eds). 1982. Mammal species of the world: A taxonomic and geographic reference. Joint Venture of Allen Press, Inc., and Assoc. Syst. Collect., Lawrence, Kansas. IX+694 pp. [ Links ]
Hughes, P.M., R.D. Ransome & G. Jones. 1989. Aerodynamic constraints of flight ontogeny in free-living greater horseshoe bats, Rhinilophus ferrumequinum. Pp. 255-262. In: V. Hanák, I. Horácek, and J. Gaisler (eds). European Bat Research 1987. Charles Univ. Press, Praga. [ Links ]
Humphrey, S.R. 1975. Nursery roosts and community diversity of neartic bats. J. Mamm. 56:321-346. [ Links ]
Humphrey, S R.& F.J. Bonaccorso. 1979. Population and community ecology. Part III. Pp. 409-441. In: R.J. Baker, J.K. Jones, Jr., and D.C. Carter (eds). Biology of bats of the New World Family Phyllostomatidae. Spec. Publ. Mus., Texas Tech Univ. 16:1-441. [ Links ]
Jenness, R. & E.H. Studier. 1976. Lactation and milk. Part I. Pp. 201-218. In: R.J. Baker, J.J. Jones, Jr. and D.C. Carter (eds). Biology of bats of the New World Family Phyllostomatidae. Spec. Publ. Mus., Texas Tech Univ. 10:1-218. [ Links ]
Jones, J.K., Jr., J.D. Smith & R.W. Turner. 1971. Noteworthy records of bats from Nicaragua, with a checklist of the chiropteran fauna of the country. Occas. Papers Mus. Nat. Hist., Univ. Kansas 2:1-35. [ Links ]
Jones, G., P.M. Hughes & J.M.V. Rayner. 1991. The development of vocalizations in Pipistrellus pipistrellus (Chiroptera: Vespertilionidae) during post-natal growth and the maintenace of individual vocal signatures. J. Zool. Lond. 225:71-84. [ Links ]
Kleiman, D.G. & T.M. Davis. 1979. Ontogeny and maternal care. Part III. Pp. 387-402. In: R.J. Baker, J.K. Jones, Jr. and D.C. Carter (eds). Biology of bats of the New World Family Phyllostomatidae. Spec. Publ. Mus., Texas Tech Univ. 16:1-441. [ Links ]
Kurta, A. & T.H. Kunz. 1987. Size of bats at birth and maternal investment during pregnancy. Symp. Zool. Soc., Lond. 57:79-106. [ Links ]
Lewis, S.E. & A.E. Pusey. 1997. Factors influencing the occurrence of communal care in plural breeding mammals. Pp. 335-363. In: N.G. Solomon and J.A. French (eds). Cooperative breeding in mammals. Cambridge Univ. Press. [ Links ]
López Wilchis, R. 1989. Biología de Plecotus mexicanus (Chiroptera: Vespertilionidae) en el Estado de Tlaxcala, México. Tesis Doctoral, Facultad de Ciencias. U.N.A.M, México. 227 pp. [ Links ]
Mares, M.A. & D.E. Wilson. 1971. Bat reproduction during the Costa Rican dry season. BioScience 21:471-477. [ Links ]
O'Farrell, M.J. & E.H. Studier. 1973. Reproduction growth and development in Myotis thysanodes and M. lucifugus (Chiroptera: Vespertilionidae). Ecology 54:18-30. [ Links ]
––––––––––. 1975. Population structure and emergence activity in Myotis thysanodes and M. lucifugus (Chiroptera: Vespertilionidae) in northeastern New Mexico. Amer. Midl. Nat. 93:368-376. [ Links ]
Racey, P.A. 1982. Ecology of bat reproduction. Pp. 57-104. In: T.H. Kunz (ed). The Ecology of Bats. Plenum Press, New York. [ Links ]
Ramírez-Pulido, J., M.A. Armella & A. Castro-Campillo. 1993. Reproductive patterns of three neotropical bats (Chiroptera: Phyllostomidae) in Guerrero, Mexico. Southwest. Nat. 38:24-29. [ Links ]
Ramírez-Pulido, J. & A. Castro-Campillo. 1992. Regiones y Provincias Mastogeográficas de México. Hoja IV. 8. 8. In: Atlas Nacional de México. Sección Naturaleza. Subsección Biogeografía. Inst. Geogr. UNAM. [ Links ]
Ramírez-Pulido, J., A. Martínez & G. Urbano. 1977. Mamíferos de la Costa Grande de Guerrero, México. An. Inst. Biol., Univ. Nal. Autón. México, Ser. Zool. 48:243-292. [ Links ]
Ransome, R.D. 1973. Factors affecting the timing of births of thegreater horseshoe bat (Rhinolophus ferrumequinum). Period. Biol. 75:169-175. [ Links ]
––––––––––. 1990. The natural history of the hibernating bats. Christopher Helm, London. [ Links ]
Schaldach, W. J., Jr. 1966. Notas breves sobre algunos mamíferos del sur de México. An. Inst. Biol., Univ. Nal. Autón. México 35:129-137. [ Links ]
Scherrer, J.A. & G.S. Wilkinson. 1993. Evening bat isolation calls provide evidence for heritable signatures. Anim. Behav. 46:847-860. [ Links ]
Schmidt, U. 1987. The bats: order Chiroptera. Pp. 217-234. In: R. E. Brown and D. W. MacDonald (eds.). Social odours in mammals. Clarendon Press, Oxford. [ Links ]
Swift, S.M. 1980. Activity patterns of pipistrelle bats (Pipistrellus pipistrellus) in north-east Scotland. J. Zool. 190:285-295. [ Links ]
Tamsitt, J.R. & D. Valdivieso. 1965. Reproduction of the female big fruit-eating bat, Artibeus lituratus palmarum, in Colombia. Caribbean J. Sci. 5:157-166. [ Links ]
Tuttle, M.D. 1970. Distribution and zoogeography of Peruvian bats, with comments on natural history. Univ. Kansas Sci. Bull. 49:45-86. [ Links ]
––––––––––. 1975. Population ecology of the gray bat (Myotis grisescens): Factors influencing early growth and development. Occas. Papers Mus. Nat. Hist., Univ. Kansas 36:1-24. [ Links ]
Twente, J.W. 1955. Some aspects of habitat selection and other behavior of cavern dwelling bats. Ecology 36:706-732. [ Links ]
Villa-Ramírez, B. 1967. Los murciélagos de México. Su importancia en la economía y la salubridad. Su clasificación sistemática. An. Inst. Biol., Univ. Nal. Autón. México XVI+491 pp. [ Links ]
Wilson, D.E. 1979. Reproductive patterns. Part III. Pp. 317-378. In: R. J. Baker, J.K. Jones, Jr., and D.C. Carter (eds). Biology of bats of the New World Family Phyllostomatidae. Spec. Publ. Mus. Texas Tech Univ. [ Links ]














