Malaria is the most important anopheles-borne disease and causes great mortality worldwide. This disease is endemic to 106 countries, primarily in the tropical and subtropical regions. In 2015, there were approximately 212 million new cases and 429 000 deaths, mostly among children. Currently, half of the world population, or about 3.2 billion people, are at risk of contracting malaria.1 In Mexico, the number of cases have decreased significantly, from 7 259 in 2000 to 518 in 2016.2,3 Malaria cases are concentrated in four foci: a) Sinaloa and Nayarit, b) Durango and Chihuahua, c) Campeche and Quintana Roo, and d) Chiapas (Lacandon Forest). The latter is the main focus of transmission in the country, having registered 63.70% of the cases in 2016.3
There are 475 species of the genus Anopheles in the world, 41 of which have been incriminated for transmitting the Plasmodium parasite.4,5 In Mexico, there are 26 species of Anopheles6,7 distributed along the country, and three were incriminated as the main vectors of malaria: Anopheles pseudopunctipennis, An. albimanus and An. vestitipennis.8,9,10,11 Studies of the larval ecology of anophelines in Mexico date back to the fifties.6 However, the geographic distribution and abundance of the species have changed due to human activities such as deforestation, increase of croplands and cattle grazing areas, road building, creation of new human settlements, and water control systems, among others.12,13
Recent studies on the anopheline larvae ecology were carried out mainly in southeastern Mexico (Chiapas), pointing to An. pseudopunctipennis as the main vector in the mountain regions. Their larvae inbreed in puddles along river margins, with a positive association with the presence of filamentous green algae, and the presence of Heteranthera.14,15 While An. albimanus is the main malaria vector in the coastal plain of the Pacific and Atlantic. 6,8,16 In Chiapas, larvae of this species are positively associated to planktonic algae both in the dry and wet seasons, and negatively associated with altitude.15 During the wet season the larvae can be found in the margins of permanent water bodies, positively associated with floating plants, and during the rainy season they are positively correlated with the presence of emergent plants, particularly Cyperaceae and flood grasses.15,17 The main vector in the Lacandon Forest (Chiapas) is An. vestitipennis and its larval habitat has not been described in Mexico.6,9 Recently, the larval habitats of An. darlingi, the secondary vector in the Lacandon Forest, were studied and characterized.18 Part of the knowledge obtained in those studies has been the basis for the implementation of successful antilarval measures in Chiapas and Oaxaca, applying the technique of “Elimination of Anopheline Breeding Sites”, which consists mainly in removing green algae from rivers through community participation.19 This strategy reduced the density of An. pseudopunctipennis larvae and impacted the adult population in such a way that the numbers of malaria cases were significantly reduced.20 However, this knowledge cannot be applied equally throughout the Mexican territory because of the high environmental variability caused by its biogeography, because the northern part of the country is located in the Nearctic region, and the southern part, in the Neotropical region.21 Each biogeographical region has specific climatic, hydrologic and orographic characteristics that are unique to the larval habitats of each anopheline species.22 It is essential to know the ecology and type of larval habitats of anophelines in order to design control measures of the immature stadia using larvicides, environmental management or the removal of filamentous algae.
Materials and methods
Study área
A descriptive cross-sectional research was carried out in 19 states in Mexico from April to February 2016. The study area was selected based on previous records on Mexican anophelines.6 The area covers two main biotic regions: Nearctic and Neotropical. Ten states are located in the Nearctic region and nine in the Neotropical region, which include a wide variety of climates, topographic diversity, different land uses and vegetation. The climatic conditions in the Nearctic zone are very varied, but the predominant climates are arid and semi-arid (Koppen BW, BS), with precipitation ranging between 100 and 600 mm, and an annual median temperature ranging between 22°C and 26°C in some regions, and 18°C and 22°C in the cold and temperate areas. The Neotropical region comprises the states of southern Mexico. The climate is hot and humid with dry and rainy seasons (Koppen Aw) or tropical with year-round rains (Koppen Af), a median annual temperature of 18°C to 22°C, and precipitation ranges between 2 000 and 4 500 mm.23,24 The search for anopheline larvae was carried out at any available body of water found along the trip both inside and outside urban or rural settlements, by the side of the road, in natural and man-made habitats.
Larvae collection
Each site was visited every two to three months (from February 2012 to April 2016). The larval habitats were sampled along their margin using a 500 mL dipper, according to a standardized method.17 The water samples were poured in a white 30×30×5 cm tray in order to collect larvae of all stadia (I to IV). The larvae were counted and placed in 1 L plastic containers to be transported to the insectary (Centro Regional de Investigación en Salud Pública [CRISP]) for rearing to adulthood. The samples were collected in each habitat for at least 1 hr., between 8:00 and 14:00 hours. Google Earth was utilized in the search for water body images, and the positive sites were georeferenced using a GPS (Garmin eTrex Garmin Ltd., Kansas USA).
Larvae identification and growth
The larvae were raised in the CRISP insectary in a climate-controlled room at 25±1 °C temperature, 70±10% relative humidity and a photoperiod of 12:12 (L: D) h, using a standardized technique.25 In some cases where the distance did not allow the transportation of biological material to CRISP, an insectary was improvised in the study area under natural conditions of temperature, humidity and photoperiod. All the larvae were raised using water from the same collection site in order to minimize mortality, but taking care of using clean water free of predatory insects.26 The larvae were fed with finely ground and sterilized commercially available mouse food (Laboratory Rodent Diet 5001, LabDiet, St. Louis, MO). All the adults obtained were mounted and accounted for in a database built for later analysis. Larval densities (LD) were quantified as total number of larvae/the number of dips.18 All specimens were identified using the Wilkerson and Strickman (1990) keys, based on morphological characteristics.7
Characterization of larval hábitats
Several water bodies were visited, selecting those that were positive and negative for anopheline larvae. Each larval habitat was characterized in situ determining the following parameters: water depth, turbidity, plant cover (%), amount of detritus, presence of algae, light intensity, vegetation type, amount of predators, water movement, habitat stability, altitude, and hydrologic type. Water depth was measured using a graduated string with a lead weight, and was categorized in three strata: 0-200 cm, 201-400 cm, and 401-600 cm. Turbidity was determined according to color as “clear or turbid”. Plant cover was classified in the following groups: 0-30%, 31-60%, and 61-100%. Detritus, composed of twigs, dry leaves and dead insects, was determined according to presence in the habitat as “low, moderate or high”. The algae were characterized as “present or absent”. Light intensity was measured according to the amount of vegetation surrounding the habitat and categorized as “sunny, partially sunny, or shaded”. Vegetation was categorized as “emergent, floating/submerged, or none”. Emerging plants include aquatic vegetation and submerged terrestrial vegetation. Predators were categorized by larval habitat according to abundance: low (≤5 predators), moderate (6 to 10 predators) or abundant (≥11 predators). Water movement was determined as “stagnant, slow, or fast”. Habitat stability was considered permanent if the water body persisted throughout the year and temporary if it was only present during part of the year. Altitude was determined by portable GPS as follows: 0-759 meters above the sea level (masl), 760-1 519 masl and 1 520-2 279 masl. The hydrologic type was classified according to the nature of the water body.18
Data analysis
A one-way analysis of variance was used (Anova) to compare the absolute larval index (ALI) between hydrologic types, and for species abundance, the results from the identification of the obtained adults were used. A multiple logistic regression analysis was used to determine the association between environmental variables and the occurrence of An. pseudopunctipennis and An. albimanus larvae. The presence of larvae was categorized as one (1) and the absence of larvae was categorized as zero (0). Data were analyzed using statistical software SPSS 20.0 (SPSS, Inc., Chicago, IL). We determined the species richness with Fisher’s α test (number of species found in each state and type of habitat), using the software “Species Diversity & Richness version 4”.27,28 Geographic distribution maps of the species were created utilizing the QGIS software Version 2.18.1.
Results
Composition and abundance of anopheline species
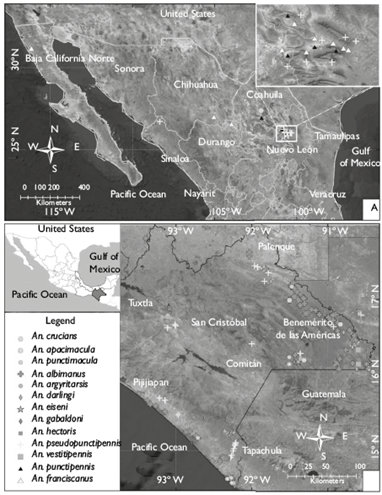
In this study, 19 states of the Mexican Republic were examined, selecting 169 larval habitats: 91.94% were natural, and the rest were artificial; 149 tested positive for anopheline larvae, and 20 larval habitats tested negative (figure 1). All water bodies, positive and negative, were sampled throughout the study. Lagoons and fast flowing rivers were the main negative habitats. Of the total positive larval habitats, 21 were located in the Nearctic region and 128 in the Neotropical region. In the Nearctic region of Mexico, five anopheline species were found: An. pseudopunctipennis, An. albimanus, An. franciscanus, An. punctipennis and An. crucians (figure 2). Of these species, An. franciscanus and An. punctipennis were specific to this biogeographic region; the rest of the species were found across Mexico. In central Mexico, the transition between the Nearctic and Neotropical regions (the states of Morelos and Puebla), only An. pseudopunctipennis was detected, in river margins. Eleven species were found in southern Mexico: An. pseudopunctipennis, An. albimanus, An. vestitipennis, An. darlingi, An. punctimacula, An. crucians, An. hectoris, An. apicimacula, An. gabaldoni, An. argyritarsis, and An. eiseni. The southern state of Chiapas presented 112 aquatic habitats and 11 mosquito species, in the Lacandon Forest area and the Soconusco area in the coastal plain (figure 2).
A total 21 687 larvae of 13 Anopheles species were captured and reared until adulthood with 84.48% of breeding success, i.e. obtaining 18 322 adults. The most abundant species throughout the study was An. pseudopunctipennis (52.92%), followed by An. albimanus (39.14%) and An. franciscanus (5.29%), which comprise 97.35% of the total specimens. The remaining 2.54 % is constituted by the least abundant species: An. punctipennis (1.0%), An. vestitipennis (0.70%), An. darlingi (0.49%), An. Punctimacula (0.13%), An. crucians (0.11%), An. hectoris (0.11%), An. apicimacula (0.08%), An. gabaldoni (0.03%), An. argyritarsis (0.01%), and An. eiseni (0.01%) (figure 3). An. pseudopunctipennis had a significantly higher ALI (±StD) (14.05±21.11), compared to other species (F=1.897; df=11; p< 0.05) (table I). As for the spatial distribution of the main malaria vectors in Mexico, An. albimanus was found in 13 states, An. pseudopunctipennis in 10 states and An. vestitipennis was found only in two states in southern Mexico (figure 4).

Figure 1 Study area indicating the positive and negative sampling sites of anopheline larvae. February 2010 to April 2015. Mexico
Habitat diversity and larval abundance
Twelve hydrologic types were found positive for anopheline larvae in Mexico. The most common hydrologic types were river margins (29.53%), rain puddles (16.77%), ponds (12.75%) and river pools (10.73%). The least frequent were swamps (6.71%), streams (6.71%), ditches (4.02%), pools (4.02%), lagoons (3.35%), gravel pits (2.68%), irrigation canals (2.01%), and drinking troughs (0.67%). The average ALI (±StD) was 14.55 larvae per dip (l/d) (±25.28) with a range of 0 to 153.0 l/d. The greatest ALI averages (±StD) were found in river pools 19.40 l/d (±19.46), ponds 18.99 l/d (±35.75), river margins 18.97 l/d (±31.19), gravel pits 17.65 l/d (±9.13), streams 17.20 l/d (±27.41) and ditches 16.25 l/d (±28.89). No significant differences were found in the absolute larval index (ALI) between hydrologic types (F=0.748; df=11,137; p=0.690) (table I).
Larval presence and associated parameters in An. Pseudopunctipennis and An. Albimanus
The multiple logistic regression analysis showed a significant negative association between the presence of An. pseudopunctipennis and water turbidity (ß=-1.342; 232 Wald=6.122; p=0.013) and the amount of detritus (ß=-2.206; Wald=3.642; p=0.050) (table II). In contrast An. albimanus had a significant positive association with water turbidity (ß=1.344; Wald=4.256; p=0.039) and a negative correlation with altitude (ß= −235 3.445; Wald=5.407; p=0.020) (table III).
Richness and diversity of anopheline species
The diversity of Anopheles species in 19 states exhibited a general Fisher’s α value of 1.77. The state with the most species richness was Chiapas (n=11), followed by Coahuila (n=5). In other states, only 1-3 species were detected. The greatest diversity index was found in Chiapas (Fisher’s α=1.20), and the lowest diversity index was found in Chihuahua (Fisher’s α=0.26) (table IV). The greatest richness was found in streams (n=11), followed by river margins (n=9) and ponds (n=6).
Table I: Average adult density (±StD) of anophelines obtained by hydrologic type, in 19 States of Mexico. February/2010 to April/2015
| Hydrologic type | n | Ap | Aa | Af | Apu | Av | Ad | Apc | Ac | Ah | Api | Ag | Aar | Ae | ||||||||||||||||||||||||||||
| River margin | 56 | 18.35±27.43 | 9.65±16.80 | 4.83±0.28 | 5.00 | 0 | 0.01±0.008 | 0.55±0.07 | 0.55±0.63 | 2.00 | 0.30±0.28 | 0.50 | 0.10 | 0.10 | ||||||||||||||||||||||||||||
| Rain puddle | 25 | 1.20±0.90 | 6.87±17.65 | 0 | 0 | 2.10 | 0.07±0.03 | 0.10 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Pond | 19 | 23.62±28.53 | 11.91±16.60 | 0 | 0 | 0.65±0.77 | 0.10±0.06 | 0.50 | 0.10 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| River pool | 16 | 16.93±14.81 | 4.80±5.18 | 2.00 | 5.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Swamp | 10 | 0.53±0.58 | 5.76±2.51 | 0 | 0 | 0 | 0 | 0 | 0.40 | 0 | 0.80 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Stream | 10 | 10.46±11.65 | 3.93±3.61 | 2.30±1.85 | 2.76±0.49 | 1.00 | 0.18±0.15 | 0 | 0.40 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Ditch | 6 | 0.87±1.03 | 3.25±1.06 | 70.00 | 0 | 2.33±1.75 | 0.25±0.17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Ground pool | 6 | 1.70±0.70 | 5.44±3.88 | 0 | 0 | 0 | 0.21±0.08 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Lagoon | 14 | 0 | 2.85±3.04 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Gravel pit | 4 | 14.25±6.35 | 2.95±2.41 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Irrigation canal | 3 | 0 | 11.6±16.60 | 0 | 0 | 0.75±0.35 | 0 | 0.60 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Drinking trough | 1 | 0 | 0 | 1.20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||
| Total | 149 | 14.05±21.11 | 7.54±13.45 | 9.69±21.26 | 3.66±1.27 | 1.43±1.23 | 0.47±0.36 | 0.46±0.20 | 0.40±0.36 | 2.00 | 0.46±0.35 | 0.50 | 0.10 | 0.10 |
Ap=An. pseudopunctipennis; Aa=An. albimanus, Af=An. franciscanus, Apu=An. punctipennis, Av=An. vestitipennis, Ad=An. darlingi, Apc=An. punctimacula, Ac= An. crucians, Ah=An. hectoris, Api=An. apicimacula, Ag=An. gabaldoni, Aar=An. argyritarsis, Ae=An. eiseni.
Table II: Multiple logistic regression analysis of the larval abundance and environmental parameters for Anopheles pseudopunctipennis in Mexico. February 2010 to April 2015
| Variables | Coefficient(ß) | S.E. | Wald | df | Sig | Exp(ß) | CI95% Exp(ß) | |||||||||
| Lower limit | Upper limit | |||||||||||||||
| Interception | 1.126 | 2.557 | 0.194 | 1 | 0.660 | |||||||||||
| Water depth | ||||||||||||||||
| 0-200 | 0 | 0 | ||||||||||||||
| 201-400 | -1.156 | 0.000 | 1 | 0.315 | 0.315 | 0.315 | ||||||||||
| 401-800 | 0 | 0 | ||||||||||||||
| Turbidity | ||||||||||||||||
| Clear | -1.342 | 0.542 | 6.122 | 1 | 0.013 | 0.261 | 0.090 | 0.757 | ||||||||
| Turbid | 0 | 0 | ||||||||||||||
| Coverage % | ||||||||||||||||
| 0-30 | -0.564 | 0.808 | 0.487 | 1 | 0.485 | 0.569 | 0.117 | 2.771 | ||||||||
| 31-60 | -0.592 | 0.945 | 0.393 | 1 | 0.531 | 0.553 | 0.087 | 3.525 | ||||||||
| 61-100 | 0 | 0 | ||||||||||||||
| Detritus | ||||||||||||||||
| Low | -1.148 | 0.692 | 2.756 | 1 | 0.097 | 0.317 | 0.082 | 1.230 | ||||||||
| Moderate | 0 | 0 | ||||||||||||||
| High | -2.206 | 1.156 | 3.642 | 1 | 0.050 | 0.110 | 0.011 | 1.061 | ||||||||
| Algae | ||||||||||||||||
| Absent | 0.656 | 0.675 | 0.943 | 1 | 0.331 | 1.927 | ||||||||||
| Present | 0 | 0 | ||||||||||||||
| Light intensity | ||||||||||||||||
| Sunny | -0.694 | 0.892 | 0.605 | 1 | 0.437 | 0.500 | ||||||||||
| Sun/shade | -1.320 | 0.976 | 1.829 | 1 | 0.176 | 0.267 | ||||||||||
| Shade | 0 | 0 | ||||||||||||||
| Vegetation | ||||||||||||||||
| Emergent | -0.007 | 1.362 | 0.000 | 1 | 0.996 | 0.993 | 0.069 | 14.325 | ||||||||
| Floating/submerged | 0.385 | 1.396 | 0.076 | 1 | 0.783 | 1.469 | 0.095 | 22.675 | ||||||||
| None | 0.119 | 1.471 | 0.007 | 1 | 0.935 | 1.127 | 0.063 | 20.137 | ||||||||
| Predators | ||||||||||||||||
| Low | 0.452 | 0.539 | 0.705 | 1 | 0.401 | 1.572 | 0.547 | 4.517 | ||||||||
| Moderate | 0 | 0 | ||||||||||||||
| Abundant | -1.121 | 1.315 | 0.727 | 1 | 0.394 | 0.326 | 0.025 | 4.291 | ||||||||
| Water movement | ||||||||||||||||
| Stagnant | 0 | 0 | ||||||||||||||
| Slow | 0 | 0 | ||||||||||||||
| Fast | 0.052 | 1.165 | 0.002 | 1 | 0.964 | 1.053 | 0.107 | 10.344 | ||||||||
| Habitat stability | ||||||||||||||||
| Permanent | 0.417 | 0.706 | 0.348 | 1 | 0.555 | 1.517 | 0.380 | 6.054 | ||||||||
| Temporary | 0 | 0 | ||||||||||||||
| Altitude | ||||||||||||||||
| 0 -759 | 1.264 | 1.018 | 1.541 | 1 | 0.214 | 3.540 | 0.481 | 26.056 | ||||||||
| 760 -1 519 | 0 | |||||||||||||||
| 1 520 -2 279 | 0.926 | 1.558 | 0.353 | 1 | 0.552 | 2.524 | 0.119 | 53.487 | ||||||||
| Hydrologic type | ||||||||||||||||
| Ditch | -1.439 | 1.488 | 0.935 | 1 | 0.333 | 0.237 | 0.013 | 4.381 | ||||||||
| Drinking trough | 20.230 | 0.000 | 1 | 6.104E-5 | 6.104E-5 | 6.104E-5 | ||||||||||
| Gravel pit | -19.127 | 7 132.062 | 0.000 | 1 | 0.998 | 4.934E-9 | 0.000 | |||||||||
| Pools | -1.223 | 1.488 | 0.675 | 1 | 0.411 | 0.294 | 0.016 | 5.439 | ||||||||
| Irrigation canal | 18.154 | 8 502.676 | 0.000 | 1 | 0.998 | 76 627 495.02 | 0.000 | |||||||||
| Lagoon | 18.715 | 6 306.399 | 0.000 | 1 | 0.998 | 134 229 308.46 | 0.000 | |||||||||
| Pond | 0.663 | 1.232 | 0.290 | 1 | 0.590 | 1.941 | 0.174 | 21.703 | ||||||||
| Rain puddle | 1.471 | 1.299 | 1.282 | 1 | 0.257 | 4.355 | 0.341 | 55.594 | ||||||||
| River margins | -0.752 | 1.031 | 0.532 | 1 | 0.466 | 0.472 | 0.063 | 3.555 | ||||||||
| River pools | -1.395 | 1.242 | 1.262 | 1 | 0.261 | 0.248 | 0.022 | 2.826 | ||||||||
| Creeks | -0.408 | 1.304 | 0.098 | 1 | 0.755 | 0.665 | 0.052 | 8.572 | ||||||||
| Swamps | 0 | 0 | ||||||||||||||
Sig: significant; df: degrees of freedom
Table III: Multiple logistic regression analysis of larval abundance and environmental parameters for Anopheles albimanus in Mexico. February 2010 to April 2015
| Variables | Coefficientß | S.E. | Wald | df | Sig | Exp(ß) | CI95% Exp(ß) | |||||||||
| Lower limit | Upper limit | |||||||||||||||
| Interception | 1.317 | 2.580 | 0.261 | 1 | 0.610 | |||||||||||
| Water depth | ||||||||||||||||
| 0-200 | 0.054 | 0.797 | 0.005 | 1 | 0.946 | 1.056 | 0.221 | 5.040 | ||||||||
| 201-400 | 0 | 0 | ||||||||||||||
| 401-600 | 0 | 0 | ||||||||||||||
| Turbidity | ||||||||||||||||
| Clear | 1.344 | 0.652 | 4.256 | 1 | 0.039 | 3.836 | 1.069 | 13.760 | ||||||||
| Turbid | 0 | 0 | ||||||||||||||
| Coverage % | ||||||||||||||||
| 0-30 | -0.005 | 0.922 | 0.000 | 1 | 0.995 | 0.995 | 0.163 | 6.058 | ||||||||
| 31-60 | -0.703 | 1.229 | 0.327 | 1 | 0.568 | 0.495 | 0.045 | 5.509 | ||||||||
| 61-100 | 0 | 0 | ||||||||||||||
| Detritus | ||||||||||||||||
| Low | 1.528 | 0.829 | 3.394 | 1 | 0.065 | 4.609 | 0.907 | 23.421 | ||||||||
| Moderate | 0 | 0 | ||||||||||||||
| High | 1.421 | 1.287 | 1.219 | 1 | 0.270 | 4.140 | 0.333 | 51.534 | ||||||||
| Algae | ||||||||||||||||
| Absent | -0.544 | 0.835 | 0.424 | 1 | 0.515 | 0.581 | 0.113 | 2.985 | ||||||||
| Present | 0 | 0 | ||||||||||||||
| Light intensity | ||||||||||||||||
| Sunny | 0.225 | 0.937 | 0.058 | 1 | 0.810 | 1.252 | 0.199 | 7.865 | ||||||||
| Sun/shade | 0.843 | 0.966 | 0.762 | 1 | 0.383 | 2.324 | 0.350 | 15.422 | ||||||||
| Shade | 0 | 0 | ||||||||||||||
| Vegetation | ||||||||||||||||
| Emergent | 0.234 | 0.694 | 0.114 | 1 | 0.736 | 1.264 | 0.324 | 4.923 | ||||||||
| Floating/submerged | 0 | 0 | ||||||||||||||
| None | 0.651 | 0.944 | 0.475 | 1 | 0.491 | 1.917 | 0.301 | 12.204 | ||||||||
| Predators | ||||||||||||||||
| Low | -0.157 | 0.637 | 0.061 | 1 | 0.805 | 0.854 | 0.245 | 2.980 | ||||||||
| Moderate | 0 | 0 | ||||||||||||||
| Abundant | -16.759 | 7 018.994 | .0000 | 1 | 0.998 | 5.27E8 | 0.000 | |||||||||
| Water movement | ||||||||||||||||
| Stagnant | 0 | 0 | ||||||||||||||
| Slow | 0 | 0 | ||||||||||||||
| Fast | 1.886 | 1.335 | 1.996 | 1 | 0.158 | 6.594 | 0.482 | 90.252 | ||||||||
| Habitat stability | ||||||||||||||||
| Permanent | -1.456 | 0.828 | 3.092 | 1 | 0.079 | 0.233 | 0.046 | 1.182 | ||||||||
| Temporary | 0 | 0 | ||||||||||||||
| Altitude | ||||||||||||||||
| 0 - 759 | -3.445 | 1.486 | 5.407 | 1 | 0.020 | 0.032 | 0.002 | 0.581 | ||||||||
| 760 - 1 519 | 0 | 0 | ||||||||||||||
| 1 520 - 2 279 | 15.470 | 0.000 | 1 | 5 230 658.306 | 5 230 658.306 | 5 230 658.306 | ||||||||||
| Hydrologic type | ||||||||||||||||
| Ditch | 1.531 | 1.993 | 0.590 | 1 | 0.443 | 4.621 | 0.093 | 229.885 | ||||||||
| Drinking trough | 34.453 | 0.000 | 1 | 9.180E-14 | 9.180E-14 | 9.180E-14 | ||||||||||
| Gravel pit | -19.824 | 7 540.604 | 0.000 | 1 | 0.998 | 2.457E-9 | 0.000 | |||||||||
| Pools | 0.969 | 1.935 | 0.251 | 1 | 0.617 | 2.635 | 0.059 | 116.950 | ||||||||
| Irrigation canal | -17.419 | 9 242.478 | 0.000 | 1 | 0.998 | 2.722E-8 | 0.000 | |||||||||
| Lagoon | 2.077 | 1.674 | 1.540 | 1 | 0.215 | 7.984 | 0.300 | 212.396 | ||||||||
| Pond | -1.091 | 1.623 | 0.452 | 1 | 0.501 | 0.336 | 0.014 | 8.079 | ||||||||
| Rain puddle | -3.157 | 1.936 | 2.660 | 1 | 0.103 | 0.043 | 0.001 | 1.891 | ||||||||
| River margins | 0.808 | 1.380 | 0.343 | 1 | 0.558 | 2.244 | 0.150 | 33.555 | ||||||||
| River pools | 0.791 | 1.505 | 0.276 | 1 | 0.599 | 2.205 | 0.115 | 42.124 | ||||||||
| Creeks | -0.282 | 1.711 | 0.027 | 1 | 0.869 | 0.754 | 0.026 | 21.568 | ||||||||
| Swamps | 0 | 0 | ||||||||||||||
Sig: significant; df: degrees of freedom
Table IV: Ecologic diversity of anophelines in 19 states in Mexico. February 2010 to April 2015
| Geographic region/State | Number of habitats | Number of larvae | Species richness | Fisher’s α | ||||
| Nearctic region | ||||||||
| B. California | 1 | 10 | 1 | 0.71 | ||||
| Sinaloa | 2 | 1 160 | 2 | 0.38 | ||||
| Nayarit | 1 | 25 | 1 | 0.50 | ||||
| Chihuahua | 1 | 750 | 1 | 0.26 | ||||
| Coahuila | 3 | 959 | 5 | 1.00 | ||||
| Durango | 1 | 12 | 1 | 0.65 | ||||
| Nuevo León | 6 | 4 245 | 3 | 0.42 | ||||
| Tamaulipas | 1 | 60 | 2 | 0.65 | ||||
| Puebla | 4 | 285 | 1 | 0.33 | ||||
| Morelos | 1 | 65 | 1 | 0.39 | ||||
| Neotropical region | ||||||||
| Jalisco | 1 | 55 | 1 | 0.50 | ||||
| Colima | 1 | 32 | 1 | 0.51 | ||||
| Guerrero | 1 | 56 | 1 | 0.41 | ||||
| Oaxaca | 3 | 987 | 2 | 0.38 | ||||
| Chiapas | 112 | 11 667 | 11 | 1.20 | ||||
| Veracruz | 6 | 816 | 3 | 0.59 | ||||
| Tabasco | 1 | 88 | 1 | 0.50 | ||||
| Campeche | 1 | 65 | 1 | 0.41 | ||||
| Quintana Roo | 2 | 350 | 2 | 0.45 | ||||
| Total | 149 | 21 687 | 13 | 1.77 |
Discussion
There were 13 Anopheles species identified throughout Mexico in this study. An. pseudopunctipennis and An. albimanus were the most abundant species and occupied the greatest diversity of habitats, and both are considered the main malaria vectors in Mexico.8,11 Of the two species mentioned, An. pseudopunctipennis exhibited the greatest larval density and was widely distributed throughout the country, in accordance with the published geographic distribution.29 This species is reported to be the main vector between 200 and 500 masl, and to be associated with river margins with filamentous algae14 and positively associated with altitude in southern Mexico.15 In this national study, this species was found in an altitude range that goes from 3 to 2 279 masl, and no association was found with the altitude in the multiple logistic regression analysis. The reason for this lack of association is that this species did not exhibit at the national level a similar pattern to that reported in Tapachula, Chiapas, where populations are abundant at the mountain foothills, at an altitude above 200 masl and up to 500 masl, and is not commonly found in the coastal plain.14,15 According to the results, we infer that, nationally, the altitude range where this vector may transmit malaria could be greater than the previously reported one of 200 to 500 masl.3,10 These results agree with the findings of a study in the Yucatán Peninsula, which reports An. pseudopunctipennis in a cenote (its natural, permanent habitat) at 20 masl.30On the other hand, there is a negative association with water turbidity, which indicates that females prefer to lay their eggs in clear rather than turbid water. This coincides with a previous report from 10 countries, spanning most of the geographic range of the species, whose larvae were found in clear-water breeding sites in 90% of the cases (i.e. in 54 out of 60 larval habitats), but is not consistent with the presence of An. pseudopunctipennis in turbid water habitats contaminated with cattle feces in the city of Salinas, Ecuador, and other turbid breeding sites caused by flooding in Monterrey, Mexico.31 This study is also consistent with various studies about the presence of larval habitats with filamentous algae and their disappearance during the rainy season caused by the strong flow of water.14,31
An. albimanus was found in the greatest diversity of hydrologic types (11 out of 12), indicating that this species is generalist when it comes to its preference for larval breeding sites.32 It is the species with the broadest geographic distribution, from Chiapas to Sinaloa on the Pacific side, and from Yucatán to Tamaulipas on the Gulf side, which is consistent with previous reports.29,32 Larval habitats for An. albimanus were characterized as sunny and were positively associated with turbidity, but negatively associated with altitude, which is consistent with reports for southern Chiapas.15,17 On the Pacific side, there are breeding sites for this species all along the coast up to Sinaloa, but no positive larval habitats were found further north. This habitat limitation may be due to the high temperatures and arid climate persistent in Sonora, which is consistent with previous studies.32
Another abundant species was An. franciscanus, which was found only in northern Mexico, the Nearctic region. The geographic distribution of An. franciscanus in this study in northern Mexico apparently is not consistent with a previous report by Vargas and Martínez-Palacios (1956),6 who reported this species only in northern Baja California. At the time of the study, in 1956, the status of An. franciscanus as a species was unclear, a problem that was solved when genetic incompatibility was found to exist between An. pseudopunctipennis and An. franciscanus, and it was concluded that these were two different species.33 The literature also reported An. pseudopunctipennis var Willardi in Sonora, Chihuahua, Coahuila and San Luis Potosí, but this variety disappeared and was integrated to the species An. franciscanus.34,35 Finally the results of the present study are consistent with those studies regarding the geographic distribution reported for northern México.6In this study, this species was not found in Tamaulipas, as was previously reported,36 but was found for the first time in Nuevo León and Durango. The lack of reports for this species in Nuevo León could be derived from the lack of taxonomic studies in this geographic area, or possibly because of a range expansion derived from anthropogenic changes or climate change.12,13 Apparently An. franciscanus has little importance as a malaria vector today or in the past;34 however, it is necessary to deepen taxonomical and binomial analyses in the states of Sinaloa, Nayarit, Durango and Chihuahua, where malaria cases have been reported.3 The vector An. vestitipennis was found only in southern Mexico, in the Neotropical region, where it is one of the main malaria vectors in the Lacandon Forest.9 Larval habitats for this species in the Lacandon Forest were found in ponds, ditches and rain puddles. The most abundant larval habitat were ditches, which are caused by man-made changes in orography, because road building avoids natural river paths, allowing the formation of water bodies that are attractive for this species. The same pattern coincides in a report from Colombia, where road building favored the development of several species of Anopheles.32 Further studies are required about the incrimination of vectors in the regions of Soconusco, Chiapas, and Catemaco, Veracruz, where this species was found and its role as a malaria vector is unknown. This study describes for the first time the characteristics of the larval habitats of this species in the Lacandon Forest area in Chiapas.
The greatest diversity of anopheline species was found in the Lacandon Forest area in Chiapas, with 11 species and the highest Fisher’s α diversity value: 1.20. These results are consistent with studies in other regions of the world, where it has been confirmed that tropical zones have the greatest biodiversity of insect species.37 It must be noted that one of the main historical references of abundance and distribution of Mexican anophelines (published in 1956),6 does not mention any anopheline species for the Lacandon Forest in Chiapas. At that time in the 1950s, despite there being populated areas in the Lacandon Forest, there were few taxonomic works,38,39,40 mainly due to the difficult access to these areas.41 At present, despite the Lacandon Forest being the most important malaria focus in Mexico,3 very little has been written about vectors.9 This is the first report that exposes the high anopheline biodiversity for this area. The high incidence of malaria cases in this region could be accounted for by the high diversity of species, as was reported in Colombia, where there was a positive correlation between richness of anopheline species and risk of transmission.42,43 Another phenomenon that may cause the high incidence of malaria could be the movement of people between communities for work or business, or the emigration of people from endemic areas who are traveling north or to the United States, which results in elevated transmission risk for P. vivax and P. falciparum in this area.3,44
The second region with the greatest species diversity, with a Fisher’s α diversity index of 1.0, is the state of Coahuila, in northeastern Mexico, where we found five species: An. crucians, An. franciscanus, An. punctipennis, An. pseudopunctipennis and An. albimanus. In this area, the climate is extreme and semi-desertic (Koppen BS). This area in northeastern Mexico is free of malaria despite the high species diversity. The explanation is complex and may be attributable to environmental factors such as extreme climate with very hot summers that reach up to 45 °C and cold winters, as low as -8 °C, most human dwellings are made of concrete and use screens in the windows, compared to Oaxaca, Chiapas and Tabasco in southeastern Mexico, where most houses in the rural areas are made of plant matter or wood with palm roofs.23 On the other hand, vector characteristics such as abundance, seasonal distribution, life expectancy, degree of antropophilia and vector competition may influence the malaria transmission cycle.45,46
The success of focalized control is based on community involvement or the search and treatment of sick people and environmental management through elimination of anopheline breeding sites (the ECA, “Eliminación de Criaderos de Anofelinos”).3,19 This control strategy had a resounding success in Oaxaca in the nineties and may be used as a model to be implemented in other areas with active transmission or during new disease outbreaks. In order to ensure the elimination of malaria in Mexico, it is necessary to eliminate or manage those larval habitats at risk in or near communities, applying antilarval measures and based on the knowledge about larval ecology in every specific region and for every anopheline species.46











 nueva página del texto (beta)
nueva página del texto (beta)