The Culicidae family is represented by two subfamilies: Anophelinae and Culicinae, which include species of medical importance in the genera Anopheles (Anophelinae) and Aedes, Culex, Haemagogus, Mansonia, Psorophora and Sabethes species (Culicinae).1 Species of these genera are responsible for the transmission of many pathogens of medical importance that cause dengue, yellow fever, chikungunya, Zika, encephalitis, and filariasis, among others.2 Of these mosquitoes, Anopheles spp. And Aedes spp. are responsible for transmitting malaria and dengue, respectively, which cause the highest morbidity and mortality in humans around the world.3
The Yucatan Peninsula (YP) is a region of southeastern Mexico with an area of approximately 19 760 000 ha. It is formed, to the North, by the states of Yucatan, Quintana Roo and Campeche, and to the south, by Belize and northern Guatemala. Relatively few studies have been published on the mosquitoes of this region, the most outstanding being those of: a) Ibáñez-Bernal and colleagues,4 who reported 48 species belonging to 13 genera in the Sian Ka’an Reserve, Quintana Roo; b) the study of Ibáñez-Bernal and colleagues5 in which 45 species of mosquitoes were identified in the state of Yucatan; 68 species were found in the state of Quintana Roo, and 61 species were detected in the state of Campeche; c) the mosquitoes of two Natural Protected Areas (NPA) of Yucatan in which 11 genera and 30 species were registered;6 d) the mosquitoes of Quintana Roo,7 where 13 genera and 41 species of mosquitoes were identified, and e) a study in the state of Quintana Roo reported a taxonomic inventory of 961 specimens and 13 species belonging to four genera.8 However, there are no studies on the diversity of species in this region.
New technological tools, based on algorithms, associate the presence of species with the environmental conditions and allow estimating and projecting their potential distribution.9 Ecological niche modeling has been used to project the geographic distribution potential for vectors of a number of important arthropod-borne diseases.
The purpose of this study, therefore, was to update our understanding of the diversity of culicids of the three Mexican states of the Yucatan Peninsula, and to determine their abundance, richness, and species diversity, on a state-by-state basis and their potential distribution using ecological niche modeling methods.
Materials and methods
Study area
The study area of this project included urban, suburban and rural localities bordering the Biosphere Reserves (BR) of Calakmul and Los Petenes, located in the state of Campeche; Ría Celestún, located between Campeche and Yucatan; Ría Lagartos, in Yucatan, and Sian Ka’an, in Quintana Roo.
Study design
A cross-sectional study was carried out with four collection periods, the first from April 26 to May 28, 2016; the second, from October 8 to November 7, 2016; the third, from February 1 to March 2, 2017, and the fourth, from June 7 to July 5, 2017. Different sites were sampled in each period. In each study area, the following parameters were recorded: altitude, habitat type, vegetation type, topography, rainfall, temperature, relative humidity and geographical coordinates.
Collection methods
In each site, the following collection methods were used: 1) Larval survey, which involved inspection of all artificial water containers, as well as natural ones (ponds, lagoons, margins of rivers and streams, etc.), in search of immature stages of mosquitoes, through the use of pipettes or metal dippers (500 mL capacity); 2) Ovitraps, comprising one-liter plastic containers, which were filled with 750 mL water and had a strip of filter paper placed around the edge of the water; 3) BG-Sentinel traps, designed to catch adult mosquitoes, and 4) BDV tent traps, for host-seeking mosquitoes.10
The collected eggs were allowed to hatch, and the resulting larvae were reared to adulthood. In the field, larvae were collected in small plastic bags, and the collected adults were stored in 1.5 mL vials and then transported to the insectary of the Regional Center for Research on Public Health of the National Institute of Public Health (CRISP/INSP, Spanish acronyms) in Tapachula, Mexico, where the larvae were reared to adulthoods (28 ± 2 °C, RH: 70-80%), and the adults were taxonomically identified at species level by means of dichotomous keys.11,12 For each species, the number of individuals was recorded and abundance values were transformed (log N). Species were classified as rare species (log=0 to 1), species of intermediate abundance (log=>1-2), and abundant species (log=>2). The specimens collected were deposited in the CRISP/INSP mosquito collection and registered in the National Biodiversity Information System (SNIB/Conabio) through the Biótica 5.0 database.
Species richness and diversity
Species richness (S), Margalef’s species richness index (DMG) were calculated. Alpha diversity of species was determined by the Shannon diversity index (H’), likewise the equitability (E) and maximum diversity (Hmax) were calculated.13 These values were determined for each Biosphere Reserve and by state. Differences in the diversity values were compared by means of a randomization test.14 All index values were calculated using the Species Diversity and Richness 3.0 program (v. 3.0.3).
Ecological niche models (ENM)
A database was constructed from collections reported in Baak-Baak and colleagues,15,16 this study, and the Global Biodiversity Information Facility (GBIF, www.gbif.org/). We obtained a total of 3 458 single records for 14 species (Aedes aegypti, Ae. albopictus, Ae. taeniorhynchus, Anopheles albimanus, An. pseudopunctipennis, An. vestitipennis, Coquillettidia venezuelensis, Culex coronator, Cx. interrogator, Cx. nigripalpus, Cx. quinquefasciatus, Haemagogus equinus, Mansonia titillans, Psorophora confinnis, and Sabethes chloropterus) in the YP, Chiapas and Tabasco.
In order to avoid potential modeling bias related to an important component of model calibration, the accessible region “M”,17 a 200 km radius buffer was created around each point of occurrence of these species in order to extend the limits of the calibration region. Thirteen layers were used to construct the ENM: nine bioclimatic layers, and four topographical layers.18 Bioclimatic layers (mean annual temperature, seasonal temperature, maximum temperature, minimum temperature, annual temperature range, annual rainfall, precipitation of the wettest month, precipitation of the driest month and seasonality of precipitation) were obtained from WorldClim.19 The topographical variables (aspect, slope, topographical index and elevation) were downloaded from Hydro 1K. All layers had a spatial resolution of 30 arc-seconds (≈1 km2).
For the construction of ENM a Maximum Entropy (MaxEnt) algorithm, a machine-learning technique that combines statistics, maximum entropy and Bayesian methods, was used to estimate a suitable probability distribution incorporating restrictions imposed by environmental information.20 Occurrences points were divided into training (75%) and evaluating (25%) data.18 The parameters for MaxEnt required use of bootstrap procedures for the replicate run type, maximum interactions (N=500), features, regularization multiplier (N=2), max number of background points (N=10 000), bootstrap (N=10) and do clamping.21,22
All models were converted into binary (absence/presence) maps based on 95% of occurrence points23 in order to create a map of the richness of species of medical importance in the region.18 Models were evaluated using the partial-Receiver Operating Characteristic (partial-ROC) software, and a ratio was calculated. A total of 1 000 bootstraps runs (in which 50% of evaluation data were resampled with replacement and the area under the curve [AUC] ratios were recalculated) were used to test the hypothesis that model performance was better than random expectation. The null hypothesis was that better performance was no better than the random expectation when ≥95% of bootstrap-replicate AUC ratios were >1. Partial-ROC software is available for free download at https://kuscholarworks.ku.edu/handle/1808/10059.23,24
The total human population growth rate was generated using projections for fertility, mortality and international migration. The population growth rate in Mexico is projected to increase by 30% by 2020. After obtaining the ENM, population projections were calculated for the resident population at risk of contact with vectors for both the rural (<10 000 inhabitants) and urban (>10 000 inhabitants) categories, as reported in Moo-Llanes and colleagues,18 for 14 species of medical importance present in the YP, Tabasco and Chiapas.
Results
Abundance and species richness
A total of 10 669 individuals belonging to 15 genera and 52 species of culicids present in the YP were taxonomically identified. The state with the highest abundance of individuals was Yucatan, followed by Quintana Roo, and finally, Campeche. When comparing the abundance across Biosphere Reserves, Ría Celestún had the highest abundance of culicids, followed by Ría Lagartos, Sian Ka’an, Los Petenes, and, finally, Calakmul. The highest value for the Margalef index corresponded to Campeche (4.43) and the Calakmul reserve (4.48) (table I).
Alpha diversity
The diversity of species by state was significantly higher (randomization test,p<0.001) in the state of Campeche than in Quintana Roo and Yucatan, although the state of Yucatan had the largest number of individuals (N). This was due to the fact that Campeche had the highest equitability of the three states (E=0.57), in addition to the joint highest species richness (S=34) (table I). When analyzing the diversity of species across the five Biosphere Reserves, the Calakmul reserve exhibited the greatest diversity (p<0.001; H’= 2.65), followed by Sian Ka’an, Ría Celestún, Los Petenes and Ría Lagartos. In addition, Calakmul reserve presented the highest species richness (S=29) and the highest equitability index (E=0.79) among the reserves (table I). Across the entire YP, 52 species of culicids were recorded, of which 11 species were found to be very abundant (log N > 2.0 to > 3.0); 16 had an intermediate abundance (log N > 1.0 to 2.0); and 25 were represented by only a few individuals (log N= 0.0 to 1.0), and therefore they were considered as rare species.
Table I: Abundance, richness and diversity of culicid species of the Yucatan Peninsula. Collection periods: April 26 to May 28, 2016; October 8 to November 7, 2016; February 1 to March 2, 2017, and June 7 to July 5, 2017
| State | N | S | DMG | H´ | Variance H´ | E | Hmax |
| Campeche | 2 158 | 34 | 4.43 | 2.02a | 0.0009 | 0.57 | 3.53 |
| Quintana Roo | 2 524 | 27 | 3.20 | 1.76b | 0.0008 | 0.53 | 3.29 |
| Yucatan | 5 987 | 34 | 3.80 | 1.71b | 0.0003 | 0.48 | 3.53 |
|
Biosphere Reserve |
|||||||
| Calakmul | 515 | 29 | 4.48 | 2.65a | 0.0019 | 0.79 | 3.37 |
| Sian Ka’an | 1 161 | 24 | 3.25 | 2.06b | 0.0013 | 0.65 | 3.18 |
| Ría Celestún | 3 608 | 24 | 2.80 | 1.52c | 0.0005 | 0.48 | 3.18 |
| Los Petenes | 1 100 | 18 | 2.43 | 1.44c | 0.0013 | 0.50 | 2.89 |
| Ría Lagartos | 2 026 | 20 | 2.50 | 1.35d | 0.0008 | 0.45 | 2.99 |
N: number of individuals; S: species richness; DMG: Margalef’s species richness index; H’: Shannon diversity index; E: Evenness; and Hmax: maximum diversity. The different letters indicate statistically significant differences in H´ values (Randomization test,p<0.001).
Table II: Species of Culicidae and their abundance in the three States of the Yucatan Peninsula. Collection periods: April 26 to May 28, 2016; October 8 to November 7, 2016; February 1 to March 2, 2017, and June 7 to July 5, 2017
| Species | Campeche | Quintana Roo | Yucatan | Total | Log N∆ | |||||
| Culex trifidus | 0 | 0 | 1 | 1 | 0.00 | |||||
| Mansonia indubitans | 1 | 0 | 0 | 1 | 0.00 | |||||
| Mansonia titillans | 1 | 0 | 0 | 1 | 0.00 | |||||
| Ochlerotatus quadrivittatus | 1 | 0 | 0 | 1 | 0.00 | |||||
| Psorophora champerico | 0 | 1 | 0 | 1 | 0.00 | |||||
| Psorophora confinnis | 0 | 0 | 1 | 1 | 0.00 | |||||
| Trichoprosopon digitatum | 0 | 1 | 0 | 1 | 0.00 | |||||
| Uranotaenia lowii | 0 | 0 | 1 | 1 | 0.00 | |||||
| Anopheles apicimacula | 2 | 0 | 0 | 2 | 0.30 | |||||
| Coquillettidia nigricans | 2 | 0 | 0 | 2 | 0.30 | |||||
| Psorophora cilipes | 0 | 2 | 0 | 2 | 0.30 | |||||
| Aedes tormentor | 0 | 0 | 3 | 3 | 0.48 | |||||
| Anopheles punctipennis | 0 | 0 | 3 | 3 | 0.48 | |||||
| Culex corniger | 2 | 0 | 2 | 4 | 0.60 | |||||
| Culex corniger/lactator | 4 | 0 | 0 | 4 | 0.60 | |||||
| Culex theobaldi | 0 | 4 | 0 | 4 | 0.60 | |||||
| Psorophora ciliata | 2 | 0 | 2 | 4 | 0.60 | |||||
| Culex erytrothorax | 0 | 0 | 5 | 5 | 0.70 | |||||
| Ochlerotatus epactius | 6 | 0 | 0 | 6 | 0.78 | |||||
| Toxorhynchites moctezuma | 1 | 3 | 2 | 6 | 0.78 | |||||
| Psorophora lineata | 0 | 7 | 0 | 7 | 0.85 | |||||
| Aedes bimaculatus | 1 | 0 | 7 | 8 | 0.90 | |||||
| Ochlerotatus euplocamus | 0 | 8 | 0 | 8 | 0.90 | |||||
| Sabethes chloropterus | 3 | 5 | 0 | 8 | 0.90 | |||||
| Haemagogus equinus | 2 | 6 | 2 | 10 | 1.00 | |||||
| Aedes terrens | 12 | 0 | 0 | 12 | 1.08 | |||||
| Uranotaenia socialis | 16 | 0 | 0 | 16 | 1.20 | |||||
| Ochlerotatus sollicitans | 0 | 0 | 17 | 17 | 1.23 | |||||
| Psorophora albipes | 17 | 0 | 0 | 17 | 1.23 | |||||
| Anopheles pseudopunctipennis | 0 | 0 | 20 | 20 | 1.30 | |||||
| Culex interrogaror | 0 | 0 | 25 | 25 | 1.40 | |||||
| Wyeomyia celaenocephala | 8 | 22 | 0 | 30 | 1.48 | |||||
| Mansonia dyari | 5 | 4 | 26 | 35 | 1.54 | |||||
| Deinocerites cancer | 0 | 4 | 51 | 55 | 1.74 | |||||
| Limatus durhamii | 39 | 19 | 1 | 59 | 1.77 | |||||
| Psorophora cyanescens | 27 | 8 | 25 | 60 | 1.78 | |||||
| Psorophora ferox | 37 | 21 | 2 | 60 | 1.78 | |||||
| Culex erraticus | 7 | 59 | 7 | 73 | 1.86 | |||||
| Ochlerotatus serratus | 50 | 2 | 22 | 74 | 1.87 | |||||
| Anopheles vestitipennis | 27 | 17 | 32 | 76 | 1.88 | |||||
| Aedes cozumelensis | 75 | 10 | 2 | 87 | 1.94 | |||||
| Anopheles albimanus | 10 | 60 | 38 | 108 | 2.03 | |||||
| Culex coronator | 72 | 0 | 54 | 126 | 2.10 | |||||
| Ochlerotatus scapularis | 50 | 86 | 1 | 137 | 2.14 | |||||
| Culex taeniopus | 25 | 0 | 132 | 157 | 2.20 | |||||
| Aedes albopictus | 0 | 273 | 16 | 289 | 2.46 | |||||
| Coquillettidia venezuelensis | 19 | 56 | 348 | 423 | 2.63 | |||||
| Anopheles crucians | 2 | 0 | 488 | 490 | 2.69 | |||||
| Culex quinquefasciatus | 222 | 124 | 151 | 497 | 2.70 | |||||
| Culex nigripalpus | 21 | 26 | 599 | 646 | 2.81 | |||||
| Aedes aegypti | 761 | 1 284 | 742 | 2 787 | 3.45 | |||||
| Aedes taeniorhynchus | 628 | 412 | 3 159 | 4 199 | 3.62 |
Log N= 0-1: rare species; log N= >1-2: common species; log N= >2: abundant species.
Ecological niche models
All models were statistically significant and afforded better than random expectations, with partial-ROC ratios >1 (figure 1). The YP, the coastal region of Tabasco and Chiapas all had high species richness (figure 2a). The models of ecological niche predicted a high suitability across the YP for Ae. aegypti (figure 2b), An. albimanus (figure 2e), An. pseudopunctipennis (figure 2f), Cx. coronator (figure 2i), and Cx. quinquefasciatus (figure 2l). The smallest distribution in the study area corresponded to Cq. venezuelensis (figure 2h) on the coast of the YP and Chiapas state. In contrast, the Puuc region (intersection between Yucatan, Campeche and Quintana Roo) of the YP and the highlands of Chiapas state were not suitable for Ae. albopictus (figure 2c), An. vestitipennis (figure 2g), Hg. equinus (figure 2m), Ps. confinnis (figure 2n) and Sa. chloropterus (figure 2o). Much of the state of Chiapas was not suitable for Cx. interrogator (figure 2j), Cx. nigripalpus (figure 2k) and Ae. taeniorhynchus (figure 2d). The species with the highest risk of contact with urban and rural populations in the YP, Tabasco and Chiapas were Cx. quinquefasciatus and Ae. aegypti, while the species with the lowest risk was Cq. venezuelensis (figure 3). Only seven species exhibited more than 50% risk of vector contact with the rural population, compared to 11 species above 50% for the urban population. The species An. pseudopunctipennis, An. albimanus, and Ae. albopictus implied higher risks for the rural population compared to the urban population (figure 3).
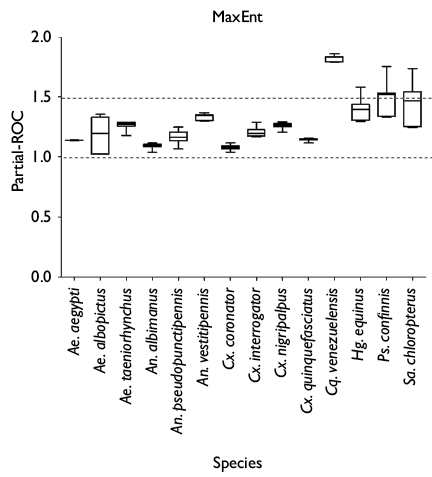
Figure 1. Partial-ROC for 14 species of culicids of medical importance using MaxEnt. Collection periods: April 26 to May 28, 2016; October 8 to November 7, 2016; February 1 to March 2, 2017, and June 7 to July 5, 2017

Collection periods: April 26 to May 28, 2016; October 8 to November 7, 2016; February 1 to March 2, 2017, and June 7 to July 5, 2017
Figure 2. Species richness and ecological niche modeling of 14 species of medical importance in the Yucatan Peninsula, Mexico. a) Species richness, b) Ae. aegypti, c) Ae. albopictus, d) Ae. taeniorhynchus, e) An. albimanus, f) An. pseudopunctipennis, g) An. vestitipennis, h) Cq. venenzuelensis, i) Cx. coronator, j) Cx. interrogator, k) Cx. nigripalpus, l) Cx. quinquefasciatus, m) Hg. equinus, n) Ps. confinnis, and o) Sa. chloropterus.
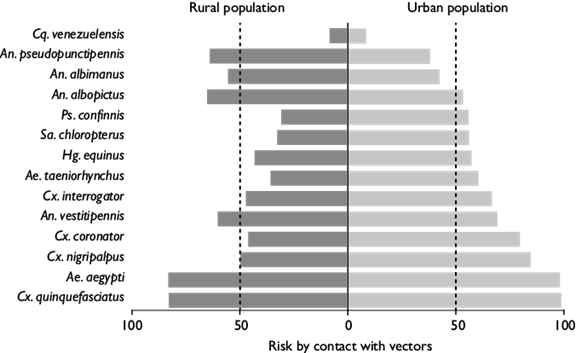
Figure 3. Risk of contact with vector by medical importance in the Yucatan Peninsula, Tabasco and Chiapas. The risk of contact was estimated as a percentage. Rural population (communities with <10 000 inhabitants) and urban population (communities with >10 000 inhabitants). Collection periods: April 26 to May 28, 2016; October 8 to November 7, 2016; February 1 to March 2, 2017, and June 7 to July 5, 2017
Discusión
A total of 52 species were registered for the YP. The state with the greatest diversity of species was Campeche, particularly the Biosphere Reserve of Calakmul.
Worldwide, the Culicidae family includes 113 genera and 3 564 species. The mosquito fauna of Mexico comprises 20 genera and between 225-247 species.5,25 In the present study, 75% (N=15) of the genera of mosquitoes reported for Mexico and 21-23% (N=52) of the species were identified in the fauna of the YP. In a previous study in eight states of the Mexican Pacific coast, 15 genera and 74 species of culicids were reported;26 these amount to the same percentage of genera reported in the present study and 30% more species. However, the results of the present study correspond only to three states of the Mexican republic.
Previous studies on culicids in the YP reported 13 genera and 41 species for Quintana Roo,7 while in the present study 14 genera and 27 species were recorded. For the state of Yucatan, Ibáñez-Bernal and colleagues5 reported 45 species, whereas Ibáñez-Bernal6 registered 11 genera and 30 species for Yucatan. Nájera-Vázquez and colleagues27 registered eight genera and 19 species of mosquitoes for the state of Yucatan; Zapata-Peniche and colleagues28 reported four genera and 12 species in the city of Mérida, Yucatan; Dzul-Manzanilla and colleagues29 registered nine genera and 22 species for the state of Yucatan; and recently, Baak-Baak and colleagues15 reported seven genera and 27 species for the same state. In the present study, the state of Yucatan was found to include 11 genera and 34 species. There have been relatively few studies in the state of Campeche, reporting 50-61 species of mosquitoes,5,30 while the present study registers 14 genera and 34 species in the state of Campeche.
Of the list of mosquitoes registered in the present study, at least 14 have been reported as vectors of human diseases, among which Ae. aegypti and Ae. albopictus have particular importance as vectors of dengue, Zika, chikungunya, yellow fever, and the Mayaro virus.31,32 The mosquitoes An. albimanus, An. pseudopunctipennis, An. vestitipennis, An. crucians and An. punctipennis are malaria vectors;33Ps. confinnis is a vector of Venezuelan equine encephalitis (VEE);34Mansonia titillans is a vector of VEE and lymphatic filariasis; Sa. chloropterus and Hg. equinus are vectors of the yellow fever virus; Ae. taeniorhynchus is a vector of VEE;34Cx. quinquefasciatus is a vector of lymphatic filariasis, West Nile Virus (WNV) and St. Louis Encephalitis Virus (SLE);35 and Cx. nigripalpus is a vector of Eastern equine encephalitis (EEE), SLE and WNV.36
The values of diversity for the culicid species reported in the present study ranged between 1.35 and 2.65, which are close to the values of 1.93-2.66 reported for the eight states of the Pacific coast of Mexico.26
Ecological niche models (ENM) have been implemented in order to understand the cycles of transmission of various diseases, as well as to predict areas susceptible to invasion by exotic species and areas of population at risk of contact with vectors, among other issues.9,18,23 The niche model of Ae. albopictus in the YP, Tabasco and Chiapas indicate that these areas are very similar in suitability (potential distribution) for these vectors, compared to the niche models presented by Campbell and colleagues9 and Pech-May and colleagues.24
In partial species distribution models, a limited number of points of occurrence can be used to generate a partial picture of the distribution.37 Therefore, by including a greater number of points, or expanding the distribution area, we can determine the distribution of these species at a finer scale, although such inferences must be interpreted with care. However, in the present study, we were able to define zones of unsuitability in the YP, Tabasco and Chiapas. Therefore, having clearly defined objectives and spatial scale should improve our interpretation of the results. In the case, of Ae. albopictus, we predicted the presence of the vector across all the YP with high probability. We also generated a high resolution map of the distribution of Ae. albopictus in the region (figure 2c). In addition, the ENM predicted the presence of the vector in Campeche. Therefore, we may expect that this vector will be registered in the state of Campeche in the near future.
A key aspect of public health studies involves the estimation of the number of people who are at risk of disease (figure 3). In urban communities, most mosquitoes (11 out of 14 species) exhibited more than a 50% risk of contact with human populations (figure 3). In rural communities, although there is still a risk of contact for Ae. aegypti, Cx. quinquefasciatus, An. vestitipennis, Ae. albopictus, An. albimanus, and An. pseudopunctipennis, it is important to note that there seems to be a more significant possibility of exposure to Ae. albopictus, An. albimanus and An. pseudopunctipennis in rural areas than in urban areas. In the case of Ae. aegypti and Cx. quinquefasciatus, our results are similar to those of Baak-Baak and colleagues,16 however, for Cx. coronator, our results represent an improved estimate of the risk of contact with vectors in the region.
In conclusion, this study represents the most recent mosquito inventory for the YP, and contributes to understanding the diversity and current distribution of mosquito species in Mexico, particularly for species of medical and veterinary importance. Such information should prove valuable for monitoring changes in species abundance, community composition and vector distribution that can be incorporated in mathematical and statistical models, and assist in the elaboration of successful programs of surveillance and control of vector populations. Of the species reported, the presence of Ae. albopictus in Yucatan is relevant,24 since it is considered to be an invasive species and a vector of numerous diseases. The individuals and species registered during the present study were cataloged in the databases of mosquitoes of Mexico’s National System for Biodiversity Information (SNIB-Conabio), which are available for public consultation.











 nueva página del texto (beta)
nueva página del texto (beta)


