In the late 1960s, before the discovery of enteric viruses, an etiologic agent could only be identified in ~20% of children hospitalized with diarrhea. In 1973, by examination of the duodenal epithelium of children with diarrhea using electronic microscopy, Bishop and colleagues visualized a 70-nm virus that was subsequently named rotavirus because of it wheel-like (Latin, rota = wheel) morphology.1The subsequent development and application of sensitive enzyme immunoassays for rotavirus detection in fecal specimens soon led to numerous studies showing the important role of rotavirus in the etiology of severe childhood diarrhea. Before the widespread global implementation of rotavirus vaccines, data from sentinel hospital-based surveillance using a standardized protocol in more than 50 countries showed that 36% of hospitalizations for childhood diarrhea were caused by rotavirus.2 Furthermore, in developing countries with suboptimal access to health care including basic hydration therapy, rotavirus is an important cause of childhood mortality, accounting for ~200 000 deaths in the year 2013 worldwide.3,4,5
Given its important etiologic role, efforts to reduce the burden of severe childhood diarrhea have targeted rotavirus. Data showing that nearly all children in both industrialized and developing countries were infected with rotavirus by five years of age indicated that interventions to improve hygiene and sanitation and provide safe food and water may not alone fully control rotavirus. This hypothesis was supported by data from Mexico showing that as childhood diarrhea deaths declined following the implementation of safe water and hygiene measures after cholera epidemics in the early 1990s, the decline was greatest for deaths during the summer months when bacterial diarrheas were more prevalent whereas winter diarrhea deaths when rotavirus was more prevalent were less affected.6Conversely, data from a classic longitudinal cohort study showed that Mexican children infected with rotavirus were partially protected from subsequent infections, with the level of protection increasing with each subsequent infection and being greatest against moderate to severe rotavirus disease.7 These findings supported the development of attenuated rotavirus vaccines that could induce protective immunity by simulating the effect of natural rotavirus infection.
In 1998, only 25 years after the discovery of rotavirus, the first rotavirus vaccine (RotaShield, Wyeth Lederle) was licensed and recommended for routine immunization of US children. However, less than one year after vaccine implementation when about 1 million US infants were vaccinated with RotaShield, this vaccine was withdrawn from the US market because it caused an excess of one case of intussusception -- a potentially serious form of bowel obstruction -- per 10 000 vaccinated infants.8,9,10This abrupt and unanticipated setback caused considerable uncertainty over ongoing rotavirus vaccine development.
Some unique biologic properties of the parent rhesus rotavirus strain in RotaShield - such as its high rates of intestinal replication and shedding in vaccinated infants, its propensity to cause fever, and its capacity to cause extra-intestinal infection in animal models - suggested that other candidate rotavirus vaccines might carry less of a risk of intussusception. However, the international community was only reassured after large and expensive clinical trials of 60 000 to 70 000 infants each conducted over the next seven years with two new rotavirus vaccines - a monovalent human vaccine (Rotarix, GlaxoSmithKline, Rixensart, Belgium) and a pentavalent, bovine-human reassortant rotavirus vaccine (RotaTeq, Merck, West Point, PA, USA) - did not show an association with intussusception.11,12In 2006, the region of the Americas was the first to implement these new vaccines and in 2009 the World Health Organization issued a global recommendation for rotavirus vaccine use.13,14
As of July 2018, a total of 90 countries globally have implemented national rotavirus vaccination programs, including 45 low income countries that have received funding support for vaccine purchase through Gavi, the Vaccine Alliance (figure 1).15 A systematic review of 48 postlicensure evaluations from 24 countries published during 2006-2016 showed that the vaccine effectiveness of Rotarix was 84, 75, and 57% in countries with low, medium, and high child mortality, respectively, and effectiveness of RotaTeq was 90 and 45% in countries with low and high child mortality, respectively.16 Despite variations in effectiveness, however, the impact of routine childhood rotavirus vaccination in reducing the burden of severe diarrhea across many settings has been rapid and substantial. A systematic review of 57 articles from 27 countries published from 2006-2016 showed that, following implementation of rotavirus vaccination, overall acute gastroenteritis hospitalizations were reduced by 41, 30, and 46% in countries with low, medium, and high child mortality, respectively, whereas hospitalizations and emergency department visits caused by rotavirus gastroenteritis were reduced by a median of 71, 59, and 60% in countries with low, medium, and high child mortality, respectively.17
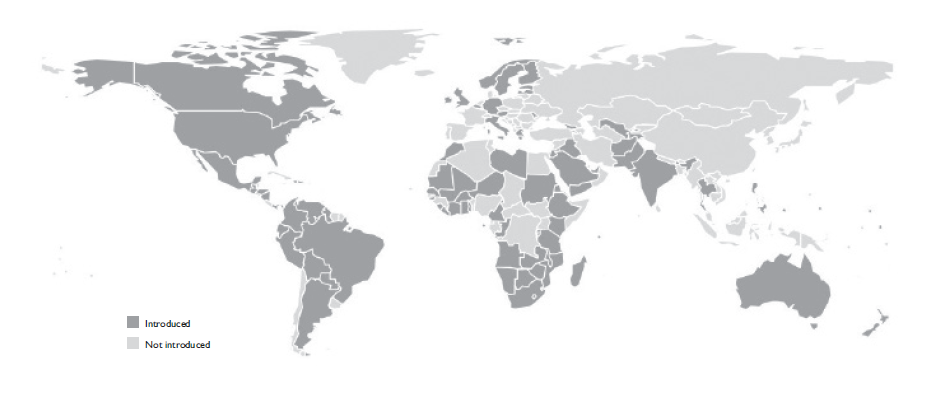
Source: International Vaccine Access Center. Current Vaccine Intro status.15
Figure 1 Rotavirus vaccine introductions globally. July 2018
Besides affirming the effect of rotavirus vaccination on reducing morbidity from diarrhea in vaccinated children, post-licensure data have expanded the evidence on vaccine benefits. First, beginning with data from Mexico, several countries in Latin America have seen declines in childhood deaths from diarrhea following rotavirus vaccination, providing the first direct evidence of the life-saving potential of these vaccines.18,19,20,21,22,23,24,25Second, in many settings, vaccination of young infants and children has led to declines in severe gastroenteritis in older unvaccinated children and adults, likely because of reduction in community transmission of rotavirus by direct protection of young children who are an important source of transmission.26,27 This phenomenon, known as herd protection or community immunity, was not anticipated with rotavirus vaccines. Finally, in addition to protection against severe gastroenteritis caused by rotavirus, some data show a reduction in risk of childhood seizures among children vaccinated against rotavirus.28,29,30,31Reports showing that wild-type rotavirus frequently causes a systemic infection with viremia and has been linked to a variety of neurologic manifestations support the biologic plausibility of this association.32,33,34,35,36
Despite these promising post-licensure data on vaccine impact, the lower effectiveness of rotavirus vaccines in high mortality settings indicates further room for improvement in vaccine performance.16 The gradient of lower rotavirus vaccine performance in settings with higher child mortality is also seen with other orally administered vaccines such as those against polio, typhoid, and cholera,37,38,39,40,41 and is likely related to factors such as passively transferred maternal antibodies, concurrent enteric infections, and infant malnutrition that inhibit the immune response to vaccination. To overcome these factors, clinical trials have evaluated approaches such as transient withholding of breast feeding at the time of vaccination, administration of additional vaccine doses, modifications to the schedule of administration of vaccine doses, and supplementation with zinc and probiotics prior to vaccination. While some of these interventions resulted in marginal improvement in vaccine immune response, the data were not convincing or consistent enough to support a programmatic recommendation for any strategy.42,43,44,45,46Research is ongoing to develop parenterally administered rotavirus vaccines that would potentially avoid the effect of factors that interfere with the performance of oral rotavirus vaccines.47
Concerns remain around the safety of oral rotavirus vaccines with regard to the risk of intussusception. While no increased intussusception risk was seen in large pre-licensure trials with Rotarix and RotaTeq, post-marketing data from several high- and middle-income countries have indicated a risk of 1 to 5 excess cases of intussusception per 100 000 infants vaccinated with either vaccine.48,49,50,51,52,53Data from these countries showing that the health benefits of vaccination far exceed this low risk of intussusception has led policy makers to make no changes to recommendations for rotavirus vaccination. Recently, the first large post-licensure assessment in low-income African countries showed no increased risk of intussusception, providing additional reassurance around the safety of rotavirus vaccines.54 While not proven, it is possible that this lack of intussusception risk in low-income settings is related to the lower efficacy and lower rate of intestinal replication of the live rotavirus vaccine virus strains in these settings.
Assuring adequate supply of rotavirus vaccines at an affordable cost for the global community is vital to achieving their full public health potential. Financial support from the GAVI Alliance has allowed eligible low-income countries to procure rotavirus vaccines at a country co-pay cost of only $0.20 per child and has greatly facilitated vaccine implementation in these countries. In early 2018, an Indian-made rotavirus vaccine (Rotavac, Bharat Biotech Limited, Hyderabad, India)55,56was pre-qualified for global procurement through the GAVI Alliance, and another vaccine (Rotasil, Serum Institute of India, Pune, India)57,58 was pre-qualified later in 2018 (table I). These two vaccines are being routinely administered to all Indian children (~26 million births per year), and their global availability will help assure stable vaccine supply and is likely to lower vaccine cost through increased competition.
table I Globally available rotavirus vaccines
| Manufacturer | RotaTeq | Rotarix | Rotavac | Rotasiil |
| Merck and Co | GSK Biologics | Bharat Biotech | Serum Institute of India | |
| Composition | Live attenuated bovine-human reassortant strains, G1, G2, G3, G4, P1[8] | Live human-attenuated rotavirus strain, G1P[8] | Live attenuated neonatal rotavirus strain, G9P[11] (aka 116E) | Live attenuated bovine-human reassortant strains [G1, G2, G3, G4, G9] |
| Route of administration | Oral | Oral | Oral | Oral |
| Dose volume | 2 mL | 1 mL | 0.5 mL (5 drops) | 2mL |
| Doses per course | 3 | 2 | 3 | 3 |
| Pre-qualification | Yes | Yes | Yes | Yes |
VVM: vaccine vial monitor
In summary, substantial progress has been made in the past decade in the implementation of rotavirus vaccines into childhood immunization programs globally, which is particularly noteworthy given the great uncertainty after the abrupt withdrawal of the first licensed rotavirus vaccine. Routine rotavirus vaccination has led to rapid and large declines in the burden of severe rotavirus diarrhea in countries using vaccine, and vaccine safety has been demonstrated through well-designed, large, post-licensure evaluations. Efforts should continue to provide countries that have not yet introduced vaccines with evidence that will allow them to assess the value of this intervention, and assuring adequate and affordable rotavirus vaccine supply for the global market. Together with other interventions such as provision of safe food and water, improving environmental sanitation, promotion of breastfeeding, and prevention of childhood malnutrition, rotavirus vaccines will have a substantial impact in reducing the morbidity and mortality from severe childhood diarrhea worldwide.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.











 nueva página del texto (beta)
nueva página del texto (beta)


