Introduction
Despite concrete global efforts on primary and secondary prevention, lung cancer is the most lethal malignancy worldwide, causing over 1.6 million deaths yearly. It is also the most common cancer in men worldwide (1.2 million), and is considered a global public health problem.1 Interestingly, the incidence differs according to geographic regions, with the highest incidence rates present in Europe and Asia.1 The landscape of this malignancy is even more complex given the enormous healthcare burden, high costs and expenditure associated with the disease,2 especially because most (58%) lung cancer cases occur in underdeveloped countries.3 Another distinguishing factor of lung cancer is that it displays the lowest five years survival rate (5-18%), compared to other highly incident malignancies (breast, colorectal, prostate, skin and stomach cancer). In fact, lung cancer mortality more than doubles that of colorectal cancer, the second most common cause of cancer related deaths.1,2
Lung cancer is closely related to tobacco use; however, this varies according to the histologic subtypes. ADC of the lung arises mostly in patients with no previous significant tobacco exposure; in contrast SCC arises almost exclusively in former or current smokers. 4 To further complicate this relationship, recent changes of cigarette composition are thought to cause differences in both risks associated with smoking and lung cancer, and in the histologic subtypes associated with exposure.5,6All of this together added to the decreases in smoking prevalence since the 1950s7 have led to significant changes in the lung cancer histology distribution, with ADC leading as the most common. Nevertheless, SCC is responsible for 400 000 incident cases per year worldwide; as a consequence, research in this field has focused on the molecular background of the disease in order to identify potential drug targets.8,9
Due to the predominant role tobacco plays on SCC carcinogenesis, this malignancy is characterized by genomic complexity and high overall mutational load. The Cancer Genome Atlas (TCGA) has identified various genomically defined subsets of SCC. This has led to the development and investigation of targeted agents that can impact the therapeutics of this disease.8 Additionally, despite multiple studies, to date there are no accepted prognostic gene signatures for risk stratification in terms of recurrence or survival, consequently limiting the selection of patients that would require adjuvant therapy.4
In this review, we highlight the current knowledge of molecular targets, ongoing clinical trials of targeted agents, and actionable mutations with matching drugs in lung SCC.
Gross molecular biology of SCC precursors and preinvasive lesions
The molecular background in SCC has been thoroughly studied. Most of the studies investigate the process of genetic changes that appear in pulmonary squamous precursor and pre-invasive lesions (carcinoma in situ, CIS). Immunohistochemical studies have demonstrated presence of cell cycle associated proteins such as proliferating cell nuclear antigen (PCNA), MIB1 and michrosome maintenance protein 2 (MCM2) on squamous dysplasia (SD) and CIS, indicating an increased cell cycle activity.9 Also, smoking status seems to have an effect on cell cycle activity, an increasing tobacco consumption is correlated with increased cell cycle activity while smoking cessation causes it to diminish.10
Not much is known about the activity of EGFR and its signaling pathways (RAS/RAF/ MAPK, JAK/STAT and PI3K/AKT/mTOR) during the transition from dysplasia to CIS and squamous neoplasia. EGFR protein is present in normal bronchial epithelium, as well as in bronchial basal cell hyperplasia and metaplasia. Tobacco smoke phosphorylates and amplifies PI3K causing it to activate, and upregulates AKT in severe bronchial squamous dysplasia.11 In comparison to oncogene activity on SD/CIS, there is much mature data for loss of tumor suppressor genes (TSG) activity in the precursor lesions of SCC. TP53 is a tumor suppressor gene that has a role in controlling cell cycle activity, by promoting p21; CDK4/Cyclin D1 complex is repressed, which in turn promotes Rb phosphorylation and thus cell cycle activity is suppressed.12 Despite the heterogeneity of studies, the deregulation of P53 in the progression from normal epithelium to hyperplasia, SD and CIS has been a consistent finding. The P53 alteration causes bcl2 expression deregulation and a decrease in bax, increasing cell cycle activity and resistance to apoptotic mechanisms. Interestingly, the actual loss of P53 gene (by mutation or chromosomal deletion in 17p [LOH, loss of heterozygocity]) is much less common in SD/CIS than in SCC, and is detected in only 10% of high grade lesions.13,14
Abnormalities in p53 related genes and proteins are less studied in SD and CIS. p63 overexpression and P63 gene amplification is frequently present in high grade SD/CIS or initially invasive disease.15 On the other hand, there is insufficient data to make any conclusion regarding p21, mdm2 or p14 expression in SD/CIS.16 In contrast, p16 (suppressor of multiple growth promoter complexes) is commonly lost in SD/CIS through decreased protein expression and/or diminished gene function caused by deletion of 9p21 or epigenetic silencing by P16 gene promoter hypermethylation.17 Although there is great variability between studies, there is a trend of increasing p16 deregulation as the grade of atypia rises. Cyclin D1 overexpression is present initially in early inflammatory stages of SD, and upsurges as the number of high-grade lesions increases.18 In the case of Rb protein, the loss of Rb is a frequent alteration in small cell lung cancer (SCLC), but it happens to be rare in SD/CIS or in SCC.19 Other putative tumor suppressor genes of uncertain role are also lost in SD/CIS, specially loss of expression of retinoic acid receptor (RAR-B) and fragile histidine triad (FHIT).20
As dysplasia progresses, the molecular milieu becomes favorable of cell survival. Telomerase activation prevents cellular ageing, with the human telomerase reverse transcriptase (hTERT) increasing by 40 times in invasive SCC.21 Altogether, the increase of TERT, altered bax/bcl2 ratio and decreased p53 expression converge in the common purpose of cell survival, and development of SD/CIS.22,23
Angiogenesis stimulation is also relevant to the development and establishment of lesions. For example, the subepithelial stroma in SD/CIS becomes more vascular in relation to the grade of the lesion.24 Neuropilin, KDR and ftl1, all vascular endothelial growth factor receptor (VEGFR) ligands, are increased in SD/CIS, and together with an increased expression of vascular endothelial growth factor (VEGF) contribute to the increase in vascularization.24,25COX-2 is also a proangiogenic factor that appears to be increased in higher grades of SD/CIS.26,27
Other molecular alterations in SCC genesis include those related to cell invasion and migration, which include the increased expression of matrix metalloproteinases (MMP3, 9 and 11), a decrease in MMP1 and its inhibitors TIMP1 and MMP7, thus promoting tissue invasion in CIS.28 Additional alterations include transcription factors such as Upstream Stimulatory Factors USF 1 and 2 and NF-KappaB,29 and heat shock proteins 10 and 60, which are lost throughout the transformation process.30
Other global genetic studies of SC/CIS reported aneuploidy and specific chromosomal aneusomy of chromosomes 3, 5, 6, 7 and 8.31,32 Chromosomal gains and losses in chromosomes 1q, 3p, 8p, 8q, 12q, and 17q have been shown previously in CIS using comparative genomic hybridization.32,33,34 LOH studies have shown a wide spectrum of changes in several loci, especially in 3p. This highlights that a number of possible TSG are altered in the initial process of bronchial carcinogenesis. These changes are aparent even in the bronchial epithelium in smokers but increase in extent, number and frequency as SD/CIS evolves.31,35 These very early losses are present in: 3p14.2 (FHIT), 3p21 (RASSF1A, FUS1, SEMA3B), 3p22-24 (BAP1), 3p25, 9p21 (p16). Later changes include alterations in 17p13 (p53) and deletions in 8q21-23 related to focal losses in 3p and 9p.36,37
Genomic landscape of lung SCC
Whilst comprehensive genome-scale characterization has been performed in ADC,38 genetic alterations in SCC are less understood. Lung SCC is strongly associated to tobacco use with most cohorts reporting a rate of tobacco exposure in excess of 90%.39 SCC displays a somatic mutation rate and spectrum comparable to that of patients with small cell lung cancer or other smoking-related cancers. The high mutation rate in SCC is likely to result in expression of a large complement of tumor antigens, and is similar across populations from North and South America, Europe and Asia.40,41,42Interestingly, the genomic alterations in lung SCC are strikingly similar to those found in Human Papilloma Virus (HPV) negative head and neck cancers.43
Several genetic changes have been related to SCC, including amplification of p63, PI3KCA, PDGFRA, SOX2, or FGFR1 and mutation in p53, EGFRvIII, PI3KCA, NRF2, PTEN, and DDR2.38 In 2012, The TCGA identified that lung SCC is characterized by complex genomic alterations, with a mean of 165 genomic rearrangements, 360 exonic mutations and 323 segments of copy number alterations in every single tumor. The TCGA also identified statistically recurrent mutations in 11 genes (TP53, CDKN2A, PTEN, PIK3CA, KEAP1, MLL2, HLA-A, NRF2, NOTCH1, RB1 and HLA-A) and somatic copy number alterations of chromosomal segments containing SOX2, PDGFRA and/or KIT, EGFR, FGFR1 and/or WHSC1L1, CCND1, CDKN2A, NFE2L2, MYC, CDK6, MDM2, BCL2L1, EYS, FOXP1, PTEN, and NF1.8 Recent results have confirmed the TCGA findings using multiplex PCR sequencing. Most important findings in this population were driver mutations in PIK3CA, PTEN and DDR2, as well as FGFR1 amplification. Overall, 60% of patients were found to have druggable targets (figure 1).44,45
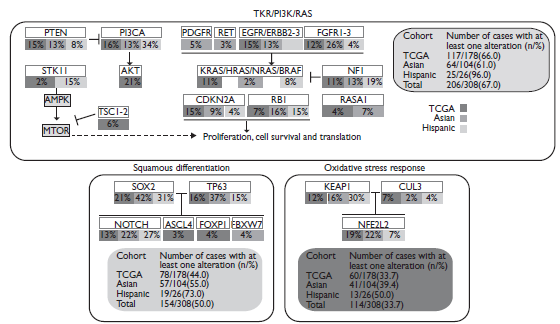
PDGFR: Platelet-derived growth factor receptor; EGFR: Epidermal Growth Factor Receptor; FGFR: Fibroblast growth factor receptors; PI3CA: Phosphoinositide 3-kinases; STK11: Serine/Threonine Kinase 11; AMPK: 5’ AMP-activated protein kinase; AKT: Serine/threonine-protein kinases 1-3, MTOR: Mammalian target of rapamycin.
Figure 1 Diagram integrating three different core pathways of lung squamous cell carcinoma (considering information of substitution mutations, truncating mutations, amplifications and deletions) from the information provided by the TCGA and by Kim and colleagues.44
Novel cytotoxic agents
Novel cytotoxic agents for SCC include Nab-paclitaxel, an agent which is combined to a human albumin molecule in order to achieve higher intratumoral concentration compared to previous taxanes due to the use of the albumin receptor 60-kDa glycoprotein (gp60). Previous clinical trials have shown that nab-paclitaxel produce higher response rates (33% vs. 25; response rate ratio, 1.313; 95%CI 1.082 to 1.593; p=0.005). Unfortunately, there was no significant benefit in terms of PFS or OS. Notably, in the patients with SCC the nab-paclitaxel regimen achieved much higher ORR than the control group (41% vs. 24; response rate ratio, 1.680; 95%CI, 1.271 to 2.221; p<0.001).46 SCC has an aberrant caveolin-1 expression; this may explain the better efficacy of nab-paclitaxel in the squamous histology.47 There is an ongoing clinical trial (NCT00729612) that is evaluating the role of SPARC, ceveolin-1, and microRNAs as predictive biomarkers in patients with advanced SCC treated with carboplatin and nab-paclitaxel. Multiple clinical trials have proven the clinical relevance of nab-paclitaxel in the treatment of NSCLC, some with special emphasis on SCC in particular populations such as the elderly.48,49
Another promising compound for SCC is the second-generation platinum compound Nedaplatin, which has shown a better toxicity profile with lower rates of renal toxicity and emesis compared with cisplatin. Nedaplatin has shown to have better response rates in the SCC subtype compared to non-squamous histologies (ORR, 55.6% in squamous histology vs. 34.4% in non-squamous histology). These results suggest there is a more potent activity of nedaplatin against SCC. In a randomized phase 3 study for advanced SCC patients, nedaplatin plus Docetaxel achieved a significantly prolonged OS compared to cisplatin plus Docetaxel (median OS 13.6 vs. 11.4 months, respectively; HR 0.81; P=0.037), though no significant differences were observed between nedaplatin and cisplatin arms in terms of PFS (median PFS, 4.9 vs. 4.5 months; HR 0.83; P=0.050) and response rates (56 vs. 53%).50
Novel targeted agents for SCC
Anti-EGFR therapy
To date, it is known that EGFR activating mutations are exceptionally rare in SC, though, loyal to its epithelial cancer nature, it keenly expresses EGF and a subset demonstrates EGFR amplification.8 Cetuximab, the monoclonal antibody (mAbs) against the EGFR protein, in combination with cisplatin/vinorelbine had a minimal survival benefit compared with chemotherapy alone in patients with stage IIIB/IV NSCLC in the FLEX study (OS 11.3 vs. 10.1 months; P=0.044).51 In a preplanned subset analysis, a minimal survival benefit was seen in SCC (OS, 9.0 vs. 8.2 months). High tumor EGFR expression (immunohistochemistry score [H-score] ≥200) and amplification, were more common in SCC than in non-SCC histology, seem to serve as predictive biomarkers for the correct patient selection of patients that would obtain a clinical benefit from treatment with chemotherapy plus cetuximab [FISH+ (11.8 versus 6.4 months; HR 0.56; P=0.01); high EGFR expression H-score ≥200 9.3 versus 6.9 months; HR 0.64; P=0.03), and both FISH+ and high-EGFR expressing tumors (12.3 versus 4.9 months; HR 0.32; P=0.0004).52
Necitumumab is another anti-EGFR mAbs. It was evaluated specifically in lung SCC in a large phase III trial (SQUIRE).53 1 093 patients with advanced SCC were randomized to gemcitabine-cisplatin with or without necitumumab. OS was significantly increased in necitumumab-treated patients (11.5 versus 9.9 months, HR 0.84, 95%CI 0.74-0.96) and PFS was also significantly increased (HR 0.85, 95%CI 0.74-0.98). A further analysis showed that addition of necitumumab to chemotherapy resulted in significant improvements in OS (11.7 versus 10.0 months; HR 0.79; 95%CI 0.69, 0.92; p = 0.002) for the vast majority of patients whose tumors expressed EGFR (95%), but not in patients without EGFR expression (5% of patients; HR 1.52). FISH status was determined for 51% of patients in the study, there was also a trend for greater necitumumab benefit in patients with EGFR FISH+ tumors (12.6 versus 9.2 months; HR 0.70; 95%CI 0.52, 0.96), but no benefit in patients with EGFR FISH- tumors (11.1 versus 10.7 months; HR 1.02; 95%CI 0.80, 1.29).53,54,55
Use of mAbs against EGFR is limited in the SCC setting, due to limited efficacy and lack of robust biomarkers for patient selection.56 However, ESMO guidelines include necitumumab combined with cisplatin-gemcitabine as an option for first-line treatment of SCC.57
Other studies, including the LUX-Lung 8 phase III trial, have focused on SCC patients with wild-type EGFR. In this study, previously treated SCC patients were randomized to receive either erlotinib or Afatinib, an irreversible pan-EGFR blocker. Results from this study showed that patients treated with Afatinib had a significantly longer PFS and disease control rate (DCR). Further, stratification of patients using Veristrat (VeriStrat Good, VS-G) demonstrated a significant benefit in terms of OS, which was maintained in a multivariate analysis independently of ECOG or best-response to first line chemotherapy.58,59Other secondary analyses have also identified that among LUX-Lung 8 study patients, those with mutations in the ERBB family assessed by NGS and presence of mutations in HER2 had a favorable PFS and OS when treated with Afatinib compared to erlotinib.60
Antiangiogenics
Ramucirumab (antiangiogenic VEGFR2 mAbs antagonist) is used in combination with Docetaxel for NSCLC patients with a good ECOG PS (0-2) based on the results from the phase III REVEL study,61 which showed an improvement in OS present in both the squamous and non-squamous subtypes. As opposed to bevacizumab, the use of ramucirumab was not associated with increased rates of hemoptysis or other pulmonary hemorrhage events in the SCC subgroup.
FGFR
Several mechanisms result in imbalanced FGFR signaling, which can lead to NSCLC development by either supporting tumor angiogenesis or promoting cell proliferation. Main mechanisms include overexpression caused by amplification or aberrant transcriptional regulation, interchanging between alternatively spliced isoforms, FGFR mutations, FGFR fusion proteins (more than 10 fusion proteins have been identified for FGFR1), increased ligand availability, and impaired down regulation of FGFR activity.62 In NSCLC cell lines with 8p12 amplification, small-molecule FGFR kinase inhibitors arrested cell growth and stimulated apoptosis, which confirms the oncogenic activity of FGFR1.63,64AZD4547 is a potent and selective inhibitor of FGFR 1-3, it is active against FGFR-deregulated and amplified tumors in preclinical models.65 The phase 1b trial, includes 15 patients with previously treated stage IV FGFR1-amplified lung SCC that were treated with AZD4547 80 mg oral bid. The most common treatment related adverse events were gastrointestinal and dermatologic. A single patient with high FGFR amplification (FISH ratio > 2.8) achieved a partial response and 4 had stable disease.65 More recently, results of a sub-study from the lung-MAP clinical trial were reported. This phase II study evaluated AZD4547 in patients with FGFR-altered chemotherapy refractory SCC. The majority of the patients had FGFR1 amplification. AZD4547 showed an acceptable toxicity profile but minimal activity. Only one of the 28 patients who received AZD4547, harbored an FGFR3 S249C mutation and showed an unconfirmed partial response (PR).66 This study was closed for futility, but at least two more phase II trials continue to evaluate the role of this compound in FGFR altered lung SCC. Other compounds currently under study for FGFR include BGJ398 [NCT01004224], LY2874455 and BAY1163877, they have a similar toxicity profile and have managed marginal stability of the disease in few patients.67 GSK3052230 is a novel FGF ligand trap that binds to all of the mitogenic FGF ligands, it blocks FGF cell proliferation in vitro and FGF- (and VEGF-) induced angiogenesis in vivo. The phase 1 trial included 39 patients with advanced solid tumors (three with NSCLC) treated with escalating doses of weekly doses of GSK3052230 for four weeks.68 Preliminary results showed that GSK3052230 elicited a good tolerance with most frequent AEs being diarrhea, fatigue, and nausea. In addition, 41.7% achieved stable disease.68
Multiple multikinase non-selective FGFR inhibitors including nintedanib, cediranib, ponatinib (AP24534), lucitanib (E3810), pazopanib, regorafenib, brivanib, orantinib, ENMD-2076, FGF401 and ODM-203 are in development, some with promising early results, others with negative phase 3 clinical trials.
PI3K pathway inhibitors
The PI3K pathway is a major signaling pathway that plays a role in cell survival and proliferation.8 Somatic mutation or amplification of PIK3CA, that affects the p110 catalytic subunit, is more prevalent in lung SCC than ADC.69 The incidence of PIK3CA mutation is approximately 8-16% and amplification is 33-43% in SCC. In phase I trials, response rate to PI3K/AKT/mTOR pathway inhibitors was significantly higher for patients with PIK3CA mutations than for those without documented mutations (35 vs. 5%, P< 0.001). Recent results among SCC showed that taselisib generates a PR in patients with PIK3CA E545K gene alteration (5% RR, 95%CI 1-24%) while 13 additional patients experienced disease stability. Median PFS was 2.5 months (95%CI 1.7-4.5) and grade 3 AEs included five patients each with hyperglycemia or diarrhea, and three with lymphopenia.70 The tumor suppressor PTEN has phosphatase activity and inhibits the PI3K/AKT/mTOR signaling pathway. In the case of SCC, the incidence of PTEN mutation/deletion was reported to be 15-28%.71,72This alteration is associated with a poorer response to erlotinib in EGFR-mutant NSCLC as well as with intrinsic resistance to immunotherapy.72,73
PI3K/AKT/mTOR pathway inhibitors may be effective in tumors with alterations in PTEN. PIK3CB has been reported in PTEN-deficient tumors.69 Further, in preclinical models of solid tumors, the inhibition of PIK3CB reduced Akt phosphorylation and prevented tumor progression; suggesting that the selective inhibition of PIK3CB may be effective in the treatment of PTEN-deficient tumors.74 Parallel inhibition of PI3K through AKT has also generated intriguing results in silico and in vivo models. Previously, Lara et al. reported the efficacy and safety of erlotinib plus MK-2206 (highly selective AKT inhibitor) in advanced previously treated NSCLC. In a combined population of patients with wild type EGFR, a disease control rate (DCR) between 40 and 47% was seen. Median PFS was of 4.6 months, and mean AEs were rash, diarrhea, fatigue, and Mucositis.74 More research is required to elucidate the role of PI3K and AKT inhibitors in lung SCC.
PDGFRA amplification/mutation
Platelet-derived growth factor receptor (PDGFR) is a tyrosine kinase, two subtypes are currently known: PDGFRA and PDGFRB. It plays a critical role in cell proliferation and angiogenesis. The amplification of the chromosomal segment of 4q12 harboring PDGFR- has been reported in ∼9% of lung SCCs.8,75 Several multi-targeted TKIs targeting PDGFRA, such as sorafenib, sunitinib, and imatinib, have been tested previously in NSCLC. Disappointingly, the addition of sorafenib to platinum-based chemotherapy failed to improve survival, and actually increased mortality in a subset of lung SCC, in a randomized phase III study.76
PARP-1
Poly (ADP-ribose) polymerase-1 (PARP-1) is an important DNA repair enzyme, implicated in base excision repair and single-strand break (SSB) repair. When PARP-1 is inhibited, accumulated unrepaired SSBs are converted to double-strand breaks (DSB), hence inducing cell death.77 PARP-1 is significantly up-regulated at the mRNA level in multiple cancer types, including lung SCC. Paul and colleagues showed that BRCA1-deficient NSCLC cells BRCA1-deficient were more sensitive to PARP-1 inhibition. Furthermore, he demonstrated that BRCA-deficient, platinum-resistant cells still remained sensitive to PARP inhibition. The authors propose that BRCA-deficient cells bypass the BAX and BAK apoptotic proteins, and that cell death occurs independently of mitochondrial induced apoptosis.78 Several PARP inhibitors are currently undergoing clinical trials in lung cancer. Recently, the results of a randomized, placebo controlled phase II study in NSCLC were reported. Patients with advanced or metastatic NSCLC are randomized to receive carboplatin/paclitaxel plus veliparib or placebo, 77 of the 158 patients enrolled had lung SCC. For the intent-to-treat population median PFS and OS was higher with veliparib, however the differences were not statistically significant.79 The improvement in PFS and OS was only present in patients with squamous histology (6.1 versus 4.1 months [HR 0.77] and 10.3 vs. 8.4 months [HR 0.71], respectively).
DDR2
The receptor tyrosine kinase Discoidin Domain Receptor Tyrosine kinase 2 (DDR2) is mutated in approximately 4% of patients with lung SCC. DDR2 is a receptor for extracellular collagens that activates a complex signaling network involving SHP-2 as well as SRC and MAP kinases.80 It regulates epithelial-mesenchymal transitions (EMT). Multitargeted kinase inhibitors like dasatinib, imatinib, nilotinib, and ponatinib suppres the proliferation DDR2 on cancer cell line models.81 Dasatinib, the most potent of these inhibitors, has been studied in multiple lung cancer clinical trials.82 While some responses to dasatinib have been reported in patients with the DDR2 S768R mutation, its associated toxicity has limited its investigation.
BRAF
BRAF mutation is oncogenic since it causes constitutional serine/threonine kinase activation. According to a recent meta-analysis, there was no significant difference in BRAF mutation rate in former or current smokers and never smokers (OR: 0.95, 95%CI 0.45-2.02). Nevertheless, several studies reported an association between BRAF mutation and tumor histology. BRAF mutations were detected 4% of adenocarcinomas and 0.58% of lung SCC.83 Paik and colleagues found no difference in OS for BRAF-mutated patients when compared with other EGFR mutated, ALK-mutated, or KRAS-mutated subpopulations.84 Marchetti and colleagues, described that patients with the BRAF V600E mutation had shorter disease free survival (DFS) and OS compared with wild-type and nonV600E mutations.85 In vitro preclinical studies have identified that vemurafenib and trametinib are effective single agents in BRAF V600E mutant cells, and trametinib in non-V600E mutants also.85 The combination of vemurafenib and trametinib increased tumor cell death, showing that the combination is more effective at least in this particular model. Two other MEK inhibitors (PD0325901 and CI-1040) have also shown activity in in vitro and in vivo preclinical models of NSCLC with BRAF V600E. Recently, Planchard et al. published the results of a phase II trial that included 36 patients treated with first-line dabrafenib plus trametinib, with an ORR in 23 (64%, 95%CI 46-79), with 6% of the cases achieving a CR and 58% a PR. Unfortunately, all cases presented one or more AEs and 69% had one or more grade 3 or 4 event, including hypertension and liver enzyme increase.86
Conclusion
The recent expansion of our knowledge on the genomic landscape of lung SCC has led to the identification of potential driver mutations, including FGFR1 amplification, PIK3CA mutation, PTEN mutation/deletion, PDGFRA amplification/mutation, DDR2 mutation, and BRAF mutation. The impact of these mutations on the response to the matching targeted therapy should be validated in the near future.











 nueva página del texto (beta)
nueva página del texto (beta)


