Introduction
Lung cancer remains the leading cause of cancer-related deaths among males and has increased in women in the last decades.1 Lung cancer in women has become an important health problem, surpassing mortality from breast cancer and being the first cause of cancer-related death in EEUU and in some countries from Europe.1,2,3,4,5Interestingly, a higher incidence among young women compared to men has been observed in the last decades in EEUU, however, this increase in incidence among women cannot be attributed solely to an increase in tobacco use, due to the fact that young women and men show a similar pattern in terms of smoking behavior, nonetheless the increased incidence of lung cancer in women is notorious.6 Moreover, an increased incidence of lung cancer has also been reported in Latin American women, who present a lower smoking index compared to European and American women.7,8 These data suggest that even though tobacco use remains an important risk factor for developing lung cancer, women face other risk factors, which also appear to play an important role in lung carcinogenesis. Furthermore, a higher percentage of women who develop lung cancer are never smokers compared to the low percentage of non-smoking men who present with this pathology (53 vs. 15%).9,10Other risk factors that might partially explain the increased incidence of lung cancer in women include: wood smoke exposure,11cooking oil fumes exposure12 or second hand exposure to cigarette smoke,13 however exposure to these risk factors does not fully explain the increased incidence of lung cancer in women. In the last years, it has been reported that estrogen and progesterone play an important role in lung cancer in women, mainly in the adenocarcinoma subtype, activating carcinogenic pathways.14,15Also, several studies have shown that hormone replacement therapy based on estrogen and progestin can increase lung cancer incidence and mortality.16,17
Additionally, lung cancer presentation, behavior and response to treatment seem to differ between women and men. For instance, the median age for lung cancer diagnosis in women is lower compared to men. Women with lung cancer more often have no history of smoking and the predominant subtype is adenocarcinoma, frequently associated with mutations in epidermal growth factor receptor gene (EGFR). In addition, women show better response to chemotherapy and longer overall survival, regardless of the clinical stage at diagnosis, compared to men.18,19Recently, it has been observed that non-small cell lung cancer (NSCLC) tumors are more immunogenic in men and consequently male patients respond better to immunotherapy compared to women.20 All these differences in lung cancer by sex suggest a role of sexual hormones in lung cancer; however, differences by hormonal status in women (premenopausal/postmenopausal) have been poorly studied. Nevertheless, when characteristics of premenopausal women have been analyzed, they show that these patients are diagnosed in advanced stages, with less differentiated tumors, distant metastases and worse prognosis compared to postmenopausal women. This information suggests that lung cancer is not only influenced by sex, but also by hormonal status.21,22
Several studies have focused on reporting the differences by sex and despite this information, these differences are poorly understood, and the results of studies are contradictory. The controversy in these studies might be due to the inherent characteristics of the population analyzed, differences in the main risk factors, mutational tumor profile, genetic characteristics of patients and hormonal status. With a better understanding about the role of sexual hormones in lung cancer, it is important to investigate differences by hormonal status in Latin America population.23
Nowadays, it is recognized that sex and sexual hormones influence lung cancer. Due to the impact that these differences have in future treatment strategies, it is important to comprehensively characterize them. In this study, we sought to investigate the differences in clinical features and survival of NSCLC patients by sex and hormonal status, in a Mexican population.
Materials and methods
Patient selection
We retrospectively reviewed demographic, clinical, molecular and pathological data of 1 104 NSCLC who attended at the National Cancer Institute of Mexico (Incan) between May 2008 through March 2017. All included patients had a histologically confirmed diagnosis of NSCLC and molecular genotype available in their clinical file (EGFR and Kirsten rat sarcoma virus oncogene [KRAS] mutation status). Information including sex, age, and menopausal status at diagnosis was collected. Menopause was defined according to the international menopause guideline24 as the permanent cessation of menses for 12 or more months in the absence of chemotherapy. Patients who had undergone oophorectomy were excluded from this study. According to standardized guidelines for smoking measurement,25 we defined any patient who smoked 100 or more cigarettes in his or her lifetime and those who currently smokes, as a smoker. A person was defined as a non-smoker if he or she had either never smoked at all, or had never been a daily smoker and had smoked less than 100 cigarettes in his or her lifetime. Smoking index was obtained by multiplying the number of cigarettes smoked per day by the number of years the patient reported that he/she had smoked [(# cigarettes per day)(years smoking)/20) and reported as pack-year]. Self-reported wood-smoke exposure while cooking was recorded and the wood smoke exposure index was calculated by multiplying the number of daily hours exposed by the number of years’ exposure.11
Outcome measurement
Clinical baseline characteristics included age, sex, hormonal status (premenopausal/postmenopausal), weight, histologic tumor type, disease stage, smoking status, wood-smoke exposure, exposure to asbestos, ECOG performance status, metastatic sites, and mutation profile for EGFR and KRAS. Overall survival (OS) was defined as the time from histological diagnosis until death or last follow-up.
Statistical analysis
For descriptive purposes, continuous variables were summarized as arithmetic means with standard deviations, while categorical variables were summarized as frequencies and percentages. The chi2 or Fisher’s exact tests were used to assess the significance among categorical variables. The primary endpoint was OS, defined as the time from histological diagnosis until death or last follow-up. Median OS was estimated using the Kaplan-Meier’s method, whereas the log-rank test was used for making comparisons among subgroups. A multivariate Cox regression model was used to adjust for potential confounders and hazard ratios (HR) were calculated along with their corresponding 95%CI as a measure of association. Statistical significance was determined as p≤0.05 using a two-tailed test. SPSS software version 21 (SPSS Inc., Chicago, IL) was used for all statistical analysis.
Results
Characteristics for the entire population of NSCLC patients
Out of 1 104 patient records reviewed for this study, 582 (52.7%) were men and 522 (47.3%) women. The median age was 60.6 years (+12.9). According to risk factors, 55.7% were ever smokers, while 38.5% of the patients had history of wood smoke exposure, and 10.5% had exposure to asbestos; 72.9 % of all patients have an ECOG <1. Most patients had adenocarcinoma histology (84.3%) and 98.9% presented with advanced or metastatic disease (stages IIIB or IV) at diagnosis. Nearly thirty percent of patients (29.6%) had EGFR mutations and 10.2% had KRAS alterations (table I).
Table I Clinic-pathological characteristics of patients treated at the National Cancer Institute (Incan) in Mexico City from 2008-2017 (N=1 104)
| All patients % (n/N) | Sex | p-Value | ||||||
| Female | Male | |||||||
| (n=522) % (n/N) | (n=582) % (n/N) | |||||||
| Age (years) | ||||||||
| Mean (+SD) | 60.6 (12.9) | 59.8 (13.4) | 61.2 (12.5) | 0.068 | ||||
| BMI (Kg/m2) | ||||||||
| Mean (+SD) | 24.8 (4.6) | 25.1 (4.9) | 24.6 (4.2) | 0.036 | ||||
| BMI groups | ||||||||
| Normal ( <25 kg/m2) | 55.2 (609/1 104) | 51.6 (269/522) | 58.5 (340/582) | |||||
| Overweight (25 - 29.9 kg/m2) | 32.4 (358/1 104) | 32.4 (169/522) | 32.4 (189/582) | |||||
| Obese (30+ kg/m2) | 12.4 (137/1 104) | 16.0 (84/522) | 9.1 (53/582) | 0.003 | ||||
| Tobacco exposure | ||||||||
| Absent | 44.3 (489/1 104) | 68.0 (355/522) | 23.0 (134/582) | |||||
| Present | 55.7 (615/1 104) | 32.0 (167/522) | 77.0 (448/582) | <0.001 | ||||
| Tobacco index | ||||||||
| Mean (+SD) | 34.4 (167.9) | 17.7 (21.8) | 40.4 (195.3) | 0.133 | ||||
| Wood-smoke exposure | ||||||||
| Absent | 61.5 (679/1 104) | 50.0 (261/522) | 71.8 (418/582) | |||||
| Present | 38.5 (425/1 104) | 50.0 (261/522) | 28.2 (164/582) | <0.001 | ||||
| Exposure to asbestos | ||||||||
| Absent | 89.5 (988/1 104) | 90.8 (474/522) | 88.3 (514/582) | |||||
| Present | 10.5 (116/1 104) | 9.2 (48/522) | 11.7 (68/582) | 0.178 | ||||
| Tumor histologic type | ||||||||
| Adenocarcinoma | 84.3 (931/582) | 88.9 (464/522) | 80.2 (467/582) | |||||
| Squamous | 15.7(173/1 104) | 11.1 (58/522) | 19.8 (115/582) | <0.001 | ||||
| Disease stage | ||||||||
| II - IIIA | 1.1 (12/1 104) | 1.2 (6/522) | 1.1 (6/582) | |||||
| IIIB - IV | 98.9 (1 092/1 104) | 98.8 (516/522) | 98.9 (576/582) | 0.866 | ||||
| ECOG performance status | ||||||||
| 0-1 | 72.9 (805/1 104) | 76.2 (398/522) | 69.9 (407/582) | |||||
| >2 | 27.1 (299/1 104) | 23.8 (124/522) | 30.1 (175/582) | 0.020 | ||||
| Brain metastases at diagnosis* | ||||||||
| Absent | 53.6 (535/999) | 54.7 (262/479) | 52.5 (273/520) | |||||
| Present | 46.4 (464/999) | 45.3 (217/479) | 47.5 (247/520) | 0.487 | ||||
| Lymphatic nodes metastases at diagnosis* | ||||||||
| Absent | 81.8 (817/999) | 79.1 (379/479) | 84.2 (438/520) | |||||
| Present | 18.2 (182/999) | 20.9 (100/479) | 15.8 (82/520) | 0.037 | ||||
| Adrenal glands metastases at diagnosis* | ||||||||
| Absent | 95.5 (954/999) | 96.0 (460/479) | 95.0 (494/520) | |||||
| Present | 4.5 (45/999) | 4.0 (19/479) | 5.0 (26/520) | 0.431 | ||||
| EGFR mutation status‡ | ||||||||
| WT EGFR | 70.4 (286/406) | 61.2 (134/219) | 81.3 (152/187) | |||||
| EGFR sensitizing mutation | 29.6 (120/406) | 38.8 (85/219) | 18.7 (35/187) | <0.001 | ||||
| KRAS mutation status‡ | ||||||||
| KRAS (-) | 89.8 (256/285) | 89.8 (132/147) | 89.9 (124/138) | |||||
| KRAS (+) | 10.2 (20/285) | 10.2 (15/147) | 10.1 (14/138) | 0.987 | ||||
SD: standard deviation, BMI: body mass index, ECOG: eastern cooperative oncology group, WT: wild-type, EGFR: epidermal growth factor receptor, KRAS: Kirsten rat sarcoma virus oncogene.
* Estimation over 999 patients with stage IV disease and known metastases at diagnosis.
‡Estimations over the number of patients tested for each molecular status.
Characteristics of NSCLC patients according to sex
The relative frequency of obese patients was substantially higher in women (16%) than in men (9%), p=0.003, likewise, female patients showed a higher body mass index (BMI) in comparison with their male counterparts (24.6 vs. 25.1; p=0.036). In addition, women had higher frequencies of wood-smoke exposure (50 vs. 28.2%; p=<0.001), EGFR-sensitizing mutations (38.8 vs. 18.7%; p=<0.001) and better ECOG performance status (≤1) (76.2 vs. 69.9%; p=0.020). By contrast, men showed higher frequencies of tobacco smoking exposure compared to women (70 vs. 32%; p=<0.001). We did not find differences among men and women in terms of age, histology, disease stage or KRAS mutation status. Table I shows the clinic-pathological characteristics by sex.
Factors associated with the overall survival of patients
The median OS for the entire cohort was 21.6 months. The univariate analysis showed that the factors associated with OS were: sex, weight, smoking history, ECOG performance status, liver metastases and alterations in KRAS. The OS was prolonged in women compared to men (27.6 vs. 18.4 months, respectively [p=0.021]) (figure 1). Similarly, overweight patients had better results in terms of OS compared with normal weight or obesity (28.4 vs. 17.4 vs. 22.4 months, respectively; p=0.045). Patients with tobacco exposure had a shorter OS compared to never smoker patients (18.1 vs. 28.2 months; p=0.002). On the other hand, patients with an ECOG <2 (23.5 vs. 14.5 months; p=0.006), absence of liver metastases, and wild type KRAS (38.9 vs. 15.7 months; p<0.001) had better OS. The multivariate analysis showed that ECOG performance status (HR: 0.4, 95%CI (0.2-0.7); p=0.001) and KRAS mutation (HR: 2.1, 95%CI (1.1-3.8); p=0.017) were independently associated with OS (table II).
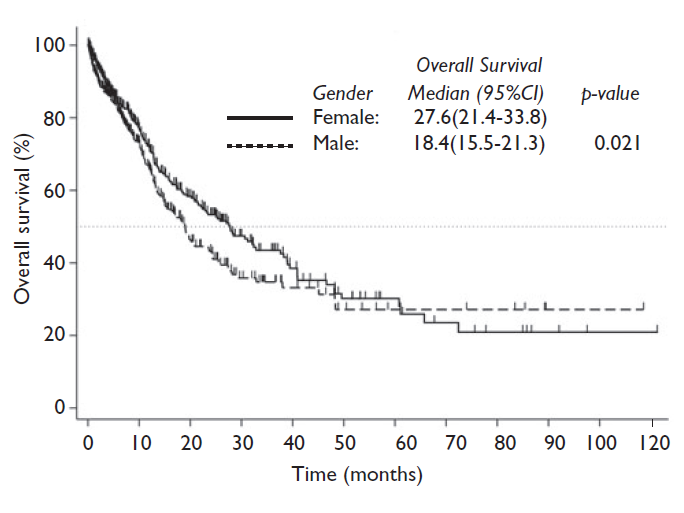
Figura 1 Overall survival in months according to gender, in Mexican population with non-small cell lung cancer. 2008-2017
Table II Univariate and multivariate analysis of the OS of patients treated at the National Cancer Institute (Incan) in Mexico City from 2008-2017 (N=1 104)
| Univariate analysis | Multivariate analysis | |||||||
| Median (95%CI) | p-Value | HR (95%CI) | p-Value | |||||
| Overall | 21.6 (17.8-25.4) | |||||||
| Sex | ||||||||
| Female | 27.6 (21.4-33.8) | |||||||
| Male | 18.4 (15.5-21.3) | 0.021 | 0.9 (0.5 - 1.4) | 0.582 | ||||
| BMI groups | ||||||||
| Normal ( <25 Kg/m2) | 17.4 (13.9-20.9) | |||||||
| Overweight (25 - 29.9) | 28.4 (21.0-35.8) | |||||||
| Obese (30+ Kg/m2) | 22.4 (9.4-35.4) | 0.045 | 0.9 (0.7 - 1.4) | 0.880 | ||||
| Tobacco exposure | ||||||||
| Absent | 28.2 (19.1-37.3) | |||||||
| Present | 18.1 (15.1-21.1) | 0.002 | ||||||
| Wood-smoke exposure | ||||||||
| Absent | 19.2 (15.7-22.8) | |||||||
| Present | 27.4 (21.3-33.5) | 0.221 | ||||||
| Exposure to asbestos | ||||||||
| Absent | 23.5 (19.5-23.4) | |||||||
| Present | 16.8 (13.7-19.9) | 0.165 | 1.0 (0.4 - 2.4) | 0.955 | ||||
| Disease stage | ||||||||
| II-IIIA | 8.5 (6.9-10.2) | |||||||
| IIIB-IV | 21.5 (18.2-24.7) | 0.220 | ||||||
| ECOG performance status | ||||||||
| 0 - 1 | 23.5 (19.2-27.7) | |||||||
| >2 | 14.5 (10.5-18.5) | 0.006 | 0.4 (0.2 - 0.7) | 0.001 | ||||
| Liver metastases at diagnosis | ||||||||
| Absent | 20.8 (17.1 - 24.5) | |||||||
| Present | 13.6 (8.9 - 18.3) | 0.043 | 1.6 (0.8 - 3.3) | 0.188 | ||||
| EGFR mutation status | ||||||||
| WT EGFR | 27.9 (16.5 - 39.2) | |||||||
| EGFR sensitizing mutation | 38.0 (28.9 - 47.1) | 0.215 | ||||||
| KRAS mutation status | ||||||||
| KRAS (-) | 38.9 (30.4 - 47.4) | |||||||
| KRAS (+) | 15.7 (12.3 - 19.2) | <0.001 | 2.1 (1.1 - 3.8) | 0.017 | ||||
CI: confidence interval, HR: hazard ratio, BMI: body mass index, ECOG: eastern cooperative oncology group, EGFR: epidermal growth factor receptor, WT: wild-type, KRAS: Kirsten rat sarcoma virus oncogene
Characteristics of women with NSCLC by hormonal status
Among 522 female patients with NSCLC analyzed, 120 were premenopausal (23%) and 402 postmenopausal (77%). Table III shows the characteristics the female sex population according to hormonal status. The relative frequency of wood-smoke exposure was substantially higher in postmenopausal than premenopausal women (52.5 vs. 41.7%, p=0.037). Likewise, postmenopausal patients showed a higher wood-smoke exposure index (113.2 vs. 50.6; p=0.006) and tobacco smoking index (19.8 vs. 10.2; p=0.017) compared to premenopausal women. By contrast, premenopausal women showed higher frequencies of exposure to asbestos in comparison to postmenopausal patients (16.7 vs. 7.0%; p=0.001). We did not find differences among women according to their hormonal status in terms of BMI, tobacco exposure, histology, disease stage, ECOG performance status, sites of metastases (brain, lung, liver, bone, lymphatic nodes, and adrenal glands) nor by EGFR or KRAS mutation status (table III).
Table III Baseline characteristics of women patients treated at the National Cancer Institute (Incan) in Mexico City from 2008-2017 (N=522)
| Hormonal status | ||||||
| Premenopausal | Postmenopausal | p-Value | ||||
| (n=120) | (n=402) | |||||
| % (n/N) | % (n/N) | |||||
| Age (Years) | ||||||
| Mean (+SD) | 41.8 (7.3) | 65.2 (9.6) | <0.001 | |||
| BMI (Kg/m2) | ||||||
| Mean (+SD) | 25.4 (5.2) | 25.1 (4.8) | 0.556 | |||
| BMI groups | ||||||
| Normal (<25 kg/m2) | 51.7 (62/120) | 66.9 (207/402) | ||||
| Overweight (25-29.9 kg/m2) | 34.5 (41/120) | 42.0 (128/402) | ||||
| Obese (30+ kg/m2) | 13.8 (17/120) | 20.9 (67/402) | 0.717 | |||
| Tobacco exposure | ||||||
| Absent | 70.0 (84/120) | 67.4 (271/402) | ||||
| Present | 30.0 (36/120) | 32.6 (131/402) | 0.594 | |||
| Tobacco index | 10.2 (18.7) | 19.8 (22.2) | 0.017 | |||
| Wood-smoke exposure | ||||||
| Absent | 58.3 (70/120) | 47.5 (191/402) | ||||
| Present | 41.7 (50/120) | 52.5 (211/402) | 0.037 | |||
| Wood-smoke expossure index | 50.6 (70.3) | 113.2 (154.7) | 0.006 | |||
| Exposure to asbestos | ||||||
| Absent | 83.3 (100/120) | 93.0 (374/402) | ||||
| Present | 16.7 (20/120) | 7.0 (28/402) | 0.001 | |||
| Tumor histologic type | ||||||
| Adenocarcinoma | 90.0 (108/120) | 88.6 (356/402) | ||||
| Squamous | 10.0 (12/120) | 11.4 (46/402) | 0.659 | |||
| Disease stage | ||||||
| II - IIIA | 0.8 (1/120) | 1.3 (5/402) | ||||
| IIIB - IV | 99.2 (119/120) | 98.7 (397/402) | 1.000 | |||
| ECOG performance status | ||||||
| 0-1 | 78.3 (94/120) | 75.6 (304/402) | ||||
| >2 | 21.7 (26/120) | 24.4 (98/402) | 0.534 | |||
| Brain metastases at diagnosis* | ||||||
| Absent | 53.5 (61/114) | 55.1 (201/365) | ||||
| Present | 46.5 (53/114) | 44.9 (164/365) | 0.770 | |||
| Lung &pleural metastases at diagnosis* | ||||||
| Absent | 55.3 (63/114) | 61.9 (226/365) | ||||
| Present | 44.7 (51/114) | 38.1 (139/365) | 0.205 | |||
| Liver metastases at diagnosis* | ||||||
| Absent | 92.1 (105/114) | 92.3 (337/365) | ||||
| Present | 7.9 (9/114) | 7.7 (28/365) | 0.938 | |||
| Bone metastases at diagnosis* | ||||||
| Absent | 74.6 (85/114) | 71.8 (262/365) | ||||
| Present | 25.4 (29/114) | 28.2 (103/365) | 0.562 | |||
| Lymphatic nodes metastases at diagnosis* | ||||||
| Absent | 74.6 (85/114) | 80.5 (294/365) | ||||
| Present | 25.4 (29/114) | 19.5 (71/365) | 0.170 | |||
| Adrenal glands metastases at diagnosis* | ||||||
| Absent | 95.6 (109/114) | 96.2 (351/114) | ||||
| Present | 4.4 (5/114) | 3.8 (14/365) | 0.793 | |||
| EGFR mutation status‡ | ||||||
| WT EGFR | 65.3 (32/49) | 60.0 (102/170) | ||||
| EGFR sensitizing mutation | 34.7 (17/49) | 40.0 (68/170) | 0.502 | |||
| KRAS mutation status‡ | ||||||
| KRAS (-) | 86.2 (25/29) | 90.7 (107/118) | ||||
| KRAS (+) | 13.8 (4/29) | 9.3 (11/118) | 0.476 | |||
SD: standard deviation, ECOG: eastern oncology group, EGFR: epidermal growth factor receptor, KRAS: Kirsten rat sarcoma virus oncogene
*Estimation over 479 patients with stage IV disease and known metastases at diagnosis
‡Estimations over the number of patients tested for each molecular status
Factors associated with overall survival among women NSCLC patients
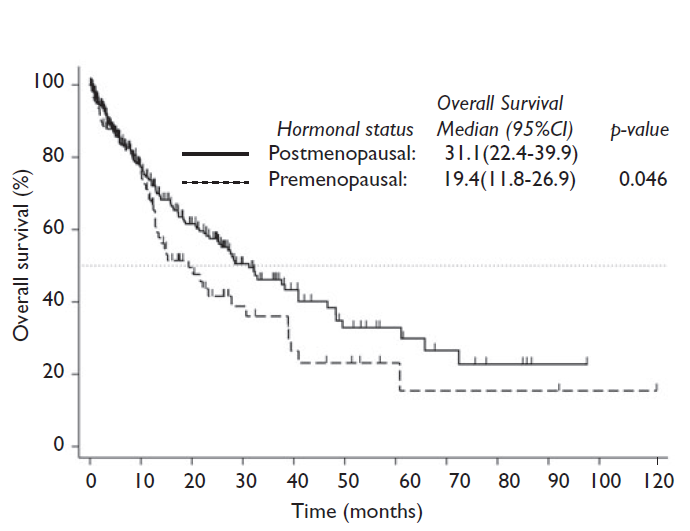
Median OS for women was 27.6 months; in the univariate analysis the factors associated with a better OS were hormonal status (postmenopausal vs. premenopausal) (31.1 vs. 19.4 months; p=0.046) (figure 2), tobacco exposure (never vs. ever) (32.1 vs. 18.5 months; p=0.037), ECOG performance status (<2 vs. 2+) (27.9 vs. 18.5 months; p=0.022), adrenal glands metastases (no vs. yes) (27.6 vs. 10.7; p=0.023) and KRAS mutation status (absent vs. present) (37.6 vs. 15.8 months; p=0.007). In the multivariate analysis, the independently associated factors with OS were tobacco exposure (HR: 1.4, 95%CI (1.0-1.9); p=0.031), ECOG performance status (HR: 01.7, 95%CI (1.2-2.4); p=0.005) and KRAS mutation status (HR: 2.1, 95%CI (1.1-4.0); p=0.022) (supplementary table I).26
NSCLC among EGFR (+) women by hormonal status
Among 522 female patients, only 219 patients were tested for EGFR mutation status, among them only 85 (38.8%) patients harbored an EGFR mutation (17 [20%] were premenopausal and 68 [80%] were postmenopausal women). We did not find any differences between premenopausal and postmenopausal EGFR-mutated women in terms of BMI, tobacco, wood-smoke and, asbestos exposure, histology, disease stage, ECOG performance status, sites of metastases (brain, lung, liver, bone, lymphatic nodes, and adrenal glands) nor by KRAS mutation status (supplementary table II).26
Factors associated with the overall survival among EGFR- mutated women
Median OS for women harboring EGFR mutations was 32.4 months; in the univariate analysis the factors associated with a better OS included a good ECOG performance status (<2 vs. 2+) (38.9 vs. 12.9 months; p=0.012) and absence of adrenal glands metastases (no vs. yes) (32.4 vs. 8.3; p=0.009). We did not find differences in OS when evaluating by hormonal status, age, BMI, tobacco exposure, wood-smoke exposure, exposure to asbestos, disease stage, sites of metastases (brain, lung, liver, bone, lymphatic nodes, and adrenal glands) nor by KRAS mutation status. None of the previously mentioned characteristics were independently associated with OS in the multivariate analysis (supplementary table III).26
NSCLC among wt-EGFR women by hormonal status
Among 522 female patients, only 219 were tested for EGFR mutation status; among them, 134 had wt-EGFR (32 [23.9%] premenopausal and 102 [76.1%] postmenopausal). Premenopausal wt-EGFR women were more likely to have a history of exposure to asbestos (25% vs. 3%; p<0.001). We did not find differences among premenopausal and postmenopausal wt-EGFR women by the other analyzed variables (supplementary table IV).26
Factors associated with the overall survival among wt-EGFR women with NSCLC
Median OS for women without an EGFR mutation was 32.1 months. In the univariate analysis the factors associated with OS were liver metastases (no vs. yes) (27.9 vs. 18.7 months; p=0.044) and KRAS mutation status (absent vs. present) (37.6 vs. 15.8 months; p=0.002). We did not find any significant differences in OS for the other analyzed factors. None of the characteristics were independently associated with OS in the multivariate analysis (supplementary table V).26
Discussion
Lung cancer presentation, behavior and response to treatment depend on several factors including grading and staging of the disease, molecular and histological tumor features, and recently it have been proposed that sex and hormonal status are also associated with tumor behavior and survival of NSCLC patients, however, this information is still controversial. We reported differences in lung cancer presentation and OS according to the sex and hormonal status in a Mexican population of NSCLC.
We observed that women presented higher frequencies of wood-smoke exposure, EGFR sensitizing mutations, better ECOG performance, had a higher frequency of obesity and, as a result, higher BMI compared to men who instead exhibited a higher smoking index compared to women. OS was also higher in women compared to men, as well as in patients who were overweight, never smokers, had a good ECOG performance status, a wt-KRAS molecular status and were free of liver metastases.
In Mexico, as in other Latin-American countries, smoking habit does not appear to be the main risk factor for the development of lung cancer in women, since a higher percentage of women with NSCLC are never smokers. Previously it had been reported that wood-smoke exposure was an important risk factor to develop lung cancer in non-smoking Mexican women,27,28 we observed that this factor remains relevant in the etiology of lung cancer in women to this day. In addition EGFR-mutations have also been associated with patients who are never smokers and women,29 which was also confirmed by our results. Nowadays patients with lung adenocarcinomas that exhibit EGFR sensitizing mutation are treated with targeted therapy based on Tyrosine Kinase Inhibitors (TKIs) which produce a high response rate as a first-line treatment.30 Moreover, obesity and high BMI were recently correlated with a reduced risk of death from lung cancer31 consistent with the data reported in the present study. The characteristics exhibited by women with lung cancer in our population, such as lower smoking index, higher frequency of EGFR sensitizing mutations, better ECOG performance status and higher frequency of obesity as well as high BMI, could explain the better OS we observe in women compared to men.
Analysis by hormonal status showed that postmenopausal women exhibited a higher wood smoke exposure and wood smoke exposure index as well as tobacco-smoking index compared to premenopausal women, who exhibited higher asbestos exposure. No differences were observed in tobacco exposure, BMI, histology, disease stage, ECOG performance status, sites of metastases and mutation in KRAS and EGFR by hormonal status. Nonetheless an interesting finding was the fact that postmenopausal women presented a better OS compared to their premenopausal counterparts. Older postmenopausal women survived a median of 31.1 months while younger premenopausal women survived only 19.4 months. The lower OS observed in premenopausal women could be explained by the differences in estrogen levels between premenopausal and postmenopausal women and the influence of this hormone in lung carcinogenesis.
Previous studies which have considered hormonal status among women with lung cancer have reported that premenopausal women presented with more advanced stage-disease at the time of diagnosis, less differentiated tumors, distant metastases and had a worse prognosis21,22 compared to postmenopausal women and men. Although our results did not show significant differences in disease stage, ECOG performance status and metastatic site by hormonal status, premenopausal women exhibited a statistically significant lower OS compared to postmenopausal. In Mexico as in other countries, lung cancer continues to be diagnosed at advanced stages both in women and men; it is likely that this delay in diagnosis and therefore treatment, as well as other problems concerning hospital admission, as well as the low percentage of premenopausal women analyzed in this study could conceal the probable differences in lung cancer metastases and stage at diagnosis by hormonal status. However, our study supports that there are differences in lung cancer behavior, presentation and prognosis not only by sex but also by hormonal status, which can be explained by the response of lung cancer to steroid sexual hormones.14,32,33 Recently lung cancer is being considered as a hormone-dependent cancer, since NSCLC tissues and cell lines exhibited strong estrogen (ER) and aromatase enzyme expression;14,32the estrogen pathway has been related to carcinogenic pathway activation and lung cancer progression.34,35 Probably the lower circulating estradiol levels present in postmenopausal women compared to premenopausal, explain the differences in terms of OS, however it is important to conduct a prospective study where hormonal biochemical characteristics (e.g. circulating estrogen level, exogenous estrogen intake, expression of estrogen receptor and aromatase in tumor) can be evaluated.
On the other hand, hormonal status does not appear to influence EGFR mutational profile, since no differences were observed between premenopausal and postmenopausal women or by BMI, tobacco exposure, wood-smoke exposure, exposure to asbestos, histology, disease stage, ECOG performance status, sites of metastases and KRAS mutation status. Also no difference was observed in terms of the OS among EGFR-mutated women. Recently a functional relationship and a crosstalk between the estrogen and EGFR pathways in lung adenocarcinoma have been observed.36,37 NSCLC cells stimulated with estradiol resulted in EGFR pathway activation and EGFR activation also increased the expression and activity of the aromatase enzyme in NSCLC cells.14,38 Although premenopausal women usually exhibit higher circulating estrogen level compared to postmenopausal women and men, it is probably that the level of estrogen in tumor microenvironment as well as the ER tumor expression are important factors to consider in terms of stimulating EGFR expression as previously reported.38,39Accordingly, it is important to conduct a prospective study to evaluate the hormonal characteristics of each patient as well as hormone tumor status (aromatase and ER tumor expression, levels of estrogen in tumor microenvironment, etc.), in relation to EGRF expression, activity and mutation profile.
Finally, this study supports the differences by sex and also by hormonal status in lung cancer presentation and sustains the relevance that sexual hormones have in the course and prognosis of lung cancer, since women exhibited higher OS compared to men and premenopausal women showed a significantly lower OS compared to postmenopausal women. Due to the differences that lung cancer exhibited by sex and hormonal status it is important to consider not only women and men as it has previously been done, but also premenopausal and postmenopausal women in futures studies. Moreover the identification of hormonal markers in tumors from patients with lung adenocarcinoma would be relevant in order to design new treatment schemes based on anti-hormone therapy as has been recently proposed.40,41,42











 nueva página del texto (beta)
nueva página del texto (beta)