Introduction
Men who have sex with men (MSM),1 people with HIV,2,3 homeless people (many of whom participate in survival sex), female sex workers,4 transgender women5 and rape victims6 are at high risk for human papillomavirus (HPV) infection and consequently developing cancers associated with chronic HPV infection including cervical, vaginal,7 vulvar,8 and anal cancers in women, cancer of the penis,9 anal cancer10,11 in men, and cancer of the oropharynx,12 tongue, and tonsils in both men and women.13,14,15 In part, this is related to increased life expectancy among people with HIV (given greater anti-retroviral treatment coverage).16
Some countries have implemented HPV vaccination policies that include men. This is based on promotion of protection against an increased risk of HPV infection, universal increase in coverage and a gender equity perspective.17 Since population-level introduction of HPV vaccines, there has been a dramatic impact in areas with over 60% vaccination coverage. Decreases of approximately 90% have been reported for HPV-6/11/16/18 infections, over 90% for new cases of genital warts, 45% for low-grade cytological abnormalities, and 85% for anogenital high-grade histological lesions.18
The HPV FASTER concept combines HPV vaccination with HPV screening. It is based on the HPV vaccine’s19 high efficacy and the high sensitivity of high-risk HPV testing for primary detection of anogenital cancer precursor lesions20 (as documented by our research group in more than 200 000 Mexican women).21,22,23,24 This strategy is effective because HPV vaccination among women and men over a wide age range offers protection to those not currently infected and protects against subsequent re-infections.16 Thus, a combined HPV vaccination and screening strategy will potentially: 1) mitigate the demand for timely screening tests by expanding screening intervals; 2) improve the cost-benefit balance of secondary prevention programs, and 3) provide greater protection and quality of life to more people by reducing the burden of diseases attributable to HPV. This intervention can save many lives over the next 30 years and be more cost-effective than traditional approaches.
Recently, the US vaccination advisory group recommended HPV vaccination among sexually abused children, recognizing their increased risk of HPV infection.25,26 The US Centers for Disease Control recommends administering the HPV vaccine to subjects with immunosuppressive conditions, HIV infection, and men who have sex with men.27 HPV vaccination in HIV-positive people is safe and reduces the incidence of HPV-associated cancers, including anal cancer, which is increasing in these populations.28,29 Moreover, some research has shown that vaccination against HPV in populations with HIV is cost-effective.30,31
HPV serotypes 16 and 18, found in the HPV bivalent and tetravalent vaccines, have an estimated relative contribution to invasive cervical cancer of at least 70% in Latin America.32 These vaccines are highly effective,33 provide high levels of immunogenicity,34 have acceptable security profiles35 and have been incorporated into public vaccination policies with 3-dose schedules over a 6-month period36 based on pharmaceutical industry recommendations.
Alternative schedules of population vaccinations have been implemented in spite of the conflict of interest with the pharmaceutical industry. The first of these schedules was conducted in Quebec, Canada where the extended 0-6-60 month schedule was promoted for girls under 16 years.37 Later, in Mexico, a similar vaccination program recommended by a group of experts coordinated by the National Institute of Public Health was initiated.38,39 Mexico was a pioneer in adopting a 2-dose alternative vaccination schedule in April 2014. Currently, there is evidence that alternative schedules are not inferior to the traditional schedule in terms of immunogenicity.40 In 2012,41 Switzerland adopted a 2-dose vaccination schedule against HPV in girls under 15 years old under assuming age was the main mediator of the protection induced by the vaccine and not the number of doses. This assumption would explain why the vaccine was the main inducer of IgG responses, plasma cells, and memory B cell formation and why 2 doses administered with a 6-month interval provided similar protection during the initial years of vaccination.
In April 2014, the Council officially pronounced the adoption of a vaccination schedule against HPV with only 2 doses with a periodicity of 0 and 6 months. The justification for this resolution, in which Mexico was a pioneer and was subsequently officially recommended by the WHO as a vaccination policy at a global level, was that the immunological responses of girls aged 9 to 11 years after two doses of HPV vaccine were similar to or greater than those obtained after three doses in women aged 16 to 26 years.
The benefits of this decision are logistical and economic; increasing coverage and providing greater flexibility in the application of the second dose. Moreover, the savings generated from not administering a third dose are considerable. Alternative schedules with two doses and schedules with a third dose at 60 months in girls 9-11 years of age can offer a) improvements in the immune response in the medium- and long-term, because there is evidence that a new exposure to HPV increases the expression of antibodies logarithmically, and b) advantages in its administration, since it is easier to organize these schedules within the framework of schools and consequently provides a better opportunity for equity in obtaining greater coverage and adherence to complete schedules in the captive population before leaving compulsory schooling. However, although the recommendation is plausible from an immunological perspective, there is no evidence from clinical trials of efficacy to support this determination for ethical reasons.
Currently, a paradigm shift is perceived toward the application of a single dose of vaccine against HPV. Research groups at a global level are evaluating the hypothesis that men and women who receive one versus two doses do not have a higher prevalence of HPV and that consequently the effect of the intervention is equivalent.
In this regard, demonstration studies are being conducted in population universes to evaluate the effect of a single dose of HPV vaccine. The strategy is to evaluate the effect of administering 1 versus 2 doses in a specific community using a randomized intervention allocation strategy. In the 2-dose intervention group, the most biologically and logistically efficient approach is to administer the second intervention between 6 and 12 months after the first intervention. The outcome to be evaluated in men who have sex with men is the presence of HPV DNA in the anal canal, which is obtained and determined in the basal measurement during the administration process of the first dose. This type of intervention is feasible in Mexico due to its invaluable tradition of innovation in public policies for the prevention and control of anogenital cancer and its implementation of large demonstration studies. Currently, we can affirm that with the current evidence, vaccination of all groups at risk of anogenital cancer with a single dose will be the preamble to the attenuation of the impact of persistent HPV infections and their consequent lesions.42
Materials and methods
Hypotheses
The application of a combined HPV vaccination scheme and screening through the identification of high-risk HPV subtypes will reduce the prevalence of HPV infection in the oral and anogenital cavities in the different study groups, at 12 months post-vaccination. Consequently, the occurrence of grade 2 or higher anal intraepithelial neoplasia (AIN 2+) lesions in men and high-grade or higher cervical intraepithelial neoplasia (CIN 2+) lesions in women will decrease.
Alternative vaccination schedules of one versus two doses of prophylactic vaccines against HPV are equivalent in terms of effectiveness.
Urine and self-collected vaginal, anal canal, and oral cavity samples constitute an alternative epidemiological surveillance approach for monitoring the impact of a combined strategy HPV vaccination and screening for high-risk HPV subtypes in vulnerable populations.
Objectives
Primary objective
To evaluate the effectiveness of a combined strategy of HPV vaccination and screening for high-risk HPV subtypes to reduce the occurrence of HPV in the anogenital region and oral cavity in five highly vulnerable groups in Mexico City, Mexico: men who have sex with men, female sex workers, transgender women, subjects living in street situations, and people who have suffered rape.
Secondary objectives
To evaluate the effectiveness of alternative HPV vaccination schedules.
To evaluate the usefulness of the collection of self-collected vaginal, anal, and urine samples as alternatives for monitoring the impact of a combined strategy of HPV vaccination and screening based on the detection of high-risk HPV subtypes.
To assess the barriers to and facilitators of the introduction of a combined HPV vaccination and primary screening strategy with tests for high-risk HPV subtypes in the study groups.
Study population and procedures
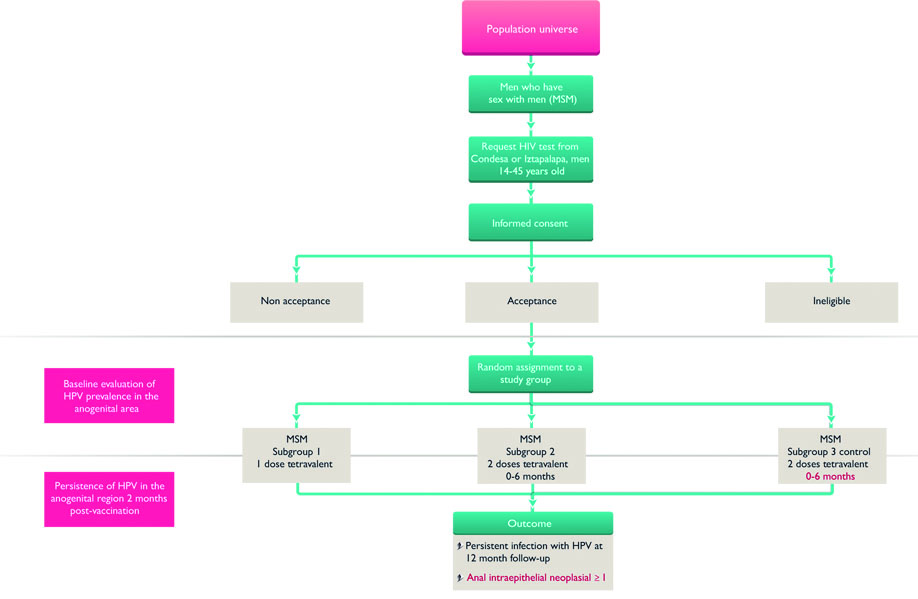
The sample sizes for the five cohorts are: 6 000 MSM, 500 transgender women, 400 homeless men and women, 400 sexual assault victims, 2 500 female sex workers (figure 1). This study is a mixed methods study combining public health interventions offering primary and secondary prevention of HPV to populations highly vulnerable to these health problems with evaluation of the interventions in a 12-month follow-up (figures 1,2,3). In addition the study includes qualitative interviews that will undergo systematic analysis on the perceptions, meanings, and individual experiences of the intervention, within the study groups.

* Anal Intraepithelial Neoplasia grade 2 or higher
‡ Cervical Intraepithelial Neoplasia grade 2 or higher
§ Syphilis, HIV, hepatitis B, hepatitis C
# Chlamydia trachomatis, Neisseria gonorrhoeae
Figure 1 HPV screening and vaccination in four vulnerable populations in Mexico City
* Sífilis, VIH, hepatitis B, hepatitis C
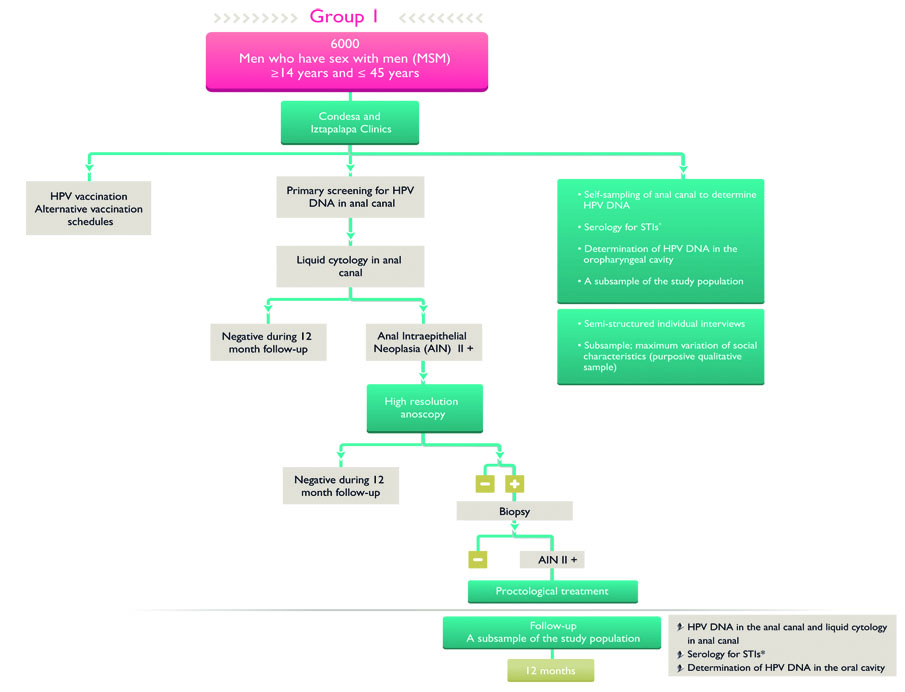
Figure 2 Vaccination subgroups and control group in men who have sex with men
A central element of the present project is a parallel study of five sentinel cohorts of the adult population ≥14 years and ≤45 years of age that is operationally coordinated by the Condesa and Iztapalapa Specialized Clinics, which belong to the health services of Mexico City and the Program of Care for rape victims. This program is coordinated by the Specialized Clinics in collaboration with the Mexico City Attorney General of Justice, especially the Victims Care Center, which is a program of the Center for Therapy in Support of Victims of Sex Crimes. We will attempt to include at least 80% of the population of each interest group in the epidemiological component of the project. Study subjects will be recruited (2018-2019) during their routine care within any program or service they participate in within the Condesa and Iztapalapa Specialized Clinics; in addition, posters will be placed within the clinics inviting potential study subjects to participate and civil society organizations which collaborate with the clinics will be visited regularly to invite persons involved in their activities to participate in the study.
For the qualitative intervention component, individuals from the five vulnerable groups will be selected for voluntary participation in qualitative semi-structured interviews. This sub-sample will be purposely selected to include the maximum variation of characteristics, and 12 to 16 semi-structured individual interviews will be conducted for each study group. These interviews will explore perceptions of HPV, experiences as users of preventive care for HPV and related cancers, acceptability of and meanings related to HPV vaccination and barriers to and facilitators of primary prevention (vaccination) and secondary prevention (screening and treatment) of HPV.
The study protocol and instruments were approved by the Research, Ethics and Biosecurity Committees of the National Institute of Public Health. All study participants will provide signed informed consent before participating in any study procedure or completing any study instruments (and consent forms will be signed by two witnesses).
Sample collection and vaccination
At the beginning of the study, all MSM who agree to participate in the study will receive the HPV vaccination in an alternative schedule for MSM of 1 versus 2 doses over a 6-month period. In addition, a control group will be screened for high-risk HPV and vaccinated at 12 months. For transgender women, women and men living on the street, and women and men who have suffered rape, a 2-dose regimen of the tetravalent HPV vaccine will be administered over a 6-month period. The additional combined strategy corresponds to screening for high-risk HPV subtypes to identify intraepithelial lesions in the transformation zone of the anal and/or cervical canals (figures 2 and 3).
Additionally, samples will be self-collected from the anal canal, oral cavity, and vaginal canal. These samples will be collected both at the baseline measurement and at 12 months after vaccination to assess changes in the prevalence and incidence of new HPV infections in the different study groups. All HPV-positive anal and cervical samples will be evaluated using liquid-based cytology; analysis will be done at the National Institute of Public Health.
On a single occasion, additional samples will be collected from a proportional sample of the different study groups (70% of MSM and 90% of women, not including transgender women) for the identification of other STIs, including Neisseria gonorrhea, syphilis, HIV, herpes simplex virus type 2, hepatitis B virus, and hepatitis C virus. Positive cases will be referred to the corresponding medical care within their respective affiliated clinics.
The HPV detection test has been installed at the National Institute of Public Health with the Cobas 4800 HPV platform. This test is a qualitative test that detects 14 high-risk genotypes using specific probes. It detects genotypes 16 and 18 individually and a pool of other types (31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) cataloged as high risk.
Cytological evaluation will be conducted for all HPV-positive samples from both the anal canal and cervix. A urine sample will be obtained, centrifuged, and prepared for DNA extraction. The sample will be stored at −20°C prior to processing for HPV detection. The techniques for these purposes are under evaluation. Blood samples (10-ml) will be collected from the study participants by vacuum venipuncture. The collected samples will be subjected to centrifugation to separate the sera into 2-ml aliquots, which will be stored in cryo-vials at −20°C prior to processing. Antibody responses to viral and bacterial etiological agents will be conducted as follows:
Hepatitis B and hepatitis C. Automated enzyme-linked immunosorbent assay. The analytical procedure will be conducted according to the corresponding manufacturer’s laboratory instructions.43,44
Syphilis. The Venereal Disease Research Laboratory (VDRL) screening test and the Serodia Treponema pallidum (TP) confirmatory test by particle agglutination. The analytical procedures will be performed in accordance with the laboratory instructions of the corresponding manufacturers.45
Herpes simplex virus type 2. Microplate immuno-enzymatic assay for the detection of type-specific viral antibodies. Confirmation of positive samples by Western blotting assay. The analytical procedures will be performed in accordance with the laboratory instructions of the corresponding manufacturers.46
In cervical samples, an automated chemo-luminescent assay for bacterial DNA detection will be performed using polymerase chain reaction (PCR). The analytical procedure will be conducted according to the corresponding manufacturer’s laboratory instructions.47
Data analysis
To estimate the effectiveness of the strategy of combining HPV vaccination with HPV screening, the differences in the prevalence of HPV and the cumulative incidence of HPV infection and/or development of CIN 2+ and/or AIN 2+ will be estimated during the first 12 months of follow-up using the Kaplan-Meier method. The time-to-event method will be applied to evaluate the time from the specific type of HPV genital positivity to the incidence of anogenital lesions harboring the same HPV subtype within the lesion. The analytical unit for this study is HPV 16/18 type-specific infection and generic high-risk strain infection.
The transcripts of individual interviews will be analyzed with through coding transcribed material, which implies the classification of pieces of the interviews into a priori and emerging categories to systematize the information and search for patterns, similarities, and differences.
Results
Women and men vaccinated against HPV and screened for HPV as a primary test in the future will have a lower prevalence and incidence of HPV infection and consequently a lower frequency of anogenital and oropharyngeal lesions caused by HPV. This decrease will greatly reduce the burden of HPV-associated neoplasms. Secondary prevention of the STIs included in this project will be achieved among individuals belonging to vulnerable groups who are the target population of this intervention. The detected STIs will be treated, and thus the negative impact on the health of these people will be reduced.
Discussion
In all of the vulnerable groups included in this proposal, the burden of diseases associated with HPV (genital warts and anogenital and oropharyngeal cancers) as well as STIs is a public health problem that has a strong negative impact on their health and quality of life. The proposal will benefit individuals of these vulnerable groups in terms of their physical and emotional health in relation to their social and sexual relationships and in general in terms of their quality of life. It will also imply a benefit in economic terms for the health services of Mexico City, since the proposed intervention has been shown to be cost-effective, especially in vulnerable populations, such as those included in this proposal.
This study has limitations: whether HPV infections occurring in adults (after onset of sexual activity) are an important cause of anogenital (or oral) cancers at a population level in vulnerable groups such as those studied here has not been fully proven, in part due to insufficient studies in some of these populations.48 However, as anogenital cancer rates are high in adults,49 including older adults,50 and high-risk (oncogenic) HPV types are also high in both younger and older adults,51 it seems likely that some of this disease burden is related to infections occurring during adulthood. Another possible limitation of the study is that, in order to effect changes in screening algorithms and populations in vaccination campaigns, policy- and decision-makers, healthcare administrators and providers will need to be convinced of the advantages, cost-effectiveness and sustainability of the combined screening and vaccination strategy for vulnerable populations which this study proposes.52,53,54
This study results from the current discussion of the need to evaluate the relevance of the introduction of vaccines that protect against HPV not only in the target population of national vaccination programs (i.e., girls and pre-adolescents) but also in vulnerable groups of men and women who are at high risk of exposure to HPV types 6 and 11, which are responsible for most genital warts, and the high-risk (oncogenic) types of HPV, including boys and girls who have suffered rape.55 With an intervention such as the one presented here, which includes the proposed actions, an organized social response will be provided to control one of the most recurrent sexually transmitted diseases, which produces anal, cervical, and oropharyngeal intraepithelial neoplasia as well as genital warts and has other impacts on the sexual, reproductive, and overall health of people.
This combined vaccination-screening strategy must be implemented in groups with very high social vulnerability. This approach constitutes the optimal organized social response to prevent and treat lesions derived from persistent HPV infections in a timely manner and the negative impact.











 nueva página del texto (beta)
nueva página del texto (beta)