Introduction
Cervical cancer can be eradicated because highly cost-effective prevention strategies exist, particularly vaccination against human papillomavirus (HPV).1 To expand the global strategy for primary prevention of cervical cancer, it will be necessary to expand the coverage of HPV vaccination, and alternative vaccination schedules should be evaluated in different contexts of low and high cervical cancer incidence rates. Given the possibility of incorporating HPV vaccine booster doses for unprecedented scenarios in populations that live in endemic areas2,3 and/or that have a high prevalence of immunosuppression due to HIV, it is necessary to consider the relevance, feasibility, and possible long-term effect of alternative schedules. There are recent examples of infectious diseases for which the incorporation of a booster dose at different time periods after the last application has been evaluated,4,5 which consists of the application of an additional dose to increase and prolong the immunogenicity of individuals.
While the efficacy and safety of HPV vaccines has been established, increasing vaccine coverage remains challenging. By 2014, only 1.4% of the population in low- and middle-income countries had a complete vaccine schedule, and 1.7% had at least one dose.6 Barriers to increase the population’s coverage by HPV vaccines include vaccine prices, difficulties in implementing multiple dose schedules, and the need to include larger age groups to allow a longer implementation time.7 Recently, calls for the evaluation of alternative and more flexible vaccination scenarios have been made, as new data suggests that shorter schedules could be non-inferior to the three-dose schedule.8,9 As in other low- and middle-income countries, Mexico requires flexible vaccine schedules to increase coverage. Alternative schedules have been studied in girls and women.* A recent study evaluated the non-inferiority of a 0-6 schedule using bHPV in girls followed by a bHPV or quadrivalent HPV (qHPV) vaccine booster at month 60, finding it non-inferior to the standard 0-2-6 schedule.10
We aimed to analyze the non-inferiority of an alternative qHPV vaccine schedule compared to the standard schedule. Through a non-randomized trial conducted in 9- to 10-year-old girls, we performed a non-inferiority analysis one month after the last dose of two schedules: the standard (0-2-6 months) and an alternative schedule (0-6-50 months). This comparison is expected to capture the peak immunogenicity elicited by either schedule. We hypothesize that, one month after the last dose, the alternative schedule will produce a non-inferior immunogenic response relative to the traditional schedule.
Materials and methods
This report is a sub-analysis of an open-label, non-randomized clinical trial (NCT01717118)27 to evaluate the immunogenicity induced by the qHPV6/11/16/18 vaccine administered using an alternative (0-6 months) and traditional (0-2-6 months) vaccination schedules.8 We made an extension of the original clinical trial, adding a booster dose at month 50 in the alternative schedule group.
Group 1. One hundred forty-five girls aged 9 to 10 years were vaccinated with an extended alternative vaccination schedule with the qHPV vaccine for the months 0-6-50.
Group 2. One hundred fifty girls aged 9 to 10 years were vaccinated with a traditional vaccination schedule with the qHPV vaccine for the months 0-2-6.
The trial was conducted following the International Conference on Harmonization Good Clinical Practice Guidelines. This study was approved by the ethical, research and biohazard committees established in the National Institute of Public Health of Mexico (INSP), received the corresponding annual authorization (registration number 883) and was registered as CAS/OR/01/CMN/113300410A0246-2779/2011 in the Federal Commission for Protection against Health Risks of Mexico (Comisión Federal para la protección contra Riesgos Sanitarios de México - Cofepris). For the following phases of the study, the amendments for the years 2010, 2011, 2012, 2013, 2014, 2015 and 2016 were approved.
Study population
This study was conducted in the metropolitan area of Cuernavaca in Mexico. The study population consisted of 9- to 10-year-old female students, recruited at 18 public primary schools selected from a group of 97 that offered a morning shift. At the time of the study, a parallel HPV vaccine study was being performed; the 18 schools were selected based on fieldwork feasibility and because the parallel study had not recruited them. The girls were divided into school clusters: the first cluster was the alternative vaccination schedule (seven schools), and the second (11 schools) was the control cluster. Fifty months later, the girls who were currently between 14 and 15 years old and in years 2 and 3 of secondary school received the third dose to evaluate the booster effect. From these groups, we recruited 150 participants to reach the predefined total sample size per arm (figure 1).
Vaccine and antibody titer determination
We used the qHPV (types 6, 11, 16, and 18) vaccine Gardasil (Merck, Whitehouse Station, NJ, USA), provided by the Mexican Ministry of Health. Peripheral blood samples were obtained at months 7, 21 and 51 after the application of the first dose from the participants in both groups to assess the antibody response. Merck cLIA testing was performed at Merck Research Laboratories as previously described,11 and antibody levels were expressed as milli-Merck units (mMU) per ml. The PsV NAb assay was performed as previously described.10 Briefly, HPV16 and HPV18 PsV containing a reporter plasmid encoding red fluorescent protein (RFP) were prepared and titrated in 293TT cells. The sera were serially diluted, mixed with 100 infectious units of the respective HPV PsV, and inoculated onto 293TT cells in microtiter plates. The cultures were read by fluorescence microscopy after four to six days. The endpoint was the highest dilution of serum that completely blocked the expression of RFP (100% neutralization). Then, 7-, 21- and 51-month samples were tested in duplicate in the same assay run, and GMTs were calculated. The subjects were considered to have positive neutralizing antibodies for the respective HPV type if the GMT was 20 and 24 mMU/ml for HPV 16 and 18, respectively, according to previous validation studies.12 The testing laboratories were blinded to the dosing regimens.
Safety
The study staff recorded safety profile assessments of local symptoms (pain and redness at the injection site) and general symptoms (fever, headache, fatigue, nausea, vomiting, diarrhea and/or abdominal pain, arthralgia, myalgia, and rash) at the next scheduled appointment after the administration of each vaccine. The staff also inquired regarding serious adverse events at each contact and among women withdrawing from the study.
Statistical analysis
The immunogenicity analysis was conducted on study participants who complied with the protocol procedures and had available data for all antibodies. Immunogenicity data (GMTs) with a 95% confidence interval (CI) were computed for HPV16/18 antibodies at months 7, 21, and 51 for both groups. Seronegative subjects were not included in the GMT computation.
The primary outcome was non-inferiority (95%CI, lower bound > 0.5) of the GMT ratios for HPV16/18 one month after the third dose, that is, at month 51 in the alternative schedule compared to month 7 in the standard schedule. Our non-inferiority definition was based on criteria previously used in immunogenicity clinical trials of the qHPV vaccine13 lincluded results from Mexican women.14 All statistical analyses were performed with STATA Version 14.0.‡
Results
One hundred fifty participants were recruited in each group. One hundred percent of the girls received the first dose in the full standard schedule group (0-2-6) and 98.6% the first dose in the alternative schedule group (0-6-50). By month 7 and 21, the loss of follow-up was 5.3% (n=8) for the alternative schedule and 2.6% (n=4) for the standard in girls. At month 51, 58 girls of the alternative group were followed-up and received the qHPV booster dose, representing 39.1% of girls who received the first dose in the alternative schedule group; in contrast, 85.3% of girls in the standard schedule were followed up by month 51.
Table I presents the GMTs and seropositive rate for HPV 16/18 of each group at months 7, 21, and 51. At month 7, a higher immune response was observed in the standard schedule group; at that point, the alternative group was non-inferior for HPV16, and marginally no-inferior for HPV18 when compared with the standard schedule (table II). One month after the third dose (month 51), the alternative group showed higher antibody GMTs compared to one month after the second dose (month 7) in girls in the standard schedule. The seropositive rate by month 7 was 100% in both groups for the HPV16 and 18 types. By month 21, the rate was above 96% except for HPV18, which decreased to 67.3% for the alternative schedule, and 87.5% for the standard group. By month 51, both HPV types’ seropositive rates increased above 99%.
Table I Seropositivity and antibody GMT for HPV16/18 in month 7, 21 and 51 in girls with traditional and alterantive schedule. Cuernavaca, Morelos, 2018
|
Girls alternative schedule (M 0,6,60) n=55 |
Girls standard schedule (M 0,2,6) n=128 |
|||||||||||||
|
HPV |
Time (months post-dose 1) |
Seropositivity % (95%CI)* |
GMT (95%CI) mMU/ml‡ |
Fold change from M7 (95%CI) |
Seropositivity % (95%CI)* |
GMT (95% CI) mMU/ml* |
Fold change from M7 |
|||||||
|
16 |
Month 7 |
100 |
6 469.54(4 341.51-9 640.65) |
- |
100 |
6 690.92 (5 271.13-8 493.15) |
- |
|||||||
|
Month 21 |
98.2 (90.3-99.9) |
464.98(344.67-627.28) |
0.07 (0.04-0.12) |
100 |
345.31 (277.29-429.87) |
0.05 (0.04-0.07) |
||||||||
|
Month 51 |
100 |
10 357.72 (8 678.03-12 362.54) |
1.60 (1.04-2.46) |
99.2 (95.7-99.9) |
712.33 (583.12-870.19) |
0.11 (0.08-0.15) |
||||||||
|
18 |
Month 7 |
100 |
672.39 (513.85-879.86) |
- |
100 |
1 077.97 (867.49-1 339.52) |
- |
|||||||
|
Month 21 |
67.3 (53.3-79.3) |
47.3 (34.22-65.4) |
0.07 (0.05-0.11) |
87.5 (80.5-92.7) |
92.9 (75.22-114.75) |
0.09(0.06-0.12) |
||||||||
|
Month 51 |
100 |
1 649.6 (1306.8-2082.32) |
2.45 (1.73-3.48) |
100 |
162.23 (136-13-193.34) |
0.15 (0.11-0.19) |
||||||||
* The cut-off values for seropositivity were 20 mMU/ml for HPV16 and 24 mMU/ml for HP18
‡ Antibodies were determined using immunoasssay (competitive Luminez Immunoassay; Merck)
HPV: human papilloma virus, CI: confidence interval, GMT: geometric mean titer, mM:mili merck unit
Table II Antibody geometric mean titer ratio between girls with alternative (0-6-50) versus girls with standard schedule (0-2-6) schedule. Cuernavaca, Morelos, 2018
|
GMT ratio alternative schedule girls/standard schedule girls* |
||||
|
HPV16 |
Month 7/7 |
0.97 (0.61-1.53) |
||
|
Month 7/51 |
1.55 (1.15 - 2.08) |
|||
|
HPV18 |
Month 7/7 |
0.62 (0.44-0.88) |
||
|
Month7/51 |
1.53 (1.12 - 2.09) |
|||
* ratio with 95% confidence interval in brackets
HPV: human papilloma virus, GMT: geometric mean titer
Table II summarizes the comparison of non-inferiority of the alternative compared to the standard schedule at months 7 and 51. At month 7, the alternative schedule was non-inferior for serotype 16 compared with the standard schedule in the same month. For serotypes 18, it was marginally inferior when compared to standard schedule (table II). The data showed non-inferiority for the alternative schedule group for HPV types at month 51 compared to the standard schedule at month 7-one month after the third dose was applied, which was when the most substantial immunogenic response was to be expected.
The adverse effects reported for the booster dose were pain at the site of injection (7%, n=4) and hypotension (2%, n=1). No serious adverse effects associated with the vaccination were reported.
The figures 2 and 3 show the geometric mean titer (GMT) ratio and confidence interval between girls with the alternative (0-6-50) and standard (0-2-6) vaccination schedules. The antibodies GMT’s show the same trend over time in both schedules until month 51 when the booster is applied, leading to the increase of antibody GMT’s in the alternative schedule group.
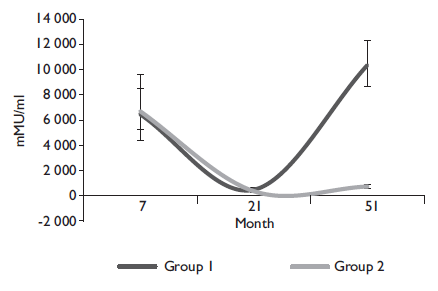
Figure 2 Anti-HPV16 geometric mean titers for month 7, 21 and 51 for girls with alternative schedule (0-6-50) (Group 1), and with traditional schedule (Group 2). Cuernavaca, Morelos, 2018
Discussion
The levels of immunogenicity observed in Mexican girls who received the qHPV vaccine extended schedule (0-6-50 months) were higher than among those who received the traditional vaccination schedule (0-2-6 months). In this respect, the effect of a booster dose is similar in magnitude to that observed when the first dose of the HPV vaccine is administered. Recently, the possibility of incorporating a third dose of the nonavalent vaccine in women who have previously received two doses of tetravalent vaccine has been established. Documentary evidence of the effect of booster doses on HPV vaccines, as in the case of Mexican women, is of particular importance from a public health perspective and in scenarios of alternative vaccination schedules because it provides support for the application of a booster dose in those scenarios of very low vaccination coverage, including 1-dose, in areas of high cervical cancer (CC) incidence or mortality or in vaccination and screening trials with HPV testing for adult women.15,16
One of the main limitations of the study is the lack of randomization, which might result in differential exposure to HPV. However, in Mexico, a small percent (<20%) of women are sexually active before age16,17 and when both groups were compared month at 7, there was non-inferiority in HPV16; thus, we can infer that both groups are homogeneous in month 7. Besides, the prevalence of the two types of HPV in the vaccine in women younger than 34 years is less than 2.2%,18 hus the results are unlikely to be a consequence of the response to exposure, through sexual intercourse; even if differential, the prevalence is low and would have a very small effect in GMT´s. We experienced substantial loss-to-follow-up in the alternative schedule group, but they were blinded to their seropositive status; thus, it is unlikely for loss-to-follow-up to be informative of immunogenicity.
The evaluation of alternative HPV vaccination schedules is necessary for low- and middle-income countries, even though second-generation HPV vaccines are highly effective,19 including in men.20 In this context, in developed countries, 11 years after the introduction of HPV vaccination, the impact on actual conditions in geographic areas with more than 60% vaccination coverage (without implying complete schedules of three doses of vaccination) has been an estimated reduction in HPV 6/11/16/18 infections of nearly 90%.21 Additionally, a reduction of up to 90% for genital warts,22 45% for low grade cytological abnormalities,23 and 85% for high grade histological lesions24 has been documented. The herd effect on unvaccinated men has been consistently reported as well.25 However, the optimal demographic protection of vaccination against HPV has not been achieved, and currently, there is significant inequity in low- and high-income countries in the control of CC. That is, lesions produced by HPV infection remain a significant cause of morbidity and mortality worldwide, and the priority need from a public health perspective is to implement universal vaccination programs against HPV with high population coverage, considering the enormous effect on the low coverage population obtained in the second and third doses.26
Alternative HPV vaccination schedules are necessary to increase coverage. They are justified because CC is still considered a disease of poverty in 2017 and is caused by persistent HPV infections in 100% of cases. During the last 25 years in Mexico, more than 135 000 deaths from CC have been officially reported, and since 2006, it constituted the second leading cause of death due to malignant tumors in women, after breast cancer. Although there has been a significant decrease in CC mortality since the 1990s, which is largely attributed to declining birth rates, there remains a large increase in the number of cases due to CC in rural and marginalized areas in Mexico; thus, optimal prevention and control responses must be implemented. For this reason, immediate actions must be taken to address what has been called the unfinished agenda: to provide an innovative public health response to the thousands of women who are currently suffering from cervical neoplasia and/or who are at risk of cervical neoplasia. According to the knowledge of the natural history of CC, women are mostly infected by HPV at the beginning of their sexual life.
In this context, the National Institute of Public Health was commissioned by the Mexican Ministry of Health to coordinate an inter-institutional group to recommend a vaccination policy against HPV. Derived from this consultation, in 2009, the National Vaccination Council of Mexico adopted, from a public health perspective, an extended schedule of universal vaccination against HPV focused on 9-12-year-old girls, with an alternative schedule of three doses at 0, 6, and 50 months -for the case of the tetravalent vaccine- which is different from that established by the pharmaceutical industry of three doses during a period of six months.
The primary objective of this recommendation was to achieve maximum protection just before sexual activity, with the hypothesis that delaying the administration of the third dose could increase the level of antibodies and simultaneously, after five years, obtain scientific evidence for not administering the third dose.
In April 2014, the Council officially ruled to adopt a vaccination schedule against HPV exclusively with two doses, with a periodicity of zero and six months. The justification is that immune response of girls nine to 11-year-old after two doses of the HPV vaccine is similar or greater than that obtained after two doses in women aged 16 to 26 years.
The benefits of this decision are logistical and economic; that is, it increases the possibility of greater coverage and provides more flexibility in the application of the second dose. In other words, alternative schedules with two doses and a third at 50 months in girls aged 9-12 years can offer: a) improvements in the medium- and long-term immune responses because there is evidence that new exposure to HPV increases the expression of antibodies logarithmically, and b) advantages in its administration because it is simpler to organize these schedules within the schools. This consequently leads to a better chance of being able to obtain greater coverage and adherence to complete schedules in a captive population before finishing mandatory schooling. However, although the recommendation is plausible from an immunological point of view, there is no evidence from clinical trials of efficacy to support that determination, particularly for ethical reasons. For this reason, the impact of HPV and the attributed abnormalities and lesions in the population of women subject to alternative schedules with two or even one dose of the HPV vaccine must be evaluated.
Conclusions
The application of an alternative three-dose booster schedule (0-6-50 months) in girls aged 9-12 years old seems to induce a non-inferior immune response than the three-dose standard schedule (0-2-6 months) one month after the last dose. Further research is needed to understand the minimal number of doses and their timing to provide the best coverage for HPV infection. However, our study suggests that using a booster dose could provide an adequate immune response, giving governments a larger period to implement the full vaccine schedule. Our results need to be replicated with a stronger methodological design, including randomization and better follow-up. Aside from this study’s methodological limitations, these findings provide initial evidence for the potential application of extended vaccine schedules in the Mexican context, where the highest coverage in girls is with one dose, and the possibility of adding a booster later in life could be relevant.











 nueva página del texto (beta)
nueva página del texto (beta)