Introduction
The burden of disease of condyloma (genital warts) has been documented, particularly in women, through epidemiological studies,1,2 population-based cohort studies3 as well as evaluation of HPV vaccine impact at the population-level and HPV vaccine efficacy at the individual level.4 It has also been estimated in external impact evaluation after introduction of anti-HPV vaccination in specific populations.5 Various studies have established that on a population level, around 5 to 10% of people have a condyloma diagnosis in their lifetime.6 Moreover, an estimated 90% of condyloma can be attributed to human papillomavirus (HPV) types 6 and 11, which are considered low-risk for developing cervical neoplasia.7 Risk for persistence of an infection increases significantly with a history of a prior episode of condyloma.8 Also, implementing national anti-HPV vaccination programs, which include protection against serotypes 6 and 11, has significantly decreased the incidence of condyloma in the population.9,10 Most documented scientific evidence on condyloma has been obtained in higher-income countries that have population records and automated clinical files, while there is very little evidence on the burden of condyloma in middle- and low-income countries.11 In this study we present the incidence rates of external genital lesions (EGL) and progression of HPV infection to EGLs, among Mexican males who participated in the HPV Infection in Men (HIM) Study.11,12
Materials and methods
Design and study population
Participants were males between the ages of 18 and 74, residing in Cuernavaca, Mexico, recruited between July 2005 and June 2009.12 The HIS Study prospectively ascertained sexual behavior by questionnaire, and collected exfoliated genital specimens for HPV genotyping every six months for a median follow-up of ~four years. A total of 1 330 men were formally recruited.13 In February 2009, a biopsy and pathology protocol was implemented. This included standardized biopsy and histopathologic confirmation procedures among men with clinical suspicion of HPV-related EGLs.13 For analysis of incident HPV, histologic analysis included men who had ≥2 visits after implementation of the pathology protocol (n=954). Close to half the men had five to seven visits (n=460; 48%); 33% (n=313) had three to four visits and 19% (n=181) had two visits.
All participants signed an informed consent form. The study protocol was approved by the research, ethics and biosafety committees of the National Institute of Public Health of Mexico.
Sample collection of the genital Surface for HPV detection
Participants underwent a clinical examination during each visit. Moistened Dacron pads were used to collect genital samples from the coronal-glans sulcus of the penis, body of the penis and scrotum.11 These samples were combined into a single sample per participant and stored at -70° C. Samples underwent DNA extraction (Qiagen Media Kit), PCR analysis, and HPV genotyping (Roche Linear Array).14 Samples that were positive for β-globin or for an HPV genotype were considered adequate and were included in the analysis. The Linear Array Assay system was used to analyze 37 HPV types, classified as either high-risk (HR-HPV: 16/18/31/33/35/39/45/51/52/56/58/59/68) or low-risk (LR-HPV: 6/11/26/40/42/53/54/55/61/62/64/66/67/69/ 70/71/72/73/81 /82/IS39/83/84/89).15
Collecting external genital lesion (EGL) samples and HPV detection
During each visit, men had an anogenital examination under a 3x lamp by a trained physician, supervised by a urologist, to detect the presence of EGLs. A tissue sample of each lesion was obtained by tangential excision. All EGLs that appeared to be related to HPV or were of unknown etiology based on visual inspection were tested for HPV and underwent histological confirmation by pathology. EGLs were classified as condyloma, suggestive of condyloma, penile intraepithelial neoplasia (PeIN), or unassociated with HPV, based on criteria described previously.16 PeIN lesions were further classified as PeIN I (low grade squamous intraepithelial lesion [SIL]), PeIN II, PeIN II/III, and PeIN III (all high grade SIL). Pathological diagnoses of EGL “suggestive of condyloma” and “condyloma” were grouped together for analysis, since the former share at least two and as many as four pathological characteristics of condyloma.
Tissues received were formalin fixed and paraffin embedded; this was done for each of the samples taken by tangential excision. DNA was extracted from these samples using the QIAamp DNA FFPE Tissue Kit (Qiagen) following the established protocol. Genotyping was performed to detect HPV DNA in sample cells using an AutoBlot 3000H (MedTec Biolab) processor, and the HPV INNO-LiPA Genotyping Extra (Fujirebio) test, which detects 28 HPV types (HR-HPV: 16/18/31/33/35/39/45/51/52/56/58/59/68; LR-HPV: 6/11/26/40/43/44/53/54/66/69/70/71/73/74/82).17
Statistical analysis
EGL incidence
Men with a prevalent lesion were excluded from this analysis. We did descriptive analysis of the demographic characteristics and sexual practices of all males in the cohort, whether or not they developed EGL during the follow-up. A specific analysis by age was performed for men who developed incident EGL within this cohort, stratified by age groups as follows: 18 to 30, 31 to 44, and 45 to 74 years.
Only the first EGL developed was included in EGL incidence analyses. Incidence was calculated from the beginning of the biopsy cohort until the date when the first EGL was detected. Person-time incidence was calculated, and 95% confidence intervals were based on the number of occurrences modeled as a Poisson variable for the total number of person-months. Kaplan-Meier curves were generated for the incidence of EGL, and EGL incidence was compared over time in all three age groups using the log-rank test. Cumulative incidence of development of an EGL was also estimated in the first 12 months of follow-up using the Kaplan-Meier method.
For specific analyses of a given genotype, all prevalent and incident lesions were included. Besides specific HPV types, positive infections for ≥1 type were included in the group of any HPV; those positive for ≥1 high-risk HPV type were included in the high-risk HPV group; and those positive for ≥1 low-risk types were included in the low-risk HPV group. Independent analyses were performed for high-risk and low-risk infections. EGLs that were positive for ≥1 high-risk HPV types and ≥1 low-risk HPV types were included in the HR/LR-HPV group.
Progression of HPV infection to EGL
Among men (without prevalent condyloma or PeIN) with an incident or prevalent genital HPV infection, the rate and proportion of men progressing to an EGL was estimated. Demographic characteristics were compared among men who developed or failed to develop an EGL using Monte Carlo estimates of exact Pearson’s chi-square test. HPV infection was described by genotype or group (any, HR-HPV, LR-HPV). Classification as any HPV type was defined as a positive test result for at least one of the 25 HPV genotypes (HPV types 43/44/74 are not detected through Linear Array Assay) using INNO-LiPA. HPV infections by a single or multiple HR-HPV types were classified as high-risk and infections by at least one of the LR-HPV types were classified as low-risk.
The cumulative incidence of EGLs at 6, 12, and 24 months and the median time to EGL development for individual HPV types was estimated using the Kaplan-Meier method for grouped datasets18 since men could have been infected with multiple HPV types within a given group; also, multiple HPV types can be detected in a single EGL, and a man may develop multiple EGLs. The global incidence rate of EGL during the study period was also calculated.
Results
Incidence of external genital lesions
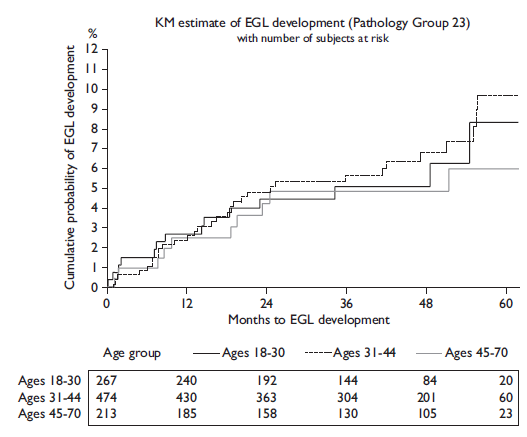
The prevalence of extra-genital lesions at baseline (during the initial visit) was 2.2%, while the prevalence of genital warts was 6.6% at baseline. EGL incidence was associated with sexual orientation (p=0.007), total number of lifetime female partners (p=0.003) and male partners (p=0.006) (table I). Overall EGL incidence rate (IR) was 1.84 (95%CI=1.42-2.39) per 100 person-years (py). The cumulative risk of EGL at 12 months was 2.9% (95%CI=1.9-4.2). The highest incidence of EGL was observed among men ages 18-30 years (IR=1.99 per 100py, 95%CI=1.22-3.25) and 31-44 years (IR=1.96 per 100py, 95%CI=1.38-2.78), although the IR did not significantly differ between the three age categories. Also, for the combined category of condyloma and its suggested diagnosis, the highest incidence rate was observed in the 31 to 44 year age group (IR=1.95 per 100py, 95%CI=1.37-2.78). Incidence of any EGL, combined condyloma, and PeIN did not significantly differ by age among men (table II, figure 1).
Table I Differences in sociodemographic characteristics and sexual behavior among Mexican men with and without an incident EGL during follow-up
|
Mexico (n=954) |
||||||||
|
Factors |
Total HIM study sample* N (%) |
No EGL incidence N (%) |
Any incident EGL N (%) |
p Values‡ |
||||
|
Age (years) |
0.39 |
|||||||
|
18 to 30 |
1 157 (38.4) |
243 (28.4) |
21 (21.6) |
|||||
|
31 to 44 |
1235 (41) |
418 (48.8) |
52 (53.6) |
|||||
|
45 to 74 |
620 (20.6) |
196 (22.9) |
24 (24.7) |
|||||
|
Years of education |
0.44 |
|||||||
|
Completed 12 or less |
1 319 (43.8) |
551 (64.3) |
56 (57.7) |
|||||
|
13 to 15 |
774 (25.7) |
74 (8.6) |
12 (12.4) |
|||||
|
Completed at least 16 |
907 (30.1) |
228 (26.6) |
29 (29.9) |
|||||
|
Refused |
10 (0.3) |
3 (0.4) |
0 (0) |
|||||
|
Missing |
2 (0.1) |
1 (0.1) |
0 (0) |
|||||
|
Marital status |
0.48 |
|||||||
|
Single |
1 148 (38.1) |
139 (16.2) |
13 (13.4) |
|||||
|
Married/cohabiting |
1 557 (51.7) |
657 (76.7) |
76 (78.4) |
|||||
|
Divorced/separated/widowed |
298 (9.9) |
58 (6.8) |
7 (7.2) |
|||||
|
Refused |
7 (0.2) |
2 (0.2) |
1 (1) |
|||||
|
Missing |
2 (0.1) |
1 (0.1) |
0 (0) |
|||||
|
Circumcised |
0.76 |
|||||||
|
No |
1 906 (63.3) |
721 (84.1) |
83 (85.6) |
|||||
|
Yes |
1 106 (36.7) |
136 (15.9) |
14 (14.4) |
|||||
|
Smoking status |
0.45 |
|||||||
|
Current |
691 (22.9) |
276 (32.2) |
38 (39.2) |
|||||
|
Former |
948 (31.5) |
248 (28.9) |
26 (26.8) |
|||||
|
Never |
1 322 (43.9) |
293 (34.2) |
30 (30.9) |
|||||
|
Missing |
51 (1.7) |
40 (4.7) |
3 (3.1) |
|||||
|
Alcohol per month |
0.87 |
|||||||
|
0 drinks |
690 (22.9) |
211 (24.6) |
27 (27.8) |
|||||
|
1 to 30 drinks |
1 293 (42.9) |
408 (47.6) |
46 (47.4) |
|||||
|
>30 drinks |
898 (29.8) |
166 (19.4) |
20 (20.6) |
|||||
|
Missing |
131 (4.3) |
72 (8.4) |
4 (4.1) |
|||||
|
Sexual orientation |
0.007 |
|||||||
|
MSW |
2 341 (77.7) |
748 (87.3) |
77 (79.4) |
|||||
|
MSM |
80 (2.7) |
8 (0.9) |
4 (4.1) |
|||||
|
MSMW |
428 (14.2) |
64 (7.5) |
13 (13.4) |
|||||
|
Missing |
163 (5.4) |
37 (4.3) |
3 (3.1) |
|||||
|
Total number of female partners |
0.003 |
|||||||
|
0 to 1 |
395 (13.1) |
115 (13.4) |
5 (5.2) |
|||||
|
2 to 9 |
1 123 (37.3) |
472 (55.1) |
42 (43.3) |
|||||
|
10 to 49 |
1 149 (38.1) |
242 (28.2) |
43 (44.3) |
|||||
|
50+ |
269 (8.9) |
14 (1.6) |
4 (4.1) |
|||||
|
Refused |
76 (2.5) |
14 (1.6) |
3 (3.1) |
|||||
|
Total number of male partners |
0.006 |
|||||||
|
0 |
2 466 (81.9) |
778 (90.8) |
80 (82.5) |
|||||
|
1 to 9 |
364 (12.1) |
65 (7.6) |
13 (13.4) |
|||||
|
10+ |
144 (4.8) |
7 (0.8) |
4 (4.1) |
|||||
|
Missing |
38 (1.3) |
7 (0.8) |
0 (0) |
|||||
* Total HIS Study sample for Mexico, Brazil and the United States (n=3 012)
‡ p values were calculated using Monte Carlo estimation of exact Pearson chi-square tests comparing characteristics of men with and without EGL
MSW=men who have sex with women, MSM=men who have sex with men, MSMW=men who have sex with men and women
Table II. Age-specific incidence of pathologically confirmed external genital lesions (EGLs) among Mexican men in the HIS Study
|
Pathological diagnosis |
||||||||||||
|
Any type* |
Condyloma |
Suggestive of condyloma‡ |
Combined condyloma§ |
PeIN# |
Other& |
|||||||
|
All ages (n=954)≠ |
||||||||||||
|
Men with incident EGL, no. |
57 |
23 |
39 |
55 |
3 |
46 |
||||||
|
Person-months |
37 169 |
37 945 |
37 972 |
37 272 |
38 697 |
37 584 |
||||||
|
Incidence rate∞ (95% CI) |
1.84 (1.42-2.39) |
0.73 (0.48-1.09) |
1.23 (0.9-1.69) |
1.77 (1.36-2.31) |
0.09 (0.03-0.29) |
1.47 (1.1-1.96) |
||||||
|
12-month incidence |
2.9 (1.9-4.2) |
1.5 (0.9-2.6) |
1.1 (0.6-2) |
2.5 (1.7-3.8) |
0.3 (0.1-1) |
2.3 (1.5-3.5) |
||||||
|
18 to 30 y (n=267) |
||||||||||||
|
Men with incident EGL, no. |
16 |
6 |
10 |
14 |
2 |
7 |
||||||
|
Person-months |
9 654 |
9 911 |
9 894 |
9 736 |
10 039 |
9 994 |
||||||
|
Incidence rate∞ (95% CI) |
1.99 (1.22-3.25) |
0.73 (0.33-1.62) |
1.21 (0.65-2.25) |
1.73 (1.02-2.91) |
0.24 (0.06-0.96) |
0.84 (0.4-1.76) |
||||||
|
12-month incidence |
3.5 (1.8-6.8) |
1.6 (0.6-4.1) |
1.6 (0.6-4.1) |
2.7 (1.3-5.7) |
0.8 (0.2-3.1) |
1.2 (0.4-3.6) |
||||||
|
31 to 44 y (n=474) |
||||||||||||
|
Men with incident EGL, no. |
31 |
14 |
22 |
31 |
1 |
27 |
||||||
|
Person-months |
19 026 |
19 347 |
19 453 |
19 047 |
19 836 |
19 080 |
||||||
|
Incidence rate∞ (95% CI) |
1.96 (1.38-2.78) |
0.87 (0.51-1.47) |
1.36 (0.89-2.06) |
1.95 (1.37-2.78) |
0.06 (0.01-0.43) |
1.7 (1.16-2.48) |
||||||
|
12-month incidence |
2.6 (1.5-4.6) |
1.7 (0.9-3.5) |
0.6 (0.2-2) |
2.4 (1.3-4.3) |
0.2 (0-1.5) |
3.1 (1.8-5.2) |
||||||
|
45 to 74 y (n=223) |
||||||||||||
|
Men with incident EGL, no. |
10 |
3 |
7 |
10 |
0 |
12 |
||||||
|
Person-months |
8 489 |
8 687 |
8 625 |
8 489 |
8 823 |
8 510 |
||||||
|
Incidence rate∞ (95% CI) |
1.41 (0.76-2.63) |
0.41 (0.13-1.28) |
0.97 (0.46-2.04) |
1.41 (0.76-2.63) |
0.0 (0.0-0.0) |
1.69 (0.96-2.98) |
||||||
|
12-month incidence |
2.5 (1-6) |
1 (0.2-4) |
1.5 (0.5-4.6) |
2.5 (1-6) |
2 (0.7-5.3) |
|||||||
|
p-valueØ |
0.63 |
0.50 |
0.72 |
0.64 |
0.30 |
0.20 |
||||||
Abbreviation: 95% CI=95% confidence interval
* Men with ≥1 incident, pathologically confirmed HPV-related EGL throughout the study period. For men with >1 EGL, incidence rates for the Any EGL category are determined for the first detected lesion; thus, men may contribute fewer person-months in this category than for specific pathological diagnoses
‡ Includes lesions suggestive but not diagnostic of HPV infection or condyloma
§ Includes both Condyloma and Suggestive of Condyloma categories
# PeIN = penile intraepithelial neoplasia (I-III)
& Includes various HPV-unrelated skin conditions, such as seborrheic keratosis and skin tags
≠ 7 men with prevalent EGLs were excluded from the initial cohort for this analysis
∞ Specified as the number of cases per 100 person-years
Ø Determined using the log-rank test and corresponding to overall differences in EGL incidence across the entire follow-up period, by age group. Values < .05 are considered statistically significant
Progression of HPV infection to EGL
Among the 954 men with at least two follow-up visits, 519 had a prevalent or incident HPV infection. In thirty-three of these men HPV progressed to a lesion with the same HPV type detected within the lesion (table III). There were no statistically significant differences between HPV-positive men who did and did not develop an EGL. Correspondingly, 31.2% of HPV6 infections progressed to HPV6-positive condyloma and 28.6% of HPV11 infections progressed to HPV11-positive condyloma (table III). In addition, the median time for progression of an infection with any type of HPV to condyloma (with DNA for that same type of HPV detected in the lesion) was 8.7 person-months. Progression from an infection with a HR-HPV type took a median time of 7.6 person-months while progression from LR-HPV types took a median time of 10.8 person-months (table IV).
Table III Comparison of characteristics among human papillomavirus-positive men who did and did not develop an external genital lesion during follow-up in the HIS Study
|
Factors |
Total*N (%) |
No EGL incidence N (%) |
Any EGL incidence N (%) |
p value‡ |
||||
|
Age (years) |
0.8280 |
|||||||
|
18 to 30 |
162 (31.2) |
152 (31.3) |
10 (30.3) |
|||||
|
31 to 44 |
243 (46.8) |
226 (46.5) |
17 (51.5) |
|||||
|
45 to 74 |
114 (22) |
108 (22.2) |
6 (18.2) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Years of education |
0.5640 |
|||||||
|
Completed 12 years or less |
333 (64.2) |
310 (63.8) |
23 (69.7) |
|||||
|
13 to 15 years |
48 (9.2) |
44 (9.1) |
4 (12.1) |
|||||
|
Completed at least 16 years |
137 (26.4) |
131 (27) |
6 (18.2) |
|||||
|
Refused |
1 (0.2) |
1 (0.2) |
0 (0) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Marital status |
0.8910 |
|||||||
|
Single |
93 (17.9) |
86 (17.7) |
7 (21.2) |
|||||
|
Married/cohabiting |
377 (72.6) |
354 (72.8) |
23 (69.7) |
|||||
|
Divorced/separated/widowed |
48 (9.2) |
45 (9.3) |
3 (9.1) |
|||||
|
Refused |
1 (0.2) |
1 (0.2) |
0 (0) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Circumcised |
0.8060 |
|||||||
|
No |
444 (85.5) |
415 (85.4) |
29 (87.9) |
|||||
|
Yes |
75 (14.5) |
71 (14.6) |
4 (12.1) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Smoking status |
0.7840 |
|||||||
|
Current |
193 (37.2) |
179 (36.8) |
14 (42.4) |
|||||
|
Former |
142 (27.4) |
132 (27.2) |
10 (30.3) |
|||||
|
Never |
165 (31.8) |
156 (32.1) |
9 (27.3) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Missing |
19 (3.7) |
19 (3.9) |
0 (0) |
|||||
|
Alcohol per month |
0.8010 |
|||||||
|
0 |
126 (24.3) |
116 (23.9) |
10 (30.3) |
|||||
|
1-30 |
246 (47.4) |
231 (47.5) |
15 (45.5) |
|||||
|
>30 |
111 (21.4) |
104 (21.4) |
7 (21.2) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Missing |
36 (6.9) |
35 (7.2) |
1 (3) |
|||||
|
Sexual orientation§ |
0.3590 |
|||||||
|
MSM |
8 (1.5) |
7 (1.4) |
1 (3) |
|||||
|
MSMW |
44 (8.5) |
39 (8) |
5 (15.2) |
|||||
|
MSW |
445 (85.7) |
419 (86.2) |
26 (78.8) |
|||||
|
Missing |
22 (4.2) |
21 (4.3) |
1 (3) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Total number of female partners |
0.0910 |
|||||||
|
0-1 |
48 (9.2) |
47 (9.7) |
1 (3) |
|||||
|
2 to 9 |
254 (48.9) |
239 (49.2) |
15 (45.5) |
|||||
|
10 to 49 |
194 (37.4) |
181 (37.2) |
13 (39.4) |
|||||
|
50+ |
12 (2.3) |
9 (1.9) |
3 (9.1) |
|||||
|
Refused |
11 (2.1) |
10 (2.1) |
1 (3) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Total number of male partners |
0.2240 |
|||||||
|
0 |
463 (89.2) |
436 (89.7) |
27 (81.8) |
|||||
|
1 to 9 |
44 (8.5) |
39 (8) |
5 (15.2) |
|||||
|
10+ |
8 (1.5) |
7 (1.4) |
1 (3) |
|||||
|
Total |
519 (100) |
486 (93.6) |
33 (6.4) |
|||||
|
Missing |
4 (0.8) |
4 (0.8) |
0 (0) |
|||||
* n=519 men participating in the HIS Study in Mexico who had >2 study follow-up visits after February 2009 and who, if they had an EGL which was suspected to be HPV-related, underwent standardized biopsy and histopathologic confirmation procedures
‡ p values were calculated using Monte Carlo estimation of exact Pearson chi-square tests comparing characteristics of men with and without EGL
§ MSW=men who have sex with women; MSM=men who have sex with men; MSMW=men who have sex with men and women
Table IV Progression of genital human papillomavirus (HPV)* infection to condyloma‡ with the same HPV type detected in the lesion among Mexican men in the HIS Study
|
HPV type |
Proportion of HPV infectionsz that progress,§ No./total (%) |
Median time# |
||
|
Any type of HPV |
36/1 103 (3.3) |
8.7 |
||
|
High-risk |
6/638 (0.9) |
7.6 |
||
|
16 |
0/86 (0.0) |
0 |
||
|
18 |
0/26 (0.0) |
0 |
||
|
31 |
1/47 (2.1) |
5.8 |
||
|
33 |
0/9 (0.0) |
0 |
||
|
35 |
0/5 (0.0) |
0 |
||
|
39 |
0/67 (0.0) |
0 |
||
|
45 |
0/34 (0.0) |
0 |
||
|
51 |
1/103 (1.0) |
8.4 |
||
|
52 |
3/72 (4.2) |
7.8 |
||
|
56 |
1/32 (3.1) |
0.4 |
||
|
58 |
0/44 (0.0) |
0 |
||
|
59 |
0/95 (0.0) |
0 |
||
|
68 |
0/18 (0.0) |
0 |
||
|
Low-risk |
30/465 (6.5) |
10.8 |
||
|
6 |
24/77 (31.2) |
14.3 |
||
|
11 |
4/14 (28.6) |
0.9 |
||
|
26 |
0/2 (0.0) |
0 |
||
|
40 |
0/26 (0.0) |
0 |
||
|
53 |
0/95 (0.0) |
0 |
||
|
54 |
1/39 (2.6) |
7.8 |
||
|
66 |
1/90 (1.1) |
17.2 |
||
|
69 |
0/5 (0.0) |
0 |
||
|
70 |
0/36 (0.0) |
0 |
||
|
71 |
0/48 (0.0) |
0 |
||
|
73 |
0/21 (0.0) |
0 |
||
|
82 |
0/12 (0.0) |
0 |
||
* DNA detected using linear array
‡ Newly acquired, pathologically confirmed EGL
§ The unit of analysis is genital HPV infection
# Median time to progression of genital HPV infection to condyloma, in person-months
The highest condyloma incidence was found in Mexican males with HPV6 (12.2 per 1 000 person-months [pm], 95%CI=8.2-18.2) and HPV11 (12.3 per 1000 pm, 95%CI=4.6-32.8). The highest cumulative incidence of condyloma at six months (44.4% 95%CI=14.3-137.8) occurred in men with HPV11. For HPV6, the cumulative incidence increased from 2.2% (95%CI=0.3-15.6) at six months, to 12.2% (95%CI=6.5-22.6) at 12 months and 14.1% (95%CI=9.0-22.1) at 24 months (table V, figure 2).
Table V. Incidence of condyloma *by human papillomavirus (HPV) type detected in the lesion‡ among Mexican men with the same HPV type detected on the genitals,§ HIS Study
|
Incidence rate≠ (95%CI) |
Cumulative incidence (%) |
|||||||
|
6m (95%CI) |
12m (95%CI) |
24m (95%CI) |
||||||
|
Any type |
1.0 (0.7-1.4) |
0.9 (0.4-2.0) |
1.6 (1.0-2.5) |
1.3 (0.9-1.9) |
||||
|
High-risk |
0.3 (0.1-0.6) |
0.5 (0.1-2.1) |
0.7 (0.3-1.6) |
0.4 (0.2-0.9) |
||||
|
31 |
0.7 (0.1-4.7) |
3.6 (0.5-25.2) |
1.8 (0.3-13.1) |
1.0 (0.1-7.2) |
||||
|
51 |
0.3 (0.0-2.0) |
0.0 (0.0-0.0) |
0.8 (0.1-6.0) |
0.5 (0.1-3.3) |
||||
|
52 |
1.2 (0.4-3.8) |
0.0 (0.0-0.0) |
2.5 (0.6-9.8) |
1.4 (0.3-5.5) |
||||
|
56 |
0.9 (0.1-6.4) |
5.4 (0.8-38.3) |
2.9 (0.4-20.8) |
1.6 (0.2-11.6) |
||||
|
Low-Risk |
2.0 (1.4-2.9) |
1.5 (0.5-3.9) |
2.9 (1.7-4.8) |
2.7 (1.8-4.0) |
||||
|
6 |
12.2 (8.2-18.2) |
2.2 (0.3-15.6) |
12.2 (6.5-22.6) |
14.1 (9.0-22.1) |
||||
|
11 |
12.3 (4.6-32.8) |
44.4 (14.3-137.8) |
33.6 (12.6-89.6) |
19.9 (7.5-53.0) |
||||
|
54 |
0.7 (0.1-5.1) |
0.0 (0.0-0.0) |
2.2 (0.3-15.7) |
1.2 (0.2-8.7) |
||||
|
66 |
0.3 (0.0-2.4) |
0.0 (0.0-0.0) |
0.0 (0.0-0.0) |
0.6 (0.1-3.9) |
||||
|
Vaccine∞ |
4.8 (3.3-6.9) |
3.4 (1.3-8.9) |
6.3 (3.7-10.6) |
6.0 (4.0-9.1) |
||||
CI= confidence interval
* Newly acquired, pathologically confirmed condyloma/suggestive of condyloma
‡ DNA detected using INNO LiPA
§ DNA detected using linear array
# Prevalent and incident genital HPV infections
& HPV types 16/18/33/35/39/45/58/59/68/26/40/53/69/70/71/73/82 did not progress to a condyloma lesion; therefore, incidence rates and cumulative incidence could not be calculated
≠ Incidence rate is cases per 1 000 person-months
∞ Vaccine HPV types 6/11/16/18

Figure 2 Kaplan-Meier (KM) curves showing differences in cumulative incidence of combined condyloma progression of HPV to condyloma by HPV type, Mexican men in the HIS Study
Seven men developed PeIN lesions. There were three HPV-positive men who developed type-specific PeIN lesions during follow-up that had both high- and low-risk types while four had only low risk types. Four men had PeIN lesions with HPV type 16; two men had lesions with type 51; three men had type 11 and 1 man had type 6. Two of the HPV16 genital infections progressed to HPV16-positive PeIN lesions and two HPV11 genital infections progressed to HPV11-positive PeIN lesions.
The highest incidence rate of progression of HPV to PeIN occurred with HPV11 at 2.5 per 1 000pm (95% CI=0.3-17.4) (table VI). The cumulative incidence of PeIN in men with HPV11 was 12.7 % (95% CI=1.8-90.4) at six months and 6.9 % (95% CI=1.0-48.9) at 12 months.
Table VI. Incidence of penile intraepithelial neoplasia (PeIN)* by human papillomavirus (HPV) type detected in the lesion‡ with the same HPV type detected on the genitals§among Mexican men in the HIM Study
|
Incidence rate≠ (95%CI) |
Cumulative incidence (%) |
|||||||
|
6m (95%CI) |
12m (95%CI) |
24m (95%CI) |
||||||
|
Any type |
0.1 (0.0-0.3) |
0.6 (0.2-1.6) |
0.3 (0.1-0.8) |
0.2 (0.1-0.5) |
||||
|
High-risk |
0.1 (0.0-0.4) |
0.5 (0.1-2.1) |
0.3 (0.1-1.1) |
0.2 (0.0-0.6) |
||||
|
16 |
0.7 (0.2-3.0) |
4.0 (1.0-15.9) |
2.1 (0.5-8.2) |
1.2 (0.3-4.7) |
||||
|
Low-risk |
0.1 (0.0-0.5) |
0.7 (0.2-2.9) |
0.4 (0.1-1.5) |
0.2 (0.1-0.8) |
||||
|
6 |
0.4 (0.1-2.8) |
2.2 (0.3-15.4) |
1.2 (0.2-8.2) |
0.7 (0.1-4.6) |
||||
|
11 |
2.5 (0.3-17.4) |
12.7 (1.8-90.4) |
6.9 (1.0-48.9) |
4.0 (0.6-28.6) |
||||
|
Vaccine∞ |
0.6 (0.2-1.7) |
3.3 (1.3-8.9) |
1.8 (0.7-4.7) |
1.0 (0.4-2.7) |
||||
CI = confidence interval
* Newly acquired, pathologically confirmed penile intraepithelial neoplasia (PeIN)
‡ DNA detected using INNO LiPA
§ DNA detected using linear array
# Prevalent and incident genital HPV infections
& HPV types 18/31/33/35/39/45/51/52/56/58/59/68/26/40/53/54/66/69/70/71/73/82 did not progress to a PeIN; therefore, incidence rates and cumulative incidence could not be calculated
≠ Incidence rate is cases per 1 000 person-months
∞ Vaccine HPV types 6/11/16/18
Discussion
This is one of the first reports on incidence of EGLs in Mexico, as well as the frequency of PeIN. This is particularly significant, since no specific information is available on the Mexican and Latin American context regarding the burden of condyloma, or cancer precursor lesions of the penis (PeIN).
Our study in a population of healthy Mexican males indicates that anogenital HPV infection is endemic, that infection with HPV6 and 11 is high, and that these infections progress to condyloma at a high rate. In addition, along with high-risk HPV types such as type 16, these infections are the main determining factor for penile cancer and precursor lesions. The proportion of subjects with HPV types that progress to low and high grade PeIN is relatively low, yet it is relevant, since it is a precursor to penile cancer.
Condyloma has been associated with poor quality of life19 and negative psychosocial impact;20 also, treatment is costly21 and recurrence rates are high (10 to 40%).22,23 The frequency of condyloma is high in high-income countries, where it is estimated that 1 in every 10 women will have had a condyloma diagnosis before age 45.21 Thus HPV infection, including condyloma, is an important cause of morbidity and risk in public health, considering its high incidence, recurrence and persistence. In middle- and lower-income countries like Mexico, data such as that presented by the current study indicates that the situation is similar. Paradoxically, HPV can cause benign and malignant lesions that are often difficult to treat, yet infections can be prevented by vaccination. Studies in the Latin American region have shown that anti-HPV vaccination can reduce the risk of condyloma by up to 67%,24 and at present this is the only type of intervention that protects against HPV types 6 and 11,25 which cause most condyloma,24 as well as laryngeal papillomatosis26 and oropharyngeal cancer.27
The burden of condyloma has been quantified mainly in higher-income countries, where sexually transmitted infections are considered a public health problem given the scientific evidence showing their high incidence and high healthcare costs.28 In many areas, introduction of anti-HPV vaccination for males could be especially beneficial to men who have sex with men.29 However, other than the HIS Study, there are no sizeable longitudinal studies that assess the natural history of condyloma in middle- and low-income countries. As a result of this lack of scientific evidence, this public health problem is underestimated and therefore also the possible benefits of vaccination among men.
In the Mexican National Health System, most condyloma are treated in primary healthcare centers with medication.30 Recurrent lesions are referred for surgical removal, diathermia, cryotherapy or laser treatment, or to gynecology, urology and/or dermatology units. However, in this healthcare system there are no specialized clinics for sexually transmitted infections except those to diagnose, treat and follow up individuals with HIV. Consequently, in Mexico, and most likely in the Latin American region in general, it is imperative that the number of medical visits for condyloma be quantified to estimate related healthcare costs.
Vaccination of males in Mexico is justified given that the burden of disease attributed to HPV manifests not only as EGLs but as the fraction of penile cancer attributable to HPV,31 which is almost 60%. Also, oropharyngeal cancer among men (75% of which is attributable to HPV) will soon surpass cervical cancer in some populations.32 This is why an aggressive HPV vaccination and screening policy (which combines primary and secondary prevention)33 is necessary34 to decrease the burden of HPV-related diseases.35
A potential limitation of the study is that the findings are not necessarily generalizable to all men in Mexico. As HPV incidence was based on clinic visits, which occurred every six-months, this might not reflect the exact timing of infection.
Conclusion
Condyloma should be considered a public health issue, as has been documented in large longitudinal studies to characterize the natural history of HPV in women36 and men.37 Standardized guidelines for diagnosis and management of condyloma are needed.11 Current discussion has focused on whether it makes sense to introduce anti-HPV vaccines in vulnerable groups of males and females who are at a higher risk of exposure to HPV types 6 and 11, which are responsible for most condyloma, including children who are victims of sexual abuse.38 Until effective treatment for HPV infection is available, primary prevention (i.e., vaccination) will be the main strategy implemented to control this sexually transmitted infection39 and consequently EGLs and precursor lesions for cancer. An intervention that integrates both proposed actions (vaccination and standardized diagnosis and management) would constitute an organized social response to control one of the most recurrent sexually transmitted diseases, condyloma.











 nueva página del texto (beta)
nueva página del texto (beta)