Introduction
Medical devices, together with drugs and other health technologies, comprise one of the six components considered as essential for good health system performance.1 Accordingly, the regulation, assessment, and management of medical devices receive increasing attention by policy makers and health system experts involved in efforts to attain universal coverage of safe, equitable and high-quality health services.2,3,4,5 At the centre of the discussion is the improvement of outputs and outcomes of the medical device life cycle (MDLC).6 A cohesive medical devices policy, well planned, supported and coordinated among all areas represented by the MDLC stakeholders (i.e. those involved in regulation, assessment, and management) is necessary to meet the population needs and to ensure the quality of health care.6
The Mexican health system is composed by a complex structure that includes various ‘sub-systems’ in charge of health service regulation, financing and provision. Most important ‘sub-systems’ are the public social security institutes (Mexican Institute of Social Security, IMSS and Institute of Social Security and Services for State Workers, ISSSTE) and the state-level health care services (SESA), which function along a large number of private health care providers.7,8 The Ministry of Health (MOH) and its departments enact regulations and recommendations at the federal level for the public and private sector, but the ‘sub-systems’ (such as social security institutes) comply with these in different ways due to their specific legal regulations and embedment.9,10 The health system is characterized by a distribution of roles across a substantial number of actors resulting in a fragmentation of responsibilities.
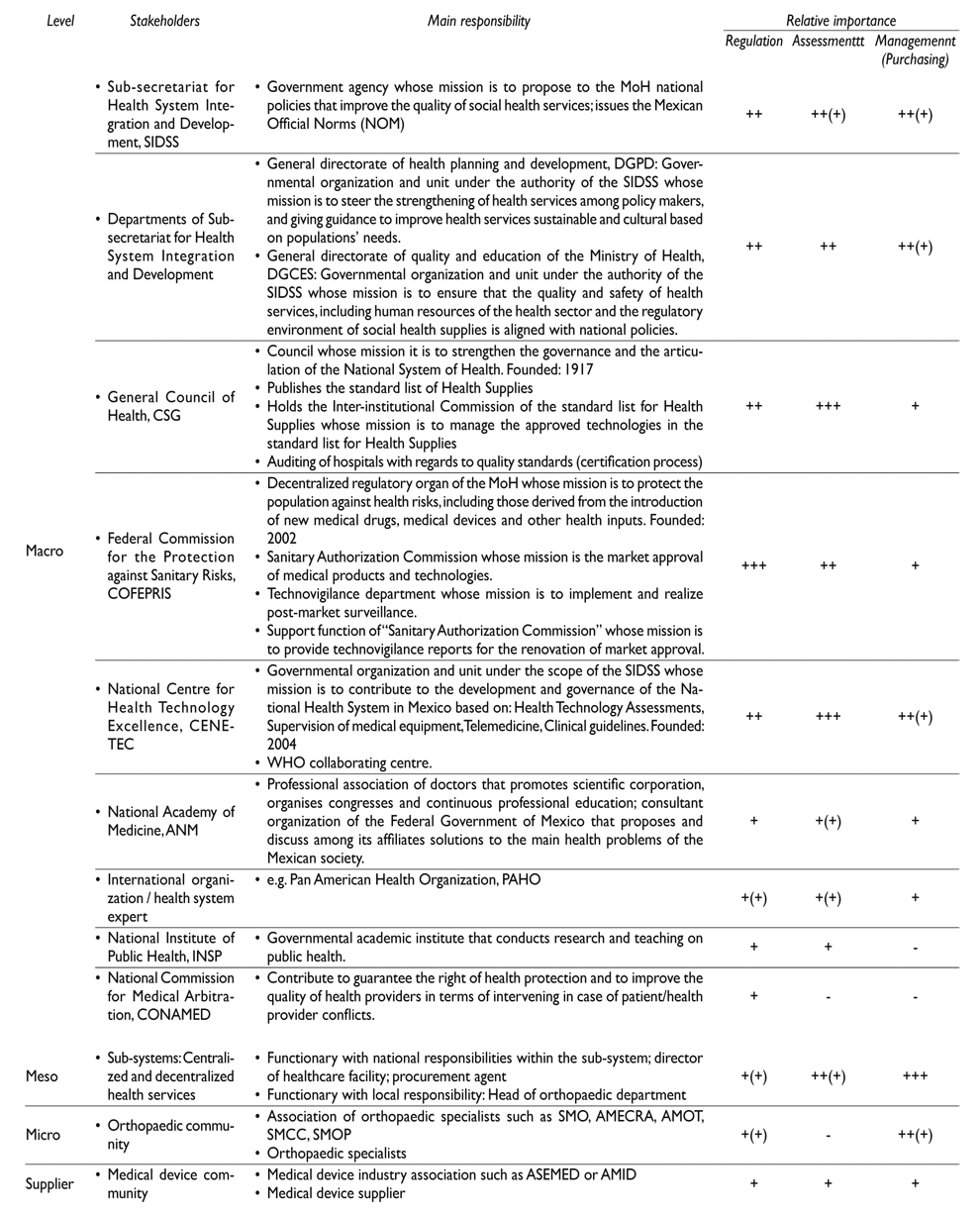
This fragmentation of roles and responsibilities is well illustrated with regard to medical devices. The General Health Council (CSG), an independent governmental organisation is responsible for overseeing the Inter-institutional Commission of the Standard List for Health Supplies; the Federal Commission for the Protection against Sanitary Risks (Cofepris), an autonomous MOH agency, is responsible for the quality and safety of new medical drugs, medical devices and other health inputs; while the National Centre for Health Technology Excellence (Cenetec), a central MOH office, is responsible for health technology assessments, supervision of medical equipment, and clinical guidelines.11 Notwithstanding their important achievements, it has been stressed that the functioning of these agencies has room for improvement; specially, with regard with their linkage with guidelines for the quality of care and with assuring value for money across all ‘sub-systems’.7 The Mexican Congress is discussing the creation of the Federal Commission for the Regulation and Surveillance of Health Care Services and Facilities,12 which could generate momentum for the uptake of a broad spectrum of aspects concerning quality in health care and this may support outcomes of the MDLC areas; therefore, the stakeholder analysis presented here comes in a timely manner.
In a series of previous studies we analysed critical aspects between and within key MDLC functions and discussed ideas about desired changes to overcome identified challenges (table I).13,14,15,16 The findings highlighted that the overall MDLC system in Mexico is not coherently outlined and set-up across the regulatory, the assessment, and the management domains of orthopaedic medical devices (OMDs), resulting in sub-optimal quality of services delivered to patients. This finding resonates with concerns raised by other authors regarding the regulation, assessment, and management of health services in Mexico at different levels of health care delivery.7,9,17,18,19
Table I. Strategies for the medical device life cycle

Source for ‘medical device life cycle’: Adapted from Development of medical device policies, WHO Medical device technical series6
Source for ’strategies’: Lingg M, Dreser A, Duran-Arenas L, Wyss K16
The aim of the present study is to review three alternative strategies to improve the regulation, assessment, and management of orthopaedic medical devices in Mexico with regard to their political feasibility.
Materials and methods
The strategies under review (table I) stem from our previous study,16 which discusses alternatives to improve the outcomes of the MDLC. In the present study, we reviewed three strategies regarding their political feasibility based on a structured stakeholder analysis to identify key stakeholders, and their potential stake, interest, position and power.20,21,22,23
Identifying stakeholders
To identify the most relevant stakeholders we did a general search in Portal de Obligaciones de Transparencia, which lists all government units or institutes and their departments and facilitates the access to government information. Further, we complemented the retrieved data by stakeholder information as provided by our previous study findings and documental analysis of information on the identified stakeholders such as recent advancements, or future projects related to the key functions of medical devices. Based on the obtained data we developed a list of most relevant stakeholders regarding medical device regulation, assessment, and management. We classified stakeholders according to four levels: macro level (normative and policy mechanism), meso level (service provision), micro level (specialists and orthopaedic associations), and supplier level (medical device suppliers and medical device industry associations). Further, we described their relative importance regarding the regulation, assessment, and management of medical devices.
Data collection
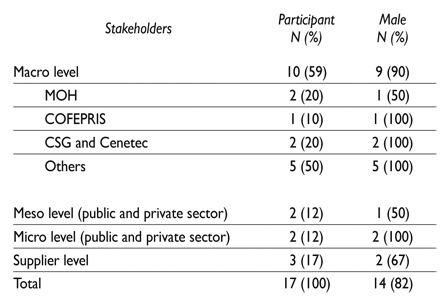
We contacted prospective participants between November 2016 and January 2017 by email and presented our study rationale and research. We used a maximum variation sampling to recruit stakeholders to achieve a balanced variety of stakeholders and diversity of data. Data collection was based on 17 structured interviews to collect data from the interviewees on themselves but also their opinions on other stakeholders (table II). For the interviews we used a questionnaire* with mainly closed-ended questions using a 5-point Likert scale. The questionnaire was previously tested among three Mexican stakeholders. We used a file-naming system and anonymised interviewees by generating a list of archival numbers.
Data analysis
We analysed the retrieved data and information on stakeholders based on four characteristics and regarding each of the three strategies (organized as sub-sections): (i) ‘Potential stake’ to distinguish between stakeholders potentially important to carrying the discussed strategy forward, considered being moderately valuing with the strategy, or not important to carrying the discussed strategy forward; (ii) ‘Interest’ to display the stakeholder’s opinion in the strategies based on advantages or disadvantages that these may bring to the stakeholder; (iii) ‘Position’ to present whether the stakeholder supports, opposes, or is neutral about the strategy; and (iv) ‘Power’ to differentiate between high to low influence regarding the realization of the strategy. Based on the stakeholder information, we discussed for each strategy its political feasibility.
Ethics
The committee for research and ethics, research division of the medical faculty of the National Autonomous University of Mexico, approved this project (Date of approval: November 4, 2014; oficio no. FMED/CI/SPLR/188/2014; dictamen proyecto: 143-2014). No formal ethical approval was needed from the ethical committee from Northwest and Central Switzerland (Switzerland), which exempted it from ethical review under Swiss law (June 24, 2014). All interviewees provided informed consent before the interview.
Results
Most relevant stakeholders
Table III displays the most relevant stakeholders. Their relative importance regarding the three strategies is decreasing from the macro to the micro levels at least for ‘regulation’ and ‘assessment’ of medical devices. Stakeholders with a relative high importance for regulation is Cofepris, for assessment are Cenetec and CSG, and for management are the ‘sub-systems’.
Analysis of stakeholders regarding strategies under review
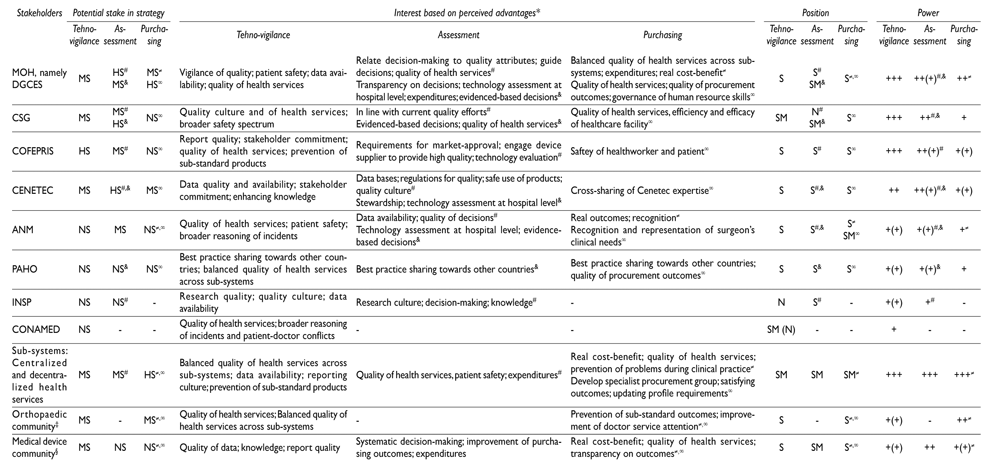
Table IV displays the most relevant stakeholders regarding each of the three strategies under review (‘potential stake’). Further, it summarizes their ‘Interest’ and indicates their ‘Position’ and ‘Power’ towards these strategies.
Table IV. Stakeholder analysis table
* Only few interviewees noted that the required increase in financial and human resources is a critical barrier
‡ Orthopaedic association and orthopaedic specialists
§ Medical device industry association and medical device suppliers
# For strategy component ‘quality benchmarks’
& For strategy component ‘technology guidance’
≠ For strategy component ‘Integral services’
∞ For strategy component ‘procurement agent’s competencies’
+++ High ++ Moderate + Low
HS Important stakeholder for carrying the discussed strategy forward MS Being considered as stakeholder moderately supporting the strategy NS Stakeholder not important for carrying the discussed strategy forward
S: support
SM: support moderately
N: neutral
OM: oppose moderately
O: oppose
Findings regarding strategy for improving ‘Regulation’
The analysis identifies Cofepris as a stakeholder with high potential stake to carrying technovigilance strengthening forward. Its ‘interest’ is relatively focused on the following advantages that this strategy would imply for their own organisation: improving report quality of e.g. incidents; achieving stakeholder support; improving quality of health services; preventing sub-standard products. Its ‘position’ was categorized as supporter of this strategy. The ‘power’ of Cofepris is being considered by different stakeholders as high, because technovigilance is one of its responsibilities.
[…] improving the quality of reports and the culture of reporting. Reports are essential and often the commitment of the stakeholders is missing […] [employee of Cofepris in a leading position of health technologies].
Cofepris is a critical organization within the Mexican health system and holds the overall responsibility for technovigilance. Other relevant stakeholders considered being moderately supporting the strategy but to be involved in one way are: MOH (in general, but particularly DGCES and Cenetec); the ‘sub-systems’ (different actors in charge of health service regulation, financing and provision); orthopaedic community; and medical device community. Their ‘interest’ is relatively focused on ‘access to data’ such as data quality and availability; stakeholder support; enhancing knowledge. Their ‘position’ was categorized between supporter and moderate supporter, and the level of their potential influence ranged from moderate to low.
Few stakeholders mentioned that the requirement catalogue for hospital certification, which is not compulsory, should include as well technovigilance as a component. In Mexico, hospital certification is aligned to the Joint Commission requirements and overseen by CSG.24
Findings regarding strategy for improving ‘Assessment’
The analysis identifies DGCES, CSG, and Cenetec as stakeholders with high potential stake to carrying forward quality attributes of medical device assessments. Their ‘interest’ is focused on the following advantages that this strategy would imply for their own organizations: relating decision-making to quality attributes of medical devices; guiding decisions on eligibility of medical devices; improving quality of health services; generating transparency on decisions; introducing technology assessment at hospital level; controlling expenditures; being in line with current quality efforts; improving data bases; creating regulations for determining quality of medical devices; assuring safe use of products; strengthening decisions about medical device eligibility. Their ‘position’ was categorized as supporter of this strategy. The ‘power’ of these three stakeholders was being considered as moderate, because clinical long-term aspects of medical devices as dynamic quality attribute haven’t been in the focus of any of these stakeholders so far.
It is essential because it represents measuring results […] it will help to know prior to purchasing a medical device its quality […] [Director of a MOH department].
Other relevant stakeholders considered being moderately supporting the strategy but to be involved in one way are Cofepris, National Academy of Health (ANM), ‘sub-systems’, and orthopaedic community (e.g. orthopaedic specialists and orthopaedic associations). Their ‘interest’ is focused on the following advantages that this strategy would imply for their own organisations: promoting transparency and evidence-based decisions. Their ‘position’ was categorized between supporter and moderate supporter of stronger quality attributes of assessments, and the level of their potential influence low to high. Overall, this would positively influence the decision-making process regarding decision-making attributes.
This would help to fight less for the lowest price and more for the quality […] [Director of medical device supplier].
Few stakeholders mentioned that such strategy requires an increase in human resources but that recent budget cuts may currently not allow allocation of required financial resources to pursue such strategy.
Findings regarding strategy for improving ‘Management’
The analysis identifies DGCES and ‘sub-systems’ stakeholders with high potential stake to carrying forward an orthopaedic specific purchasing strategy. The ‘interest’ of these two actors is focused on the following advantages that this strategy would imply for their own organisations: achieving similar level of quality of health services across ‘sub-systems’; improving the management of financial resources; achieving cost-benefit; improving quality of health services; improving quality of procurement outcomes; enhancing human resource skills; preventing problems during clinical practice; developing procurement specialists based on medical speciality for high-risk class medical devices; satisfying outcomes; updating profile requirements of procurement agents.
[…] the current situation leads to costs to solve failures caused by inferior quality […] [Ex-director of orthopaedic services at social security institute].
Their ‘position’ was categorized as supporter of this strategy. The ‘power’ of DGCES is being considered as moderate and that of social security institutes (‘sub-systems’) as high.
Other relevant stakeholders considered being moderately supporting the strategy, but to be involved in one way are Cenetec and the orthopaedic community. Their ‘interest’ was focused on the following advantages that this strategy would imply: cross sharing expertise between involved stakeholders, and prevention of sub-standard outcomes. Their ‘position’ was categorized as supporter, and the level of their potential influence low to high. Overall, this would positively influence the decision-making process regarding procurement outcomes.
[…] the procurement agent mentality is still focused on prices [Director of medical device supplier].
Few stakeholders mentioned that the role played by DGCES should be strengthened to enhance the competencies of procurement agents and to realize subsequent effects of improved outcomes of purchasing strategies such as Servicio Integral, which encompasses a range of disposable and non-disposable medical products used for surgery and merges them into one supplier service.
Discussion
The discussed strategies to improve the MDLC were largely perceived to be within the competence of central government (CSG) and MOH agencies: Cofepris, DGCES, and Cenetec. These are the different interest groups that have started looking deeper into needs that this study focuses on; however, in Mexico as in other countries the discussion about challenges or weaknesses regarding the MDLC areas develops slowly.7,12,25 For instance, at the government level the MOH is developing a new policy that aims to strengthen technovigilance across all ‘sub-systems’ of the health-system. One aspect of this policy is to integrate technovigilance into the requirement catalogue for hospital accreditation. Further, the participation of the private sector (industrial-commercial sector) in the policy-making increases and will contribute to enrich policy discussion by their interests. At the same time the participation of the public and professional associations in decision-making remains limited.13,14,15 These findings coincide largely with the literature that portrays public policy-making in Mexico as a governmental-centred process, largely impermeable to civil society participants, but increasingly influenced by the private sector.26,27We found that ANM can act as visionary for at least orthopaedic associations and therefore play an important role to change this situation in the future.
During this study we only identified significant differences regarding the power of the multiple actors to potentially influence policy (in favour). Central level government agencies were perceived as those with greater power to influence policies. In this sense, we found that the policy environment is in favour of developing such strategies and no one strategy seems to be preferred over the other. This leads to the impression that they are all politically feasible. However, organisational culture towards reform and leadership is a barrier. This came up in some of the interviews and was referred to the relation of national government offices and social security institutions regarding reforms or guidelines, for example, and how the different social security institutions adapt them. For instance, the ‘sub-systems’ tend to adapt the national standard list to their needs. Further, any strategy that intends to change processes or practices of the MDLC functions requires being included into the political agenda at federal level.
The discussed strategies are a matter of multiple actors and interviewees emphasized the role played by the social security institutions regarding the successful realization of any changes to processes and practices in order to improve outputs and outcomes of the MDLC areas.
Overall, we found that improving the MDLC is politically feasible, and may positively influence the health system performance regarding two central aspects. First, it may increase quality of care e.g. at the level of health care professionals because their clinical practice will be less affected by sub-standard medical devices and patients will benefit from orthopaedic medical devices that create less burden of revision. Second, it may support the health system’s efficiency by post-market surveillance activities that effectively identify medical devices of sub-standard clinical long-term performance, and improve decision-making through improved technology assessments.
OECD recommended specifically in its 2016 Mexico’s health systems report to improve technology assessment and regulation of medical devices, and strengthening the role of Cenetec.7 We found that good progress has been made in the authorisation and safety of new technologies through Cofepris. Still, however, not enough is known about the quality and outcomes achieved by the multiple social security institutes. A national and comprehensive approach to collect data of the quality of care remains lacking.
At the level of medical device assessment, Cenetec has the potential to be strengthened in its role and take on more extensive responsibilities in e.g. producing Health Technology Assessments (HTAs). Analyses should not just be applied to new treatments but to existing ones as well, to encourage value for money across the system. Rather than just focussing on services for the uninsured provided by the MOH health services, Cenetec’s remit should expand to cover the social security institutes as well. The expansion of Cenetec’s role will require increased investment, and modification of its legal status may also be necessary. Currently, it operates as a subsidiary unit within the MOH and is limited in its ability to contract with external bodies. Re-establishing CENETEC as an independent and decentralized office would solve this issue.
Limitations
This study has several limitations. We used the stakeholder analysis as foresight, which deals with uncertainty but helps as well to probe system boundaries. Additionally, the way in which we conducted and interpreted the analysis is not value free and potentially may have resulted in some bias. The stakeholder analysis did not reveal any group that presented itself as opposed to any of the three strategies. In-depth analysis of each strategy is necessary to assess direct or indirect improvements of cost-benefit aspects of health technologies, patient safety, workforce, quality of health care and performance of organisational processes that aim at discussing them in greater detail in the context of financial and human resources of the identified ‘main stakeholders’ for each strategy.
Conclusions
This study used a stakeholder analysis as foresight to better understand positions of different groups and institutions to changes to current processes and practices of the MDLC for medical devices. Only some of the findings that our research has produced have been discussed in the literature before. This research is novel in terms of its specific focus on key MDLC functions and on orthopaedic medical devices. Further, it was timely because some of the presented themes are currently undergoing policy discussion in Mexico.
The MDLC system in Mexico is not coherently outlined and set-up across the regulatory, the assessment, and the management domains of orthopaedic medical devices. To date, policy makers have endeavoured to advance the regulation, assessment, and management of medical devices. However, needs for improvement are rarely analysed in a broad way, even though these functions contribute importantly to the successful implementation of health technology policies.
The coordination of changes among stakeholders is complex in Mexico due to the longstanding distribution of roles and responsibilities across multiple organisations. The fragmentation of responsibilities of the MDLC areas, which is underpinned by the health system structure, has recently received more attention from different stakeholders and is subject to the current policy discussion. The suggested changes of current processes and practices of the regulation, assessment, and management can improve outputs and outcomes of these functions and positively influence the quality of health care and health system’s effectiveness.
Furthermore, within the ‘sub-systems’ a similar fragmentation of the responsibilities of the MDLC is replicated. For example, within IMSS there have their own offices that deal with MDLC responsibilities independently from the MHO agencies.
In Mexico, discussion and proposals by interest groups are slowly gaining momentum on how to improve the regulation, assessment, and management of medical devices. We have the following recommendations to the Mexican policy makers and other stakeholders related to the MDLC: (i) initiating a working group at national level that aims at refining the discussed strategies, (ii) enhancing the communication between different interest groups as identified in this analysis at the meso and macro level, (iii) fostering the role played by ANM and its relation to professional associations.











 text new page (beta)
text new page (beta)