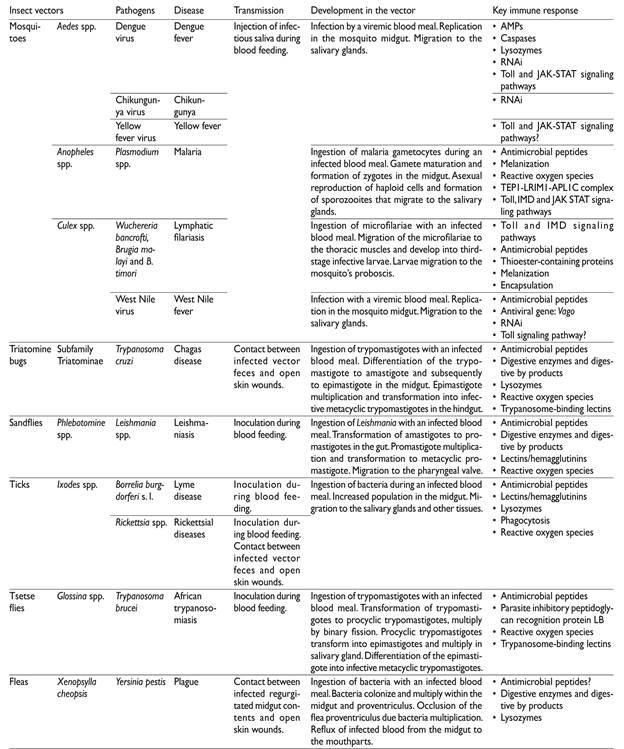
Introduction
Vector-borne diseases (VBD) cause more than one million deaths every year and represent over 17% of all infectious diseases.1 For viral infections, only symptomatic treatment is available and some drugs are effective to treat malaria, but drug resistance is increasing and no effective vaccines are available.2 The traditional chemical control of insect vectors faces insecticide resistance and high adaptability of the vectors to different climatic and environmental conditions.3,4 The recent worldwide dispersion of Zika5 and Chikungunya6 highlight the inefficiency of current control strategies. New molecular control strategies aimed at blocking pathogen transmission have been proposed, but a better understanding of pathogen-vector interactions is required.7
We conducted a search in PubMed, Science Direct, and Google Scholar databases for published studies concerning the interaction of the etiological agents of dengue, malaria, and Chagas disease with their respective insect vectors. We compiled a collection of articles from which we selected studies focused on the pathogen molecules involved in the insect invasion, their consequential immune responses, and the current knowledge of control strategies based in the pathogen transmission-blocking. We discuss here the most promising molecule candidates on the base of these interactions, using three examples of epidemiological relevance: Plasmodium - the causative agent of malaria - transmitted by Anopheles mosquitoes, Trypanosoma cruzi - the causative agent of Chagas disease - transmitted by reduviid bugs and Dengue virus [DENV] - the causative agent of dengue fever - transmitted by Aedes mosquitoes.8,9,10
All vector-borne pathogens ought to invade, multiply and produce infective forms that reach the organ that delivers them to the vertebrate hosts. Pathogens development is intrinsically associated with the insect vectors’ need of blood for growing, molting and egg production. Pathogens ingested with a blood meal, after completion of their life cycle, are transmitted in subsequent blood meals (figure 1, table I) or in the feces.
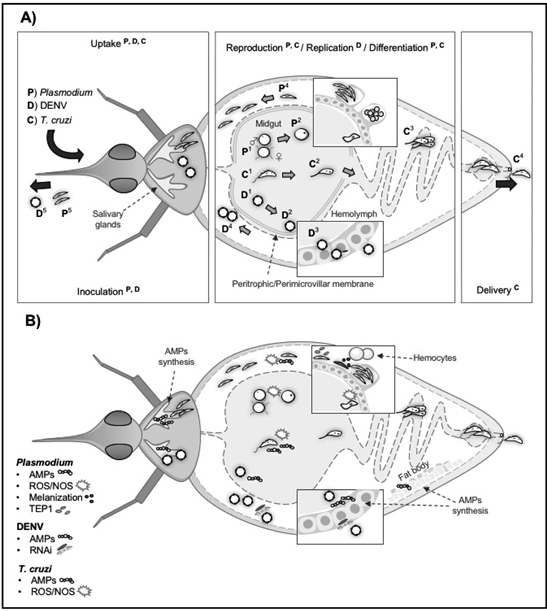
A) Development of Plasmodium, Dengue virus and Trypanosoma cruzi in their insect vectors P) Plasmodium: mosquitoes ingest gametocytes that transform into male and female gametes (P1). Gametes fuse and produce zygotes (P2). Zygotes develop into motile ookinetes that cross the perithophic matrix, invade the midgut and develop into oocysts, forming thousands of sporozoites (P3). Sporozoites released in the hemolymph invade the salivary glands (P4) where they are delivered to human hosts (P5). D) Dengue viruses ingested by the mosquito during a blood meal (D1) infect the midgut, produce viral particles (D2 and D3), that are released in the hemolymph (D4), and reach the salivary glands where they will be injected to new human hosts (D5). C) Triatomines ingest trypomastigotes (C1), they differentiate into epimastigotes (C2), and multiply in the midgut (C3). After transformation into epimastigotes, they multiply and differentiate into infective trypomastigotes in the hindgut where they are excreted with the feces (C4).
B) Immune responses raised in vectors against Plasmodium, DENV and T. cruzi Immune responses in mosquito and triatomine vectors include AMPs synthesized by hemocytes, the fat body, the midgut epithelium, and other tissues to combat DENV, Plasmodium, and T. cruzi infection. ROS/NOS are produced in both the mosquito midgut and salivary glands, which affect the Plasmodium ookinete and sporozoite invasion, respectively. These molecules are also produced in the midgut of triatomines infected with T. cruzi. Mosquito responses against parasites include melanization in the mosquito midgut to inhibit Plasmodium invasion and differentiation, and the expression of complement-like molecules TEP1 that block Plasmodium ookinete invasion and differentiation into oocysts. The main immune response against DENV is mediated by RNAi mechanism to inhibit replication in midgut and other mosquito´stissues
Figure 1 Pathogen development and induced immune responses in insect vectors
Vectors oppose microorganism with structural barriers - such as the peritrophic matrix formed in mosquitoes’ midgut 11 and the perimicrovillar membrane formed in triatomines after a blood meal.12 Constitutive prophenol oxidase cascades (PPO) leading to melanization, and the induction of reactive oxygen species (ROS) are the next line of defense and are active in the insect midgut lumen and the hemolymph that fills the haemocel cavity surrounding the insect organs.13 Insects lack the components of adaptive immunity, but possess sophisticated innate immunity responses.13 These responses are induced and active, since pathogens are detected in the midgut lumen, but exert their main activity when pathogens reach the hemolymph.13 In the haemocel, the fat body liberates lytic anti-microbial peptides (AMPs) and specialized cells in the hemolymph (hemocytes) participate in the production of PPO and ROS, as well AMPs.13 Three hemocyte types have been described in mosquitoes; plasmatocytes are involved in phagocytic removal and encapsulation of large particles whilst oxidation reactions and intermediary melanization molecules are mediated by oenocytoids.14 In triatomines seven types of hemocytes have been described, but their functions are not yet fully elucidated.15 Detection of invaders is mediated by pathogen pattern recognition receptors (PRRs) that bind to conserved motifs on microorganisms.13 This recognition induces proteolytic cascades that activate the main signal pathways (IMD, Toll, and JAK-STAT) which culminate in the translocation of transcription activators (Relish, Dorsal, and STAT) to the nucleus and their binding to gene promoters of AMPs and other effector molecules.13
Discussion
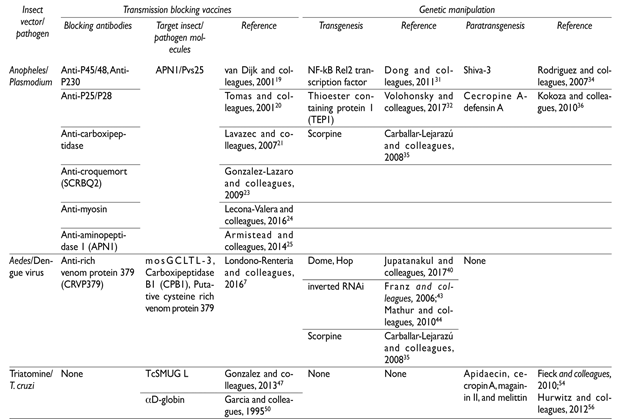
Malaria parasites. In the blood meal bolus, parasites encounter a hostile environment composed by a complex microbiota and the insect digestive enzymes.16 The bacterial population increases hundreds of times and may stimulate the mosquito’s immune response, which includes the production of AMP, ROS and nitric oxide (NO).16Plasmodium gametocytes transform to gametes and fertilization produces mobile ookinetes that interact with the insect midgut molecules, invade, and establish the infection on the midgut outer surface17 (figure 1). The interacting parasite and midgut molecules are the basis for transmission blocking vaccines (TBV), which aim at inducing host antibodies against molecules critical for parasite development and vector-parasite interactions. These antibodies would be ingested with the infected blood meal and interrupt the infection of mosquitoes.18 Candidate molecules include the surface gamete proteins P45/48, and P230 that participate in parasite fertilization19 and the ookinete surface family of proteins P25-P28 that participate in midgut invasion.20 A vaccine against Pvs25, which blocks P. vivax, is currently the leading molecule for a TBV. Candidate midgut molecules from Anopheles include carboxipeptidase (CPB), whose activity is triggered by P. falciparum.21 Other candidates include calreticulin that binds to Pvs25,22 the transmembrane protein Croquemort SCRBQ2,23 myosin,24 and aminopeptidase 1 (APN1);25 all these interact with surface parasite ligands. APN1 is highly immunogenic and conserved among anophelines, making it possible that vaccines prepared with this antigen may be active against all human malaria vectors. However, not TBV completely blocks transmission and as they do not directly protect humans, their use in public health programs is still controversial.
Evidence of the participation of the IMD pathway in the mosquito immune response to Plasmodium is supported by the prevention of the parasite development after silencing its negative regulator Caspar.26 Toll mediates the production of AMPs like attacin, cecropin, gambicin, and other defensins.27 Lysozymes are expressed in lower quantities than AMP, but they activate the phenol oxidase (PO) cascade and some exhibit anti-Plasmodium activity.28 The thioester containing protein (TEP1) is part of the complement-like mosquito system and part of the main system limiting Plasmodium infection. The midgut lesion produced by invading ookinetes, results in nitrosilation of the midgut outer surface, attracting and inducing apoptosis of hemocytes. These release microvesicles with, yet unknown, components that promote the activation of TEP1. TEP1 bound to the parasites surface participate in the parasite lysis.29 TEP1 also facilitates the elimination of many sporozoites in the hemolymph (figure 1) by granulocytes, which also participate in their melanization.30
Attempts to increase mosquito resistance to Plasmodium by inducing the overexpression of immune molecules have shown variable success. The induction of NF-kB Rel2 transcription factor (IMD pathway) in midgut and fat body of An. stephensi resulted in an incremented but not complete resistance to Plasmodium infection.31 Also, transgenic mosquitoes overexpressing TEP1 had reduced parasite numbers.32 A memory-like response phenomenon (reduction in the intensity of infection after a previous infection) has been described in anophelines re-exposed to Plasmodium.33 However, although this opens the possibility for transgenic construction of resistant mosquitoes, no specific mechanisms and molecules have been identified.
Although no direct effect of induced AMP on parasites has been documented, a synthetic cecropin-like peptide (Shiva 3) proved to be toxic to the sexual forms of P. berghei.34 Meanwhile, transgenic mosquitoes expressing scorpine, a cecropine-defensin hybrid were less susceptible to this parasite.35 These studies indicate the need for improving the efficacy of the effector molecule expression; for instance, the simultaneous expression of cecropin and defensin A completely blocked infection.36
Dengue. From an infected blood meal, DENV invade and multiply within the mosquito midgut epithelial cells, to later disseminate to other organs, reaching the salivary glands, from where they are inoculated to new human hosts in subsequent blood meals37 (figure 1). The virus envelope protein E (Ep) (antigenically different in the four DENV serotypes) interacts with several epithelium surface molecules. Three midgut molecules whose expression increases with the blood meal interact with Ep and are candidates of TBV;7 C-type lectins as mosGCLTL-3, carboxipeptidase B1 (CPB1) and the putative cysteine rich venom protein (CRVP379). CRVP379 interacts with prohibitin, a putative receptor for DENV, and antibodies against CRVP379 or silencing its coding gene blocks the mosquito infection. However, anti-dengue TBV encounters the same shortcoming as those against malaria; furthermore, these vaccines ought to be effective against the four DENV serotypes.
Several molecules are candidates for genetic manipulation of Aedes mosquitos. Although NO expressed in the mosquito midgut could inhibit DENV replication,38 this is insufficient to impede infection and no attempts for engineering mosquitos to increase its production have been made. Toll activation by DENV culminate in defensins and cecropine synthesis,39 but this is insufficient to control infection. On the other hand, recombinant scorpine inhibits DENV-2 replication, thus making it a candidate for transgenic resistant mosquitoes.35 The inhibition of JAK-STAT results in increased DENV replication; and genetically modified Ae. aegypti overexpressing Dome or Hop, upon blood feeding, activate JAK/STAT in the fat body and salivary glands inhibiting DENV infection. However, this inhibition is far from complete.40
RNA interference (RNAi) gene silencing is an important antiviral mechanism in Ae. aegypti.41 Silencing components of the RNAi pathway increases DENV replication.42 Consequently, transgenic Ae. aegypti expressing in the midgut and salivary glands inverted RNA coding for a region of the pre-membrane viral protein depicted lower susceptibility to DENV.43,44
Trypanosoma cruzi. Trypomastigotes ingested in the blood meal remain for few days in the anterior part of the insect midgut; most of them transform into epimastigotes, and move to the posterior part of the midgut. The attachment of epimastigotes to the perimicrovillar membrane (PMM) seems to be essential for parasite multiplication. Reaching the rectum, they transform into metacyclic trypomastigotes. These are discharged with the feces, usually during blood feeding.45 Parasite-PMM interactions are mediated through glycoinositol phospholipid molecules on the epimastigote plasma membrane.46 The surface of epimastigotes are covered by mucin-type glycol-conjugates and one of them, TcSMUG L, appears to mediate the interaction of the parasite with the intestinal epithelium, intercepting this interaction has been proposed for transmission blocking strategies,47 a better understanding of the molecules and mechanisms involved in vector-parasite interactions may provide more candidates for TBV.
The increase in bacteria population within the midgut after each blood meal has no effect on the parasites, but Serratia marcescens produces prodigiosin, a pigment with trypanolytic activity.48 Within the blood meal bolus, trypomastigotes agglutinated by lectins successfully develop and are highly infective, while those not agglutinated are lysed.49 The transformation of epimastigotes seems to be mediated by αD-globin, present in hemoglobin. This interacts with an epimastigote surface receptor, stimulates the parasites adenylyl cyclase and initiates their transformation into metacyclic trypomastigotes,50 providing an interesting transmission-blocking candidate based on halting the parasite cycle.
The information on triatomine immune defenses against T. cruzi is scarce; some components of Toll pathway have been identified in R. prolixus, but they lack canonical components of IMD and JAK-STAT.51 In the intestinal track, digestive enzymes have no effect on parasite survival50 and NOS expression does not eliminate infection.52 Defensins in triatomines are mostly involved in the regulation of bacterial symbionts, but it has been suggested a potential function of intestinal defensin 1 in the T. cruzi population control.53 Combination of AMPs from other insects like apidaecin, cecropin A, magainin II, and melittin, had in vitro additive toxicity for T. cruzi.54 These AMPs have been used to transform (paratransgenesis) Rhodococcus rhodnii, a symbiotic actinomycete in the lumen of triatomines.55 Triatomines carrying the transformed bacteria more effectively controlled the parasite infection.56 In this transmission blocking strategy, the parasite-toxic bacteria are transmitted to the offspring via the coprophagic behavior of the immature bug.57
Engineering strategies for genetic transformation. The overall objective of the molecular strategies to control VBD is to re-program vector genomes. The gene constructs generate alterations in the genome (gene additions or deletions) to affect the vector’s ability to transmit pathogens.58 These strategies seek the introduction of heritable modified genes into the genome of wild target vector populations. A shortcoming of these strategies is that methods which modify only one allele (one chain) of the desired gene (e.g. transposon-mediated transformation), would spread the desired trait only to half of the offspring, and it would eventually be eliminated in the wild population. Alternative approaches use endonuclease genes capable of copying themselves to both gene alleles, which are inherited to all offspring, thus spreading more efficiently through a wild population.58 One such method uses CRISPR nuclease Cas9 to cut sequences specified by guiding RNA molecules.59 Endonuclease gene drives spread through populations cutting homologous chromosomes lacking the alteration, inducing the cell to copy the endonuclease and surrounding genes into the chromosome.60
In spite of the extensive advances in identifying key candidate genes for engineering resistant insect vectors, strict methodological controls to maintain the stability of the gene construct in the insect genome and to guarantee that the gene modification will not introduce alterations to the organism as a whole (pleiotropy), or produce secondary undesirable effects on the insect fitness, reproduction or capacity to transmit other pathogens. The efficacy of these strategies to control VBD depends on not yet satisfactory gene drives capable of spreading efficiently through wild populations,61 but that will not spread to non-target species. Safety considerations should guaranty that the gene product will not harm other organisms, including humans.61
Conclusions
Approaches based in the use of antibodies or genetic manipulation against critical molecules provide several candidates for VBD control (table II). These methods are mainly focused on disrupting specific vector-pathogen interactions. Successful transgenic manipulation of mosquitoes has been achieved, but their negative relative fitness in relation to wild populations is an important limitation for their large-scale use. Despite successes of altered vectors symbionts, it remains to be seen if transformed bacteria can replace non-transformed bacteria in natural insect populations. Evidently, these novel approaches involving engineered insects and bacteria raise several ethical, legal and social implications that must be addressed before they are considered as part of integrated VBD control strategies.











 nueva página del texto (beta)
nueva página del texto (beta)