Introduction
Insects play prominent roles in supporting human welfare (as food sources and crop pollinators), a number of hematophagous arthropods transmit several infectious diseases.1 Malaria and various viral diseases transmitted by Anopheles, Aedes, and Culex mosquitoes2 affect about 1000 million people and produce about one million deaths worldwide yearly. No effective vaccines are available and the main control strategies are directed to abate mosquito vector populations and to prevent human-vector contact.3,4,5 Chemical insecticides are the main weapons used to control these vectors, but in insecticide-resistance is an important limitation. In recent decades, the interest in bio-insecticides targeting molecules that disrupt mosquito development,6 has increased.7
Neuropeptides have pleiotropic functions in insects’ physiological processes, including reproduction, development, metabolism, and behavior.8 These could be specific targets for vector control with little environmental hazards.9 Of particular interest are those engaged in mosquito metamorphosis and ecdysis, such as: Crustacean CardioActive Peptide (CCAP), Ecdysis Triggering Hormone (ETH), corazonin and bursicon. CCAP is a cyclic nonapeptide that stimulates heartbeat10 after adult emergence,11 oviduct contraction12 and plays an essential role in ecdysis and eclosion.13 Corazonin is a peptide, whose functions are associated with cardioacceleratory activity,14 diapause, circadian rhythm and induction of melanization,15 and bursicon, a heterodimer required for sclerotization, cuticle tanning and wing expansion after eclosion.16 Prior to ecdysis, corazonin is released into the haemocel and activates the release of pre-ecdysis triggering hormone (PETH) and ETH.17 ETH is a peptide that activates neurons that produce the eclosion hormone (EH), CCAP and bursicon.18 Other important neuropeptides involved in growth and development are the insulin like peptides (ILP´s), orcokinins and allatostatins. ILP´s seem to be involved in development, regulation of carbohydrate and lipid metabolism,19 diapause induction,20 stress resistance,21 regulation of sexual behaviors,22 regulation of life span23 and regulation of sleep.24 In Aedes aegypti, insulin was shown to stimulate ecdysteroid biosynthesis in the ovaries.25 Allatostatins, are potential insect growth regulators, which inhibit the production of juvenile hormone (JH);26 although it appears to have other functions, including neuromodulation, regulation of muscle contraction, and regulation of enzyme biosynthesis.27 Orcokinins are involved in the neuronal regulation of ecdysteroidogenesis28 and circadian locomotor activity29 and the control of vitellogenesis.30
Understanding the expression of these neuropeptides in developmental stages of insect vectors may open opportunities for identifying possible candidate molecules to design species-specific insecticides for biological control.9 In this paper, we examined the expression of eight neuropeptides, three associated to ecdysis, during the ontogeny of the mosquito Anopheles albimanus, one of the main malaria vectors in Mexico and Central America.31
Materials and methods
Biological samples of Anopheles albimanus
Larval, pupae and adults mosquitoes were obtained from the insectary of the Center for Research on Infectious Diseases (CISEI), National Institute of Public Health (Mexico). Larvae were grown under controlled temperature conditions (29-33°C) and humidity (70-80%), with a 12h light/12h dark photoperiod. Larvae were fed with grinded cat chow (Whiskas 0.1876 grams every 24 hours) in trays with 1.5 liters of water at 25-30°C at larval density of 200 each tray. Larval samples were taken every 24 h after egg hatching and adult mosquitoes were collected 24 hours after emergence. Because it was difficult to differentiate between larvae instars by morphology, larvae were measured and defined as first instar, larvae of 2 mm, second instar, larvae of 4 mm, third instar, larvae of 5mm and fourth instar, larvae of 6 mm in length. Groups of 8 larvae, 8 pupae and 8 adult mosquitoes were placed in 500 μl of Trizol (Ambion) and stored at -20°C until use.
Total RNA purification
Total RNA was extracted as previously described.32 Briefly, samples in Trizol were macerated with a biovortex, 10 pulses per 1 minute with a 30 second break. Samples were centrifuged for 5 min at 8 000 x g to remove the debris, the supernatant was added to 100 µl of chloroform (Sigma-aldrich), mixed and centrifuged for 15 min at 10 000 x g at 4°C, the aqueous phase was recovered and 250 µl of cold isopropanol (Sigma-Aldrich) were added, mixed and incubated at -20 °C for 1 hour. Samples were centrifuged at 10 000 x g for 10 min, the pellets were washed with 500 µl of 75% ethanol and centrifuged at 7 000 x g for 5 min; the supernatants were removed and the pellets were-suspended in 20 µl of DEPC-treated water (diethylpyrocarbonate) (Sigma-aldrich). RNA was quantified with Nanodrop and visualized using electrophoresis in agarose gels stained with ethidium bromide, to confirm integrity.
cDNA Synthesis
One µg of total RNA of each sample was treated with DNAse (ThermoScientific® ™ 200 U/µl). cDNA was synthetized, the reaction included: 250 ng oligodT (Thermo Scientific), 5X RT buffer (Promega), 2.5 mM dideoxynucleotide mixture (dNTP’s) (Promega), 10U/µl RNAse Inhibitor (RNAsin) and 5 U/µl reverse transcriptase enzyme (Promega). All samples were adjusted to a final volume of 40 µl of DEPC-treated water and incubated at 42 °C for 90 minutes. The synthesized cDNAs were stored at -70 °C until use.
Quantitative real time PCR (qPCR)
Specific oligonucleotides corresponding to CCAP, corazonin, ETH, ILP2, ILP5, allatostatin-A, orcokinin, and bursicon genes were designed using the Oligo 3.1 Program Analyzer (http://www.idtdna.com/site), based on the nucleotide sequences identified in the genome (https://www.vectorbase.org/organisms/anopheles-albimanus) and a brain transcriptome of An. albimanus.33 All quantitative RT-PCR assays were performed in duplicate with three biological replicates for each experiment. As an endogenous control, an oligonucleotide corresponding to the actin gene of An. albimanus was used. Real-time PCR assays were performed on an Applied Biosystems (ABI) Step One Plus Real-Time PCR System using the reaction mixture Master Mix (2X) SYBRGreen universal qPCR (ThermoScientific). The PCR program was: 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 seconds and 64°C for 1 min, then 95 °C for 15 sec, 64 °C for 15 sec, and 95 °C for 15 sec, for one cycle. The specificity of the SYBR green PCR signal was confirmed by a melting curve analysis and 1.2% agarose gel electrophoresis. The expression of each neuropeptide in different mosquito development stages was compared using the 2-DDCt method34 and calculated as fold increase comparing the expression of each neuropeptide in each stage against actin and tested with one-way ANOVA followed by the Kruskal-Wallis post-test (α= 0.05). Finally, graphical data were shown as percentage of expression by stage.
Results
The transcription pattern of CCAP, corazonin, ETH, ILP2, ILP5, bursicon, allatostatin-A and orcokinin neuropeptides in larvae from first to fourth instar, pupa black and mosquito was measured.
Neuropeptide expression associated to Ecdysis
The transcription pattern of CCAP, corazonin and ETH transcripts varied among the different mosquito developmental stages. The highest expression of the three neuropeptide transcripts was observed in fourth instar larvae (70.8, 76.5 and 60.2%, respectively) (figure 1). CCAP transcription increased from first to third instar larvae (1.81 to 21.3%) but, in pupae and adult mosquitoes, transcription levels decreased to 4 and 0.3%, respectively (p< 0.001) (figure 1A). The corazonin transcript had similar expression profile, in first to third instar larvae, transcription levels were 4 to 1.6%, respectively but, in pupae and adult mosquitoes, transcription levels decreased to 0.1 and 2.5%, respectively ( < 0.001) (figure 1B). The transcription of ETH increased from first to third instar larvae (0.1 to 13.7%, respectively), in pupae the transcription decreased to 1.5% but in adult mosquitoes, it increased to 14.5% (p< 0.001) (figure 1C). A schematic representation of the neuropeptide variation among mosquito stages is shown in figure 1D.
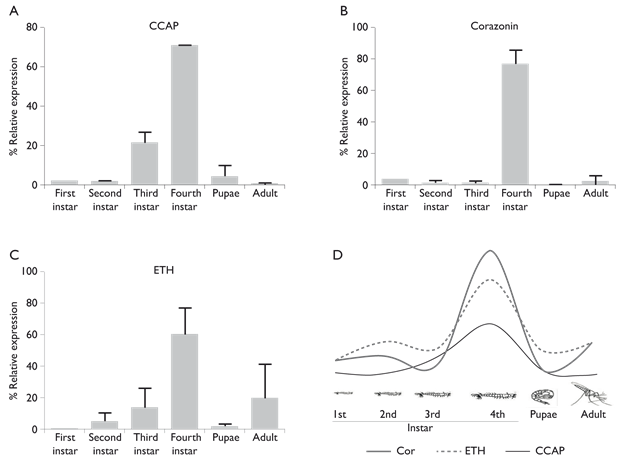
CCAP: Crustacean Cardio Active Peptide
ETH: Ecdysis Triggering Hormone
qPCR: Quantitative polymerase chain reaction
Figure 1 Variation of expression levels of CCAP, corazonin and ETH in different developmental stages of Anopheles albimanus measured by qPCR. The three neuropeptides had more 60% of expression. A) CCAP (70.8%), B) corazonin (76.5%) and C) ETH (60.2%). D) In fourth instar larvae
ILP 2, ILP5, bursicon, allatostatin-A and orcokinin
The transcription of ILP 2 was a high in most stages: 47% (first instar), 45.5% (second instar), 45% (third instar), 64.5% (pupae) and 61.6% (mosquito) (p< 0.001), the lowest transcription level of ILP2 was in fourth instar larvae (1.2%) (figure 2). Allatostatin-A and bursicon transcription occurred in fourth instar larvae. ILP5 expression decreased as the mosquito development progressed: 9, 6.5, 1.4, 0.2 and 0.1% in first, second, third and fourth instar larvae and pupae, respectively; but increased again in adult mosquitoes (6.5%) to similar values to that of fourth instar larvae (p< 0.001) (figure 2). The transcription of bursicon increased from first instar (11.2%) to third instar larvae (34.2%). Its transcription decreased in fourth instar larvae (1.5%), increased again in pupae (27.6%), but in adult mosquitoes the transcript expression was lower (18.3%) (p< 0.001) (figure 2). No detectable transcription variations during the mosquito development were observed for orcokinin and allatostatin-A. The transcription pattern of orcokinin was 15.5, 0.7, 5.3, 2, 0.4, and 0.1% in first, second, third and fourth instar larvae, pupae and adult mosquitoes, respectively. The transcription pattern of allatostatin-A was 2.3, 4.94, 2.9, 1.1, 4.2, and 5.8% in first, second, third and fourth instar larvae, pupae and adult mosquitoes, respectively (figure 2).

ILP: insuline like peptides
Figure 2 Variation of expression levels of orcokinin, ILP5, ILP2, allatostatin and bursicon in different developmental stages of Anopheles albimanus measured by qPCR. ILP2 showed higher expression in different stages (first, second, third and fourth larvae instar, pupae and mosquitoes)
Discussion
In this paper we analyzed the transcription pattern of eight neuropeptides, during the development of An. albimanus mosquitoes. Mosquitoes have a well-characterized development process from egg hatching to adults. Neuropeptides are essential during their ontogeny and together with their receptors, have been proposed as candidates for the generation of larvicides that disrupt their development. Currently, a number of peptidomimetics with c-terminal motifs PRXamide35 and FGLamide36 that confer an increase in their stability and bioavailability have been studied for their use in pest control. For example, a β-amino acid pyrokinin analog (Ac-Y[β3F]TPRLamide) accelerates an irregular pupation in the flesh fly Sarcophaga bullata.37 A PK/PBAN antagonist lead (RYF[dF]PRLa) was reported as a potent inhibitor of a sex pheromone biosynthesis in Heliothis peltigera.38 Using a diapause hormone (DH) agonistic and/or antagonistic peptidomimetics the pupal diapause of Helicoverpa zea was disrupted39 and analogs of the pyrokinin, insect kinin and insect tachykinin families showed aphicidal activity, that was better than some commercially available aphicides.40 In mosquitos, only CCAP,3 FMRFamide-like peptides (FLP),41 corazonin,42 bursicon,43 short neuropeptide F (sNPF),44 AKHI and AKHII45 have been studied during the ontogeny of An. gambiae.
This is first report of the expression of neuropeptides associated to several functions during ontogeny of An. albimanus. The high transcription of CCAP, ETH and corazonin in fourth instar larvae suggests that these neuropeptides associated with ecdysis begin their expression in this instar, and that corazonin and CCAP are required to activate ETH and start the ecdysis. Unlike these results, CCAP and corazonin are expressed in different developmental stages of An. gambiae,31 Specifically, CCAP mRNA levels increased in second instar larvae, decreased in third instar larvae and then increase to maximum levels in callow pupa stage.3 While corazonin showed a similar transcription profile in larvae of An. gambiae and An. albimanus the increase to maximum levels in An. gambiae occurred in young adults.42 We speculate that the different expression pattern of CCAP, corazonin and ETH during the ontogeny of An. albimanus and An. gambiae is due to factors associated to development, such as: temperature, food and stress; but also to different environmental adaptations of both mosquito species such as resistance to insecticides, colonization of areas with higher altitudes to 1 500 masl. The transcription expression profile of neuropeptides that we report here and other characteristics such as the short peptide sequence (CCAP, nine amino acid residues, corazonin, eleven amino acid residues and ETH, seventeen amino acid residues), the quaternary structure, the potential generation of peptidomimetics and modifications in the amino acids residues to obtain synthetic structures with desired molecular properties (increased biostability, specificity, and permeability) provide advantages to these neuropeptides for further functional studies in An. albimanus.
Orcokinin and ILP5 showed the highest transcription levels in first instar larvae, while ILP2 showed the highest transcription levels in pupae, allatostatin-A in adult mosquitoes and bursicon in third instar. On the other hand, allatostatin-A , bursicon and ILP2 showed lower transcription levels of in fourth instar larvae while orcokinin in adult mosquitoes and ILP5 in pupae. It would be interesting to conduct further functional studies and explore the possibility of blocking the expression of each of these neuropeptides during larval development of mosquito.
Although we explored eight neuropeptides and analyzed only larvae, pupae and 24 hours post-emergence adult stages, it is clear that many of the neuropeptides are expressed in different intensities or are specific to different stages; and it would be interesting to also investigate their expression in eggs and post emergence individuals. We also recognize that other important stages as eggs, brown pupae and adult stages older than 24 hours post emergency require further studies. However, the encouraging results of the present study will hopefully stimulate further studies that will explore neuropeptides as target molecules in larval control of diseases-transmitting insects. Undoubtedly detailed studies are needed to identify specific candidates to fight anophelines mosquitoes, vectors of malaria, a disease that remains a public health problem worldwide.











 nueva página del texto (beta)
nueva página del texto (beta)


