Introduction
Dengue fever is the viral illness transmitted by mosquitoes of greatest dispersion in the world in the last 50 years. Mexico is the 4th most endemic country. The State of Morelos, reported incidences of 172.82 and 42.82 cases per 100 000 inhabitants in 2013 and 2014, respectively. Both, Aedes aegypti and Ae. albopictus mosquito vectors have been collected and the four dengue virus (DENV) (DENV-1, DENV-2, DENV-3 and DENV-4) serotypes had circulated in the State.
DENV transmission is in part determined by the circulating DENV serotypes along with the population immunity against these serotypes; however, these parameters are difficult to include in routine surveillance. On the other hand a complex interaction of diverse and variable biological, social, economic, demographic and environmental factors may participate in modulating the risk of infection. Weather conditions, like temperature are directly associated with mosquito breeding and survival.1,2 These conditions, in a dynamics of seasonal and spatial interactions, influence the vectors’ capacity to transmit the viruses.3,4 Seasonal climate conditions may also introduce variability in mosquito breeding.2,5
We present herein the results of an analysis of the association of confirmed dengue case incidence and Aedes spp. abundance, estimated by a routine systematic monitoring of mosquito eggs with ovitraps, as well as the effect of climatological and geographical variables, in an endemic region ecosystem along the Apatlaco River basin in the State of Morelos, Mexico.6,7 The results indicate seasonal variations of the capacity of entomological surveillance to foreseen increments in dengue cases.
Materials and methods
Study area. An observational and ecological study was carried out during the period 2010 to 2014. The study area is located in an ecozone delimited to the North by the southern edge of Meseta Central and to the South by the basin of the Balsas River. In this area the Apatlaco River, runs with a marked North-South altitudinal gradient from 1 400 to 1 000 masl. The area, with 22.07% of the population and 18.17% of the surface of the State of Morelos, is endemic to dengue. The area was subdivided in a Northern subzone bordering with Cuernavaca (state capital), comprising the municipalities of Temixco (1 280 masl) and Emiliano Zapata (1 250 masl), and a Southern subzone bordering with the State of Guerrero, comprising the municipalities of Jojutla (860 masl), Zacatepec (910 masl), Puente de Ixtla (906 masl) and Tlaltizapan (940 masl) (table I). A total of 13 boroughs were selected, where dengue cases were detected during the previous five years (2010-2014) (figure 1 8).
Table I Population, marginalization and incidence of new cases of dengue fever by municipality, area and seasons. 2010-2014
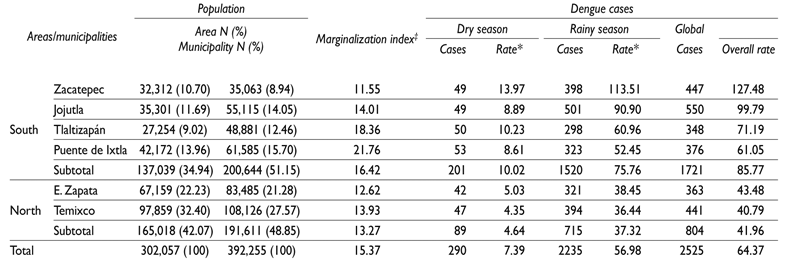
* Rate per 10,000. Censo de población de INEGI, 2010
‡ Source: reference 8
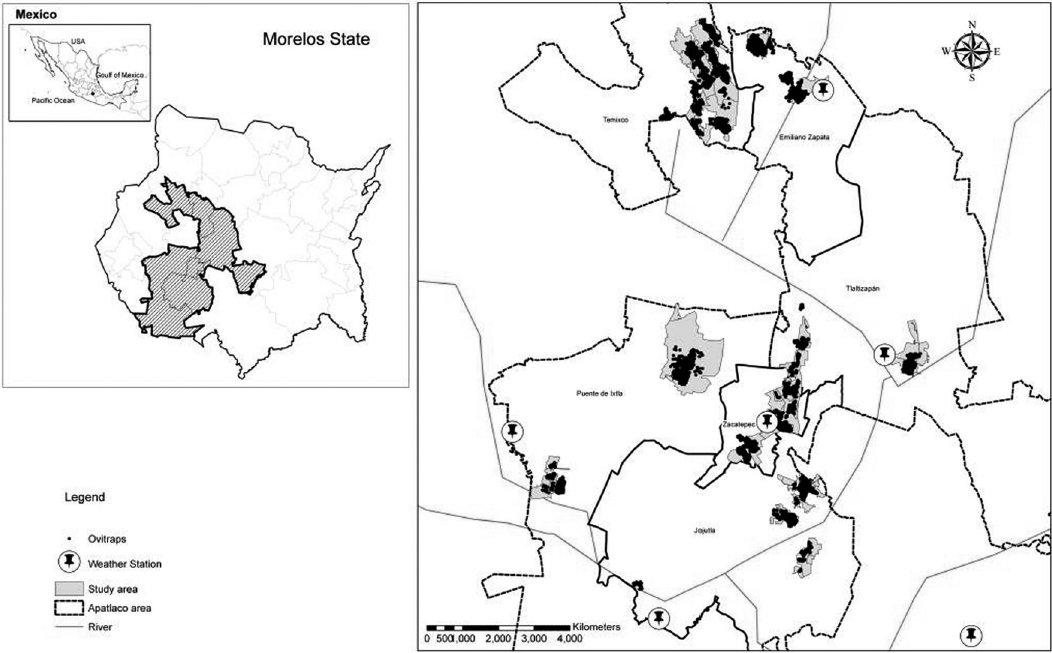
Figure 1 Study areas within the six municipalities in the Apatlaco River sub-basin (2010-2014). Within each municipality, shaded areas indicate residency of dengue fever cases included in the analysis and dark points the distribution of ovitraps. Pins indicate the location of the meteorological stations
Climatological variables. In the study area, the climate is warm with annual average temperatures of 22°C; the rainy season extends from (June to October) and the dry season from (November to May). The weekly report of temperature (average, minimum and maximum), rainfall (mm accumulated) and relative humidity (RH, percentage) were obtained from the Mexican Institute Water Technology (IMTA, acronym in Spanish).9 Data from six weather monitoring stations located within and near the municipalities of study (Tlaltizapan, INIFAP-Zacatepec, Puente de Ixtla, Emiliano Zapata, Tehuixtla-Jojutla and Tlaquitenango) (figure 1) were used. Each station has a, R sensors for temperature and relative humidity, pyrometer, and pluviometer measuring rainfall every 15 minutes. Weekly data from 2012 to 2014 (from Sunday 00.00 hours to Saturday 23:45) were captured in Excel 2013 Microsoft including temperature (average, minimum and maximum degree Celsius), pluvial precipitation (accumulated mm) and RH (percentage of present atmospheric water vapor).
Mosquito monitoring. Mosquito ovitraps were used as part of the routine State Control Program entomological surveillance. These were made of dark plastic one-liter containers with 500 mL of clean water. A fabric of 12 cm width 27 cm long, half submerged in the water (6 cm) capture the female Aedes oviposited eggs. The numbers of deployed mosquito ovitraps varied during the study period, but they covered areas where at least 77% of the population lives (figure 1). Mosquito ovitraps were installed outside houses, at the front or in the main courtyard in shaded area; previous acceptance of the inhabitants to participate in the study was obtained. Five ovitraps were installed in each square block (delimited by streets of ~100m), in houses located at the center and in houses at each side of the block.
Ovitraps were examined weekly during the entire study period (2010-2014) by trained technical personnel according to technical guidelines (National and State Mosquito Control Program). Ovitrap monitoring was not carried out in the municipalities of Temixco and E. Zapata during 2010 and 2011; and in Zacatepec during 2011.
Dengue incidence. Registered weekly data of confirmed dengue cases in the areas covered by ovitraps from 2010 to 2014 were provided by the Epidemiological Surveillance System.10,11 In this system, patients with suspected dengue infection are diagnosed using real time RT-PCR and serological IgM and IgG detection anti-dengue anti NS1 by ELISA or viral isolation.12 Patient serum samples are processed by the State Public Health Laboratories.11,12 Laboratory information is integrated into an epidemiological weekly report by sanitary jurisdiction and is linked to the weekly report of the General Direction of epidemiology.13
Statistical analysis. Means and standard errors of weekly confirmed dengue cases that occurred in areas covered by ovitraps in each municipality were registered in Excel sheets (table I). Means of the numbers of ovitraps containing eggs and of the egg numbers per block per week were compared using a t-test .The correlation between weekly climate variables and ovitraps data was estimated using in a multivariate regression model (table II). Additionally, univariate regression models were used to analyze individual variables in relation to egg numbers using Stata 14.
Results
A total of 2525 confirmed dengue cases were detected in the in the study during the study period. A total of 1955 cases (77.4%) occurred in areas covered by ovitraps. This represents 22.28% of cases in the State of Morelos during the same period. The municipal cumulative incidences per 10000 inhabitants were: Zacatepec (127.48), Jojutla (99.79), Tlaltizapan (71.19), Puente de Ixtla (61.05), Emiliano Zapata (43.48) and Temixco (40.79) (table I).
The highest incidence occurred during 2012. Zacatepec with a cumulative incidence of 98.96 Jojutla with 66.67 and Tlaltizapan with 36.41, per 10 000 inhabitants, had the highest incidence during this year. In 2013, higher incidences occurred in Puente de Ixtla with 35.07, Jojutla with 22.50, Emiliano Zapata with 20.12 and Tlaltizapan with 18.41 per 10 000. Trends in mean weekly case incidence were similar in all years and in all municipalities, but the overall incidence was higher in the southern region (µ 33.09, 5.55; 95%CI: 21.95, 44.24) compared to the northern region (µ 15.46, 2.22; 95%CI: 10.99, 19.92) (figure 2). An overall increase (7.38 times) was observed in all municipalities during the rainy seasons, compared to that of the dry seasons.
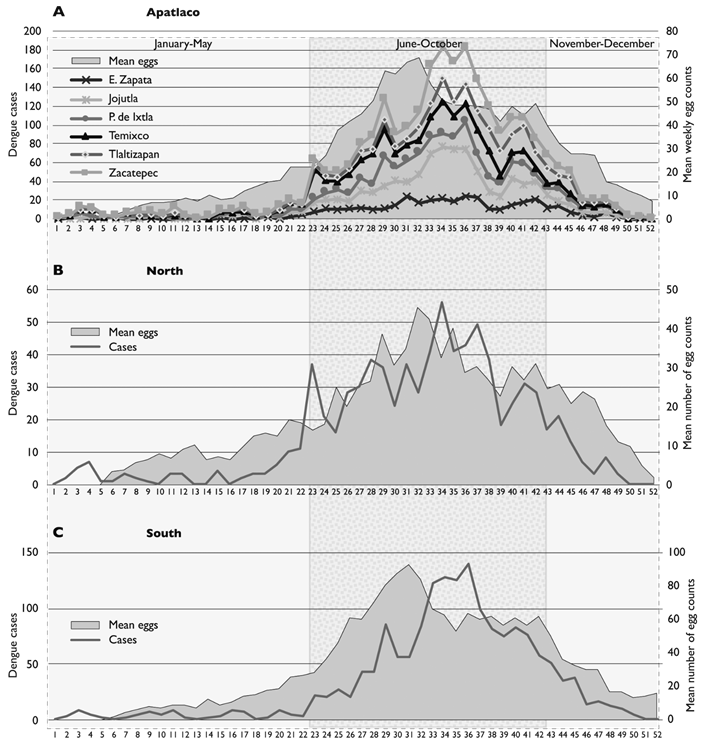
Figure 2 Weekly distribution of cases and average counts of Ae. aegypti eggs in Apatlaco, Morelos, Mexico, 2010-2014. A. weekly mean number of dengue fever cases in each of the six municipalities of the study area; grey area is the weekly mean number of egg counts in the entire study area. B. weekly mean number of dengue fever cases in the northern municipalities; grey area is the weekly mean number of egg counts in the northern area. C. weekly mean number of dengue fever cases in the southern municipalities; grey area is the weekly mean number of egg counts in the southern area. In all graphs the clean background correspond to the dry season and the shaded background to the rainy season periods
An average of 1416 ovitraps was deployed during the study period. A total of 234372 examinations and egg counts were made in the six municipalities, from 2010 to 2014; with an average of 32.83 Aedes eggs (Std. Err.= 0 1489; 95%CI: 32.537, 33.121) and weekly average of 28.33 (Std. Err.= 0 0272021; 95%CI: 28.29112, 28.38775). An upward tendency of the weekly egg number average was observed, from the first weeks of each year, which increased from weeks 23 to 43 (µ 52.34, SE 2.38; 95%CI: 47.35, 57.34), with a bimodal distribution, with a first peak during weeks 29 to 32 and another peak during weeks 40 to 43 (µ 15.78, SE 1.74, 95%CI: 12.22, 1934). The maximum peak of weekly dengue cases occurred during the weeks 34 to 36, three to four weeks after the weekly egg count peak of (weeks 29-32), with another increase of less intensity that coincided with the eggs count increase of weeks 40 to 42 (figure 2).
The municipalities Puente de Ixtla (µ 42.32, SE= 0 437; 95%CI: 41.46, 43.17), Jojutla (39.67, SE= 0.431;95%CI µ: 38.82, 40.52), Zacatepec (µ 39.49, SE= 0.44;95%CI: 38.64, 40.35) and Tlaltizapan (µ 38.73, SE= 0.47;95%CI: 37.79, 39.66) had the highest egg number averages during the study period, in comparison to Emiliano Zapata (µ 22.95, SE= 0.30;95%CI: 22.36, 23.54) and Temixco (µ 24.54, SE= 0.23;95%CI: 24.08, 24.99).
In 2010, Jojutla municipality registered the highest average egg counts (µ 72.05, SE= 4 53; 95%CI: 63,14-80.96), followed by Zacatepec (µ 59.01, SE= 3 54; 95%CI: 52.05-65.97), Tlaltizapan (µ 55.39, SE= 3 66; 95%CI: 48.20-62,58) and Puente de Ixtla µ 54.62, SE= 3 59; 95%CI: 47.58-61.66), being this year (2010) when the highest average eggs count was observed (µ 60.28 , SE= 1 93; 95%CI: 56.49-64.06). In the following three years (2012-2014), the municipalities of Temixco and E. Zapata had lower egg count averages compared to the other four municipalities. This was also true for Zacatepec (µ 37.26, SE= 1 18; 95%CI: 34.92-39.59).
During the study period, the average temperature was 23.62 oC (SE= 0.348; 95%CI: 22.563, 23.962), the average rainfall 13.017 mm (SE= 2.695; 95%CI: 7.606, 18.429) and RH of 57.53% (SE= 2 228; 95%CI: 53.054, 62.004). The annual average temperature remained constant, without significant variations. The largest rainfall precipitation was recorded in 2014 (µ 17.09, SE= 3.77; 95%CI: 9.53, 24.65) and 2010 (µ 17.06, SE= 4.42; 95%CI: 8.17, 25.94); in 2012 the lowest precipitation was recorded (µ 13.50, SE= 2.70; 95%CI: 8.06, 18.93). With significant variations in 2012 (β 3.17, t 11.11; 95%CI: 2.60, 3.74); 2010 (β 0.72, t - 7.09; 95%CI: 0.93, 0.52) and 2011 (β - 0.84, t - 5.39; 95%CI: - 1.15, - 0.52) compared to 2014. The RH had a weekly average of 57.529 % (SE= 2.228; 95%CI: 53.0544, 62.004), without variations during the study period. However a bimodal pattern was observed, with an upward tendency beginning at week 18, increasing at week 23, reaching a peak in week 26; a second rise occurred during week 35 that became more pronounced during week 43. The temperature, expressed as Daily Mean Temperature Range, (DTR) between daily mean minimum of 13.72 and 17.78 oC and maximum between 30.9 and 34.6°C, and it was mainly stable with low fluctuations below 18°C, from the week 23 until week 42. During the dry season (weeks 1 to 22 and 43 to 52), maximum temperatures ranged between 30.12 and 37.98°C, while minimum temperatures ranged between 7.18-17.96°C.
A significant correlation was documented between the weekly Aedes egg counts with DTR (- 0.8955, p < 0.0000), relative humidity (0.8693, p < 0.0000), rainfall (0.6360, p < 0.0000) and the number of confirmed dengue cases (0.8285, p < 0.000). No significant correlation occurred with the average temperature (- 0.0533, p 0.7131). The mean weekly egg counts (WEC) increased with DTR up to 18oC, but they decreased as temperature increased beyond this point (figure 3A). WEC remained similar at RH between 30 and 70% and increased as humidity increased beyond 70% (figure 3B). WEC increased as rainfall increased up to 70mm, but remain unchanged with further increases in rainfall (figure 3C). The main numbers of incident dengue cases increased as the WEC increased up to ~ 60, but no further increases were seen at higher WEC (figure 3D).
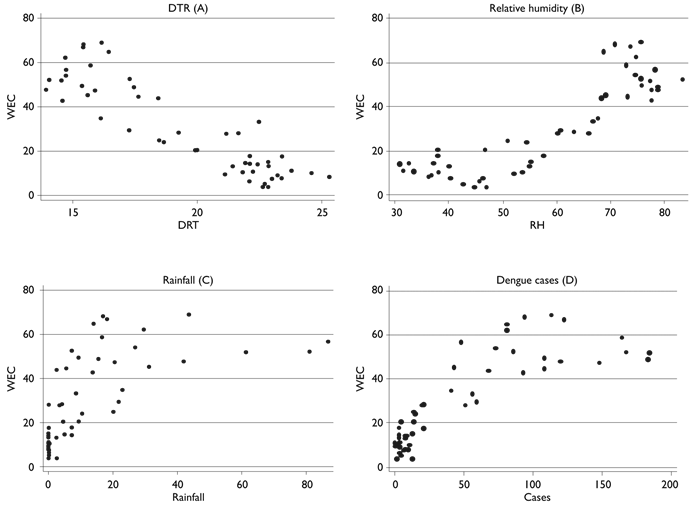
Figure 3 Scatter chart graphs of mean weekly egg counts in Ovitraps (WEC) (dependent variable Y-axis), climate indicators variation and dengue confirmed cases (independent variables, X-axis). Morelos, México, 2010-2014
The maximum peak of weekly dengue cases, occurred, at weeks, 32 to 36 and another peak of less intensity that matches the curve of eggs of Aedes, occurred at week 40 and 41. In a multivariate model, the weekly number of confirmed dengue cases (F= 32.34; R2= 0.7419), was associated with relative humidity (β 1.5047; p= 0.009) and marginally associated with eggs counts in ovitraps (β 1.043; p= 0.046). During the dry season, the occurrence of cases was better associated with the abundance of Aedes, the range of temperature and humidity (R2= 0.797: β 0.486, t 2.61 p= 0.015) compared to that during the rainy season (R2= 0.411: β 6.47, t 3.04 p= 0.008). An analysis of the correlation between the mean number of egg counts and the mean numbers of dengue cases after progressive lags in numbers of weeks, indicated that during the dry season (weeks 1 to 22 and 43 to 52 of the year) the best correlation occurred at intervals of two weeks (R2= 0.80%); whereas during the rainy season (weeks 23 to 43) the best correlation occurred at 3 to 4 weeks (R2= 0.78) (table II).
Discussion
In this study, we observed that dengue fever incidence along the Apatlaco sub-basin region followed similar yearly patterns during the five years of the study. Dengue fever incidence in all municipalities was higher during the rainy seasons compared to that of the dry seasons, but dengue fever incidence was higher in the Southern compared to that of Northern regions. The systematic assessment of Aedes spp. egg production, a measure of the vector abundance, also presented a consistent yearly pattern, with lower mosquito abundance during the dry seasons and increases during the rainy seasons in all municipalities. Overall, the Aedes mosquito abundance correlated with the number of confirmed dengue fever cases. This observation has been the base for the use of ovitrap-based monitoring the mosquito abundance to assess the risk of dengue fever transmission in endemic areas.14,15
The dynamics of climatic determinants in the geographical context explain the close association of the factors that favor mosquitos breeding and survival with the levels of intensity and temporal variations of dengue fever transmission.1,2,16,17 Taken as whole, in the study area, the weekly production of mosquito eggs was higher at a DTR range of 12 to 18 oC, but it was lower at higher temperatures. Similarly, a close association was observed with rainfall and relative humidity with egg production, but an increase of rainfall over 30mm RH around 80% was not accompanied by further increases in egg production. These conditions that vary between seasons explain the differences in dengue fever incidences between the rainy and dry season. However, it is important to notice that although egg production correlated with dengue fever incidence, no further increases in dengue fever cases occurred once the WEC increased beyond 60. Seasonal dengue fever incidence indicates that low mosquito abundance sustain low levels of dengue fever transmission during the dry season, but during the rainy season, the incidence reach a peak and then decrease, irrespective of the high mosquito abundance. This phenomenon could be explained by a depletion of susceptible people, as the epidemiological curve progresses.
The altitude gradient in the ecosystem of Apatlaco, is determinant of the variations of the climatic conditions discussed above, thus the transmission of dengue fever presents two sub-regional epidemiological patterns, which are consistent with the entomological gradient. Dengue fever incidence was higher in the southern municipalities, compared to those of the North. Accordingly, the average level of eggs was lower in the northern municipalities than in those in the South. However in both areas, dengue fever incidence followed similar seasonal patterns, with higher numbers during the rainy season.
Socio-economic factors are possibly contributing to the differences in dengue fever incidence in the sub-regions. Southern municipalities are the less developed and share social and economic conditions as well demographic tendencies (table I) (mobility and boundary and population density). Among them, Puente Ixtla has a greater number of houses without water supply, a condition that favors irregular water storage containers, increasing the probability for mosquito breeding sites, consistent with the level of eggs counts.
The urban infrastructure also may also contribute to the differences in dengue fever incidences.18 This is the case of Emiliano Zapata (Northern region) that recently presented an expansion of irregular urban settlements, along with increased immigration and population. These conditions have, very probably, favored an increase in the entomological indicators of mosquito abundance.
Planning strategies for Dengue fever prevention and control depend on the monitoring of risk factors for the transmission of the disease. The best predictive surveillance methods, so far are based on estimations of mosquito vector abundance. Monitoring mosquito abundance is largely dependent on the assessment of egg production and successful development to mosquito adulthood.19,20 Our results indicate that, although ovitrap surveillance provided good correlation between mosquito egg counts and dengue fever incidence, this correlation had better fit if a time lag between weekly ovitrap egg counts and the weekly numbers of dengue cases was introduced. This observation has been also documented in other endemic areas.21,22 This time lag represents an opportunity to use ovitrap monitoring as a predictive tool for dengue fever incidence increments. Interestingly, in the Apatlaco region, during the dry season the best predictive egg counts occurred three weeks before the occurrence of dengue fever case numbers, while during the rainy season this lag was four weeks, similar to that observed in Brazil.15 A gap of three to fourteen weeks between egg counts and the stabilization of the adult mosquito populations has been reported elsewhere.21,22,23,24
Environmental and climatic conditions could explain the abundance of vector mosquitos that during the dry season maintain egg production and mosquito adult survival to support low dengue fever. During the dry season mosquito abundance levels has little variations and may be supported by breeding sites located in domestic water containers which remain relatively unchanged. On the other hand, as the rainy season progresses, mosquito breeding sites located in containers that accumulate rain water will increase over time, and the stabilization of the adult mosquito population would take longer periods.
Understanding the conditions for dengue fever transmission in endemic ecosystems could provide better tools for prevention and prepare for control strategies. Our results indicate that in a limited endemic area (the sub-basin of the Apatlaco River) these conditions vary in space and time, on a lattice of diverse urban and socioeconomic conditions. The predictive tool provided by ovitrap monitoring, already implemented using a georeferenced platform in all endemic Mexican states25 could be improved with the incorporation of these conditions in the predictive analysis.
Conclusion
This study confirmed the correlation of Aedes spp. abundance with dengue fever incidence, and a consistent yearly pattern with seasonal variations. Vector abundance and dengue fever incidence were higher during the rainy season, compared to the dry season. The effect of climatologic variables on mosquito abundance could be explained by their effect on egg production and survival. These factors were also reflected in differences in vector abundance between Southern and Northern municipalities, but other urban infrastructure and sociodemographic factor may be participating. Seasonal differences in the time lag between egg numbers and incident dengue fever cases could be explained by the dynamics of the vector population. These observations warrant similar analysis in other endemic areas and their incorporation in the routine surveillance of dengue fever risk already in place in Mexico.











 nueva página del texto (beta)
nueva página del texto (beta)



