Introduction
Globally, breast cancer (BC) is a major growing health problem with an important worldwide variation. In Mexico it is the leading cause of female cancer with around 20000 new cases per year.1 Age at BC diagnosis differs significantly between less developed countries, where 47.37% of new BC cases occur before 50 years of age, and developed countries, where only 18.5% of new cases occur before such age.2 The lower BC incidence and younger age at onset in developing countries may be partially explained by the scarcity of BC screening programs and cancer registries in them and by the fact that these countries have a much younger population than developed ones. However, these differences across countries may also be the result of differences in exposure to environmental, reproductive and genetic BC risk factors.
Variants in cytochrome P450 1A1 (CYP1A1) and 1B1 (CYP1B1) as well as the glutathione S-transferase mu1 (GSTM1), theta1 (GSTT1) and pi1 (GSTP1), all of which codify enzymes that are involved in detoxification pathways via C-or N-hydroxylation as well as in estrogen metabolism, may be related to BC susceptibility, yet scarce information is available.3
The aim of this study was to evaluate if variants in the CYP1A1 (T3801C and A4889G), CYP1B1 (G119T), GSTM1 (indel) and GSTT1 (indel) genes, are associated with BC among Mexican women.
Materials and methods
This is a secondary report from a case-control study, conducted between 2007 and 2011 in five Northern States of Mexico (Sonora, Chihuahua, Coahuila, Nuevo León and Durango) to identify the environmental and genetic risk factors for BC in that area. Detailed information about the study design and recruitment methods is available elsewhere.4
Study population
Eligible cases were women of at least 18 years of age with no prior diagnosis of cancer, who were residents of the study areas for at least one year prior to the interview. Patients were identified in 17 public tertiary care hospitals. Population controls were randomly selected from women living in the same residence zones of cases for at least one year prior to the study, with no previous medical history of cancer, and were matched to the cases by age (±5 years).
After signing the informed consent, cases were interviewed at the hospital before receiving any specific treatment (hormonal therapy, chemotherapy and/or radiotherapy), while controls were interviewed at their homes. Information on medical and reproductive history, sociodemographic characteristics and tobacco and alcohol consumption was obtained through face-to-face interviews. Anthropometric measurements (height and weight), as well as a blood sample (10 ml), were obtained from each woman. Incentives were given in the form of grocery vouchers, to increase the response rate. The response rate among cases was 96.4% (n=1 045/1 084) and 99.9% (n=1 030/1 031) among controls. The main reason for not participating in the study was lack of interest. This study was approved by the Ethics Committee of Mexico’s National Institute of Public Health.
DNA extraction
Buffy coat was obtained from blood samples from which DNA was extracted with the Quick-gDNAMidiPrep kit (Zymo Research). Spectrophotometry was used to quantify and determine DNA purity at 260/280 nm and 260/230 nm wavelengths. DNA samples with a ratio of 1.7 to 1.9 and 2.0 to 2.2, respectively, were considered as acceptable.5 DNA integrity was visually assessed (2% agarose gels stained with ethidium bromide) in a 10% randomly chosen sample. One hundred twenty-five (31 controls and 94 cases) of the 2075 available DNA samples were not considered acceptable, yielding 952 cases and 998 controls for further analysis.
Genotyping
SNP variants in CYP1A1 and CYP1B1
SNP variants of CYP1A1: rs1048943 (A4889G), rs4646903 (T3801C) and CYP1B1: rs1056827 (G119T), were genotyped by allelic discrimination, using TaqMan assays (ABI 7900 Sequence Detection System, Applied Biosystems, Inc.), under the following conditions: denaturalization at 95°C for 10 minutes, 40 cycles of alignment to 92°C for 15 seconds and amplification at 60°C for 1 minute. Sequences from T3801C and G119T, which were not available in the Applied Catalog, were obtained from the National Center of Biotechnology Information. Conditional upon DNA availability, samples were analyzed in duplicate. Negative controls were included on each plate. Amplification failures were as follows: T>C3801 (29 cases and 20 controls), A4889G (25 cases and 11 controls) and G119T (146 cases and 131 controls), which rendered final sample sizes for subsequent analysis of: 923 cases and 978 controls for T>C3801, 927 cases and 987 controls for A4889G, and 806 cases and 867 controls for G119T.
Indel variants in GSTM1 and GSTT1
Indel variants in GSTM1 and GSTT1 were identified by Multiplex PCR with the following conditions: enzyme activation at 95°C for 15 minutes, amplification for 40 cycles at 95°C for 30 seconds, alignment at 59°C for 35 seconds and 72°C for 60 seconds, followed by an extension of 10 minutes at 72°C. Two sets of primers were used to amplify segments of 215 pb in GSTM1 and 480 pb in GSTT1.6 The reaction mixture was prepared in a final volume of 25 µl (1x) including: 20 ng/µl genomic DNA, 0.13 µl HotStarTaq polymerase (QIAGEN, INC.), 0.2 µl GSTM1 primer, 0.1 µl GSTT1 primer, 0.6 µl dNTPs and 0.2 µl DHFR (dihydrofolate reductase) as positive control. In addition, a mixture without DNA was assessed as negative control. A molecular weight marker from 100 to 3 000 pb was used (DNA Ladder, Qiagen). Genotyping was carried out by visual inspection in 10µl of amplified product by electrophoresis in agarose gels (2%). Twenty-five samples from cases and 43 samples from controls did not amplify; therefore, the final sample size for further analysis was: 927 cases and 955 controls.
Ancestry
A panel of 105 ancestry markers previously developed and validated by the Latin American Cancer Epidemiology (LACE) Consortium was used to obtain the percentage of ancestry (Native-American, European and African) on a sub-sample of 1 267 women by employing the ADMIXTURE software.7,8
Statistical analysis
Genotype distributions of interest were assessed among controls through the Hardy-Weinberg Equilibrium Test. Linkage disequilibrium was also evaluated for CYP1A1 variants, with the SNPstats software.9 Odds ratios (OR), for each reproductive, sociodemographic and lifestyle risk factor for BC considered, were obtained through unconditional logistic regression models, adjusted for age (20-24, 25-29, …. years) and years of schooling; the latter variable was used as a proxy for socioeconomic level.10 Tests for trend were performed for the corresponding variables in continuous scale.
Association between BC and each genetic variant of interest was evaluated using an unconditional logistic regression model with adjustment for age (20-24, 25-29, …. years), years of schooling, first degree relative with BC (yes, no), age at first full term pregnancy (<21, 21-24, ≥25 years, nulliparous), breastfeeding at first birth (no/nulliparous, 1-6, 7-12, >12 months), consumption of alcohol (yes, no) and tobacco (yes, no). CYPs genotypes were evaluated according to the dominant, recessive and co-dominant models. However, only those from the co-dominant model are shown in detail, since results for the other two models were similar. On a subsample of 656 cases and 611 controls, a sensitivity analysis to assess ancestry as a potential confounder was performed. Statistical analyses were conducted with Stata software, version 13 (Stata Corp., College Station, TX, USA).
Results
Due to the study design, there were no significant differences between cases and controls regarding age (approximately 54 years) and place of residence. Compared to controls, BC cases had a significantly higher educational level (8.2 vs. 5.9 years of schooling, respectively, p=<0.01) (data not included in table I). BC risk was significantly increased by nulliparity, older age at first full pregnancy, first-degree family BC history, and alcohol and tobacco consumption. In contrast, breastfeeding of first child and body mass index among pre-menopausal women were inversely associated with BC (table I).
Table I Association between breast cancer and reproductive and lifestyle risk factors in the study population. Northern Mexico, 2007-2011
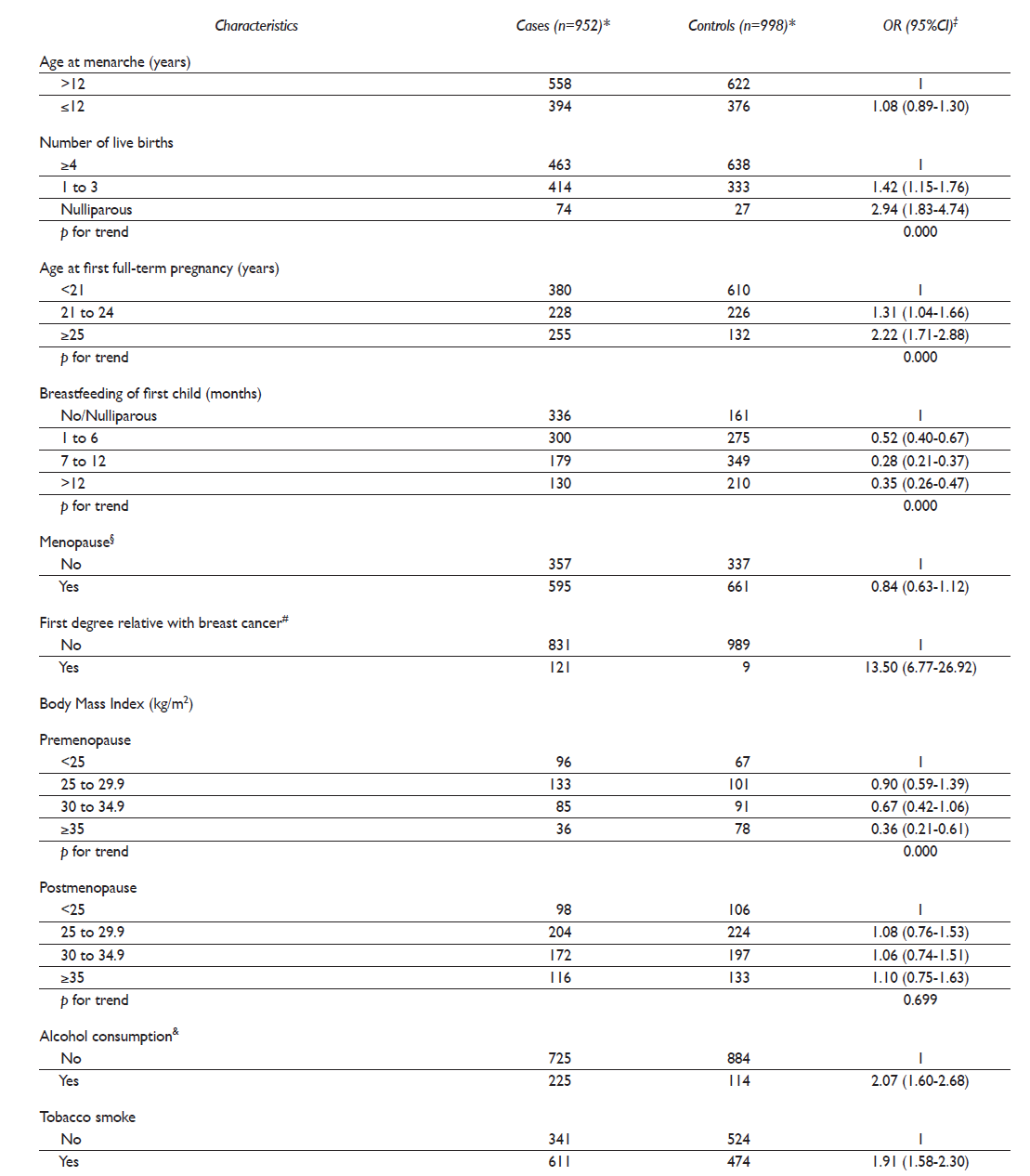
* Differences in total sample size across risk factors in cases or controls are due to missing values
‡ Separate unconditional logistic regression models were fitted for each risk factor. All ORs are adjusted for age (in five-year periods) and years of schooling
§ Menopause ≥365 days without menstrual bleeding
# Mother, sister or daughter
& Mean (SD), min-max in grams of ethanol per day, among consumers = 1.39 (4.26), 0.05 - 63.23
Regarding the association between BC and the genetic variants of interest, we only found a statistically significant association of BC with CYP1B1 (G119T) polymorphism (table II). After adjustment for other risk factors, the odds of developing BC were 1.9 (95% CI: 1.4 - 2.5) times higher in carriers of the T/T genotype in CYP1B1 than in carriers of the G/G genotype (co-dominant model) (p<0.05). The corresponding adjusted OR under the dominant (T/T + G/T vs. G/G) and recessive models (T/T vs. G/G + G/T) were 1.4 (95% CI: 1.1 - 1.8) and 1.7 (95% CI: 1.3 - 2.2), respectively (p<0.05) (data not included in table II). A marginally positive association occurred between GSTM1 deletion and BC (OR=1.1; 95% CI 0.9-1.4). A borderline significant inverse association was found between BC and GSTT1 deletion (OR= 0.77; 95% CI: 0.62 - 0.97). All genotype distributions were in Hardy-Weinberg Equilibrium. Linkage disequilibrium was observed in CYP1A1 variants.
Table II Association between breast cancer and the genetic variants of interest. Northern Mexico, 2007-2011

* Separate unconditional logistic regression models were fitted for each genetic variant. All ORs are adjusted for age (in five-year periods), years of schooling, first degree relative with breast cancer (no, yes), age at first full-term pregnancy (<21, 21-24, ≥25, nulliparous), breastfeeding at first birth (no/nulliparous, 1-6,7-12, >12 months), consumption of alcohol (no, yes) and tobacco (no, yes)
‡ Linkage equilibrium (T3801C vs A4889G, D’= 0.46, p≤0.001)
The ancestry profile was (mean ± SD): 49.8 ± 15.9% Native-American, 43.3 ± 16.9% European and 6.9 ± 5.4% African among the controls, and 46.9 ± 16.8% Native-American, 45.8 ± 17.5% European and 7.3 ± 6.2% African among the cases (data not included in tables).
Discussion
We found that carriers of the T/T CYP1B1 G119T genotype were at increased BC risk in the Northern Mexican states. To our knowledge, this is the first report from Latin America showing an increased BC risk due to the variant CYP1B1 G119T.
Our results are similar to those observed among the Asian women but not among the African-American or Caucasian women that were included in a recent meta-analysis (9453 BC cases and 10 607 controls). Among the Asian women, the OR for CYP1B1 G119T variant was 2.3 (95%CI 1.2-4.5), with the co-dominant model (T/T vs. G/G); 2.0 (95%CI 1.3-3.1) with the dominant model, and 1.8 (95% CI 1.1-3.0) with the recessive model. The prevalence of the T/T genotype in our controls was 22.8% compared to 1.1% to 11.6% among populations included in the meta-analysis and 31.0% in Brazilian males of European descent.11CYP1B1 G119T polymorphism results in a substitution of alanine by serine, which enhances the catalytic enzymatic activity in T/T genotype carriers compared to wild-type carriers; its overexpression increases the formation of quinones capable to form DNA adducts.12
We found that GSTM1 deletion seemed to marginally increase BC risk. This result is very close to that found in a meta-analysis of 15 studies that also included Asian women (OR=1.2; 95%CI 1.1-1.3), and in another pooled analysis of 61studies (OR=1.1; 95%CI 1.1-1.2).13,14 However, a recent, smaller study among Mexican women found a stronger association between GSTM1 deletion and BC risk (OR=2.1; 95%CI 1.5-3.2).15 Null genotype carriers might be at higher risk of BC because of a complete absence of GSTM1 enzyme activity in the detoxification of toxic compounds and endogenous hormones.13,16
Despite the biological evidence suggesting that the variants CYP1A1 T3801C and A4889G are related to the formation of carcinogenic metabolites17, and that GSTT1 deletion is associated with a reduced detoxification capacity, our results showed that neither of them is significantly associated to BC risk, a fact that supports the findings of several previous studies. At least four meta-analyses have shown a lack of association between T3801C variant and BC risk,18,19,20,21,22 as well as between A4889G variant and BC.18 Moreover, a non-significant association between A4889G carriers and BC has been reported among Mexican women.23 Likewise, another pooled analysis24 and two Mexican studies15,23 have found no association between GSTT1 deletion and BC risk. These findings are in contrast to the increased BC risk that has been observed in non-native American populations.14,25,26,27
Some methodological considerations should be taken into account to interpret our results. The genotype distributions of interest did not deviate from the expected values; which implies that our study population was not genetically atypical. It has been suggested that, within the Hispanic population, BC risk may differ depending on the amount of the European vs. American Indian genetic heritage. Therefore, a panel of 105 ancestry markers was determined in a subsample of 1 267 women. The analysis of this subsample showed that ancestry did not confound the results for most of the variants of interest; however, it did not help us to determine this situation, for CYP1B1 G119T variant that was not in Hardy-Weinberg equilibrium (table III). All laboratory personnel were blinded to case-control status, to reduce the likelihood of differential error; however, we cannot rule out that our estimates may be underestimated, due to non-differential errors resulting from the less than total sensitivity and specificity of the laboratory techniques.
Table III Breast cancer risk according to the genetic variants in a subsample of 1267 women: ancestry sensitivity analysis. Northern Mexico, 2007-2011

* Model 1: A Separate unconditional logistic regression models were fitted for each genetic variant. All ORs are adjusted for age (in five-year periods), years of schooling, first degree relative with breast cancer (no, yes), age at first full-term pregnancy (<21, 21-24, ≥25, nulliparous), breastfeeding at first birth (no/ nulliparous, 1-6, 7-12, >12 months), consumption of alcohol (no, yes) and tobacco (no, yes)
‡ Model 2: ORs adjusted for the same covariates in Model 1 plus percentage of ancestry (Native-American, European and African)
In conclusion, our results support the existence of genetic susceptibility for BC conferred by the variant CYP1B1 G119T among Mexican women. The potential interaction between the exposure to carcinogenic compounds and genetic susceptibility warrants further attention.











 nueva página del texto (beta)
nueva página del texto (beta)


