Introduction
The genomic integrity of a population is influenced by lifestyle, weather, diseases such as diabetes, cancer, medical treatments, nutritional status, genetic polymorphisms, alcohol consumption, cigarette smoking, metabolism of drugs and exposure to pesticides and herbicides.1,2,3,4,5,6,7,8,9 Therefore, DNA damage varies considerably from one population to another.
Mexico presents a high ethnic diversity with at least 64 indigenous (Amerindian) groups representing ~7% of the total population. These groups maintain their own social, economic, cultural and political structures; however, they live in conditions of social inequality, poverty and high marginalization. Most indigenous populations in Mexico are engaged in subsistence farming, for which they employ fertilizers and herbicides, that are inexpensive, but highly genotoxic. Furthermore, most of these indigenous populations use timber as fuel for cooking, which exposes them to high concentrations of smoke.10,11 These conditions compromise their genomic integrity.
The Buccal Micronucleus Cytome (BMCyt) assay is a minimally invasive method for studying DNA damage, chromosomal instability, cell death and the regenerative potential of human buccal mucosal tissue.2,6,12,13 The exfoliated cells of the oral mucosa reflect chromosomal aberrations generated in the proliferating basal cell layer of the epithelium, which subsequently migrate to the surface.2 The presence of MN in the cellular field represents loss of DNA,14,15 and the nuclear alterations (NAs) and the different chromatin status are used as markers of cytotoxicity.3,13
The aim of this work was to evaluate the integrity of nuclear DNA through MN and NA analysis in four Mexican ethnic groups: Cora, Huichol, Tarahumara and Tepehuanos.
Experimental section
Subjects
One hundred twenty individuals of four different Amerindian groups of Mexico were studied. The sample included 30 Tepehuanos and 30 Huicholes from the state of Durango, 30 Tarahumaras from the state of Chihuahua, and 30 Coras from the state of Nayarit (figure 1). A questionnaire was applied, and the number of subjects that smoke, number of subjects that ingest alcohol, number of subjects exposed to herbicides, dietary habits, gender and age was recorded. Sampling took place in the period from January 2009 to December 2012.

The indigenous populations originate from the states of Durango, Chihuahua and Nayarit and the sampling took place in the period from January 2009 to December 2012
Figure 1 Geographic location of the Mexican indigenous groups studied. México
Ethnicity was initially evaluated by self-identification of the subjects to the ethnic group; additionally, molecular studies were conducted to determine ancestry on each group through genotyping of 15 Short Tandem Repeats (STRs).16
The present work was performed according to the Helsinki Declaration and was approved by the Ethics and Research Committee of the Durango General Hospital of the Mexican Health Ministry. Volunteers were included in the study after they were informed of the nature of the study, and signed a consent form.
Sample
Samples were taken from oral mucosa in all the participants. The mouth of each subject was rinsed with water, and then a slide was used to collect cells from oral mucosa of the right and left cheeks. Samples were spread directly onto two separated slides previously cleaned and coded.17,18 Smears were air-dried and fixed with 80% methanol for 48 hours and then stained with acridine orange (CAS no.: 10127023; Sigma-Aldrich, St. Louis, Missouri, USA). All precoded slides were examined by the same reader, who counted the MN and NAs including binucleated cells, cells with nuclear buds, and karyolitic, karyorrhectic, condensed chromatin, and pyknotic cells and was blind to the identification of the individual. The criteria used for scoring MN and NAs were according to those described by Thomas and colleagues (2009),12 and the number of cells with MN and NAs were evaluated in 2 000 cells using an Olympus CX31 microscope equipped with epifluorescence and oil immersion objectives (×60 and ×100; Olympus, Tokyo, Japan). Results are presented as the number of cells with MN or NAs per 1 000 cells.
Statistical analysis
The results are expressed as mean ± standard deviation. Comparisons for categorical variables including herbicide exposure, smoking and alcohol consumption, were performed using chi-square and/or Fisher’s exact test. The differences in MN and NAs values were evaluated by Kruskal-Wallis and Mann-Whitney’s U test for intergroup comparison. All tests were performed using the Statistical Program for the Social Sciences (SPSS v11.0) for Windows medical pack (SPSS Chicago, IL, USA). A p value less than 0.05 was considered statistically significant.
Results
The characteristics collected through the questionnaire of all participants are summarized in table I.
Table I General characteristics of the participants. México

Inter-group differences were assessed using the chi-squared (aHuicholes vs. Tarahumaras, bCoras vs. Tepehuanos; cTarahumaras vs. Tepehuanos)
NS: not significant
Note: The indigenous populations originate from the states of Durango, Chihuahua and Nayarit and the sampling took place in the period from January 2009 to December 2012
The frequency of women was higher than men in the four studied groups. The average age ranged from 32.73 to 47.26 years and the range of body mass index was 22.73 to 26.24.
No significant differences in number of subjects that smoke were observed between groups. The highest number of subjects with drinking habits was found in Tarahumaras (table I). A significantly higher number of individuals exposed to herbicides was found in Tepehuanos (table I).
Table II shows the average and standard deviation of the number of MN and NAs numbers in the studied groups.
Table II Number of micronuclei and other nuclear abnormalities in the groups of study. México

Differences in MN and NAs values were assessed using Mann-Whitney’s U test for intergroup comparison (aTepehuanos vs. Coras, bTepehuanos vs. Huicholes; cTepehuanos vs. Tarahumaras)
Note: The indigenous populations originate from the states of Durango, Chihuahua and Nayarit and the sampling took place in the period from January 2009 to December 2012
The markers of DNA damage [MN, Cells with nuclear buds and binucleated cells] (figure 2) were present with higher frequency in the Tepehuano group compared with the other studied groups (table II).
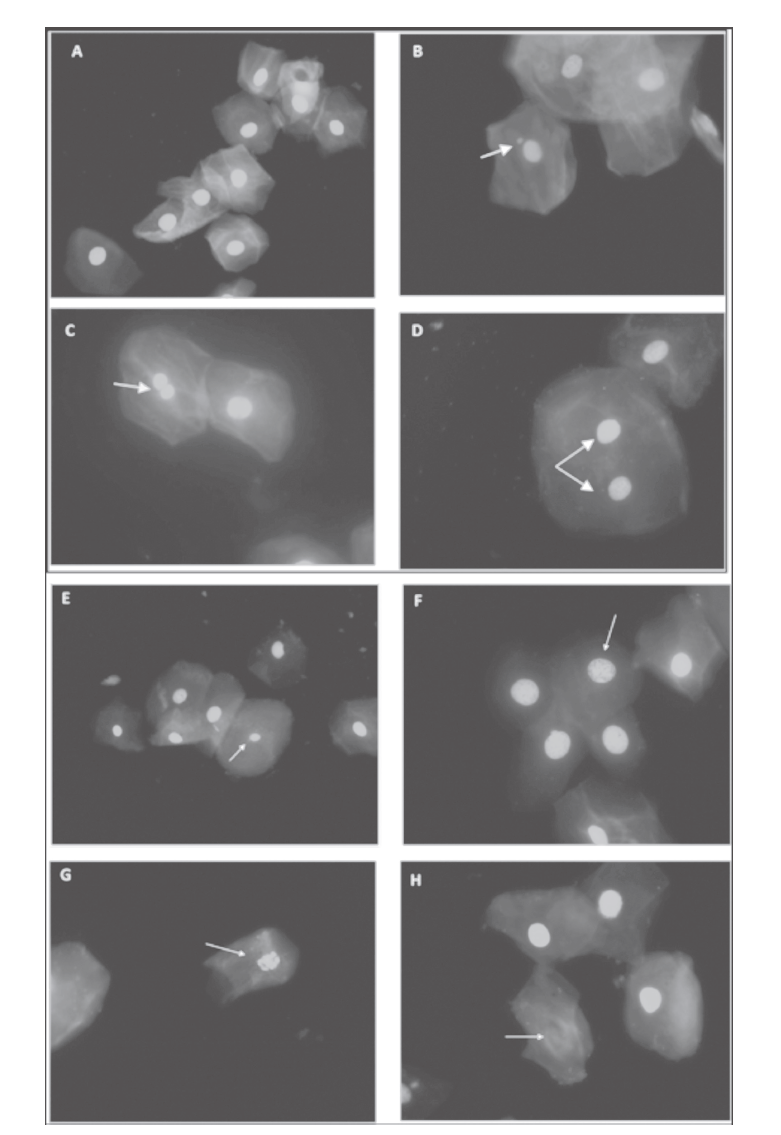
A: normal cells; B: micronuclei; C: nuclear bud; D: binucleated cells; E: pyknotic; F: condensed chromatin; G; karyorrhectic; and H: karyolytic (oil-immersion objective 60x, acridine orange stain)
Figure 2 Markers of cytotoxicity and DNA damage in buccal mucosa. México
The number of MN was significantly higher in the Tepehuano group in comparison with Coras (p = 0.004), Huicholes (p = 0.001) and Tarahumaras (p = 0.001). The number of cells with nuclear buds in the Tepehuanos was significantly higher than that observed in the other study groups (p< 0.05). In addition, the number of binucleated cells in the Tepehuanos was significantly higher than in Tarahumaras (p < 0.05).
Regarding markers of cytotoxicity or cell death [condensed chromatin cells, karyolitic cells, karyorrhectic cells and pyknotic cells] (figure 2), no significant differences between the study groups were found in the number of karyolitic cells and pyknotic cells. Conversely, the number of condensed chromatin cells was significantly higher in Tepehuanos than in Huicholes (p = 0.02); also, the Tepehuano group presented a significantly higher number of Karyorrhectic cells than Tarahumaras, Coras and Huicholes (p < 0.05). The number of condensed chromatin cells was significantly higher in the Tepehuanos group compared to Huicholes group (p = 0.02); a significantly higher number of Karyorrhectic cells was found in Tepehuanos in comparison to Tarahumaras, Coras and Huicholes (p < 0.001).
There was no correlation between the number of MN with chronological age, gender, and number of subjects exposed to herbicides, smokers and alcohol drinkers (table III).
Table III Correlation test for general characteristics of participants and micronuclei. México
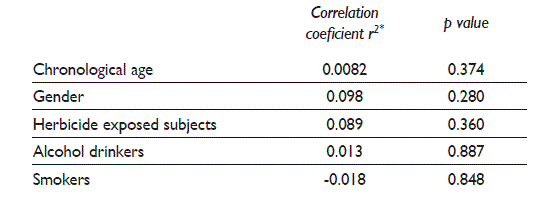
Spearman’s correlation coefficient, significance p value < 0.05 and CI95%
Note: The indigenous populations originate from the states of Durango, Chihuahua and Nayarit and the sampling took place in the period from January 2009 to December 2012
Discussion
Mexico has one of the richest ethnic, cultural and linguistic diversities in the Americas. The existing Native Mexican groups have settled in difficult-to-access areas, and retain their traditional lifestyle and language, for this reason it is important determine the impact of environment and lifestyles on DNA integrity of the Native Mexican groups.
Our results showed that the Tepehuano group presented greater number of micronuclei (DNA damage) and nuclear abnormalities (cytotoxicity) than the Huichol, Cora and Tarahumara groups (p < 0.05). The greater cytotoxic and genotoxic damage observed in 50% of Tepehuanos could be the result of a greater number of individuals exposed to herbicides than the other Amerindian groups studied.
Supporting this, some reports demonstrated that chemical agents contained in fungicides, herbicides and insecticides, increase the number of MN in exfoliated buccal cells.5,8,19,20 For example, the inhalation of glyphosate herbicide may cause DNA damage in exposed individuals.4 Also, there is evidence of cytotoxic and genotoxic effects of paraquat in human lymphocytes in vitro.21
Differences in the number of MN and NAs depend on genetic and environmental factors such as diet, age and sex.2,3,22 A higher number of MN in women compared to men was previously described,23 however in the present study such differences were not observed (data not shown).
Age can also influence the number of MN,2,24,25 since the older the subject, the greater is the number of MN.18,26 Conversely, in the present study the number of MN and NA were higher in young individuals belonging to Tepehuano group. This could be explained by the widespread use of herbicides in this group, although no correlation was found between these two variables.
In relation to smoking habits and the number of MN, the reports are very controversial, some authors described that smoking increased the frequency of MN27,28,29,30 and other authors did not observe this effect.31,32,33,34 In this study, the smoking habit was similar between the studied groups, and there was no correlation between the number of MN and number of individuals who smoke (table III).
There is evidence that ethanol increased the number of MN in exfoliated cells.35,36,37 In addition, a significant increase in the number of MN in lymphocytes of alcoholics compared with abstinent alcoholics and nonalcoholics has been reported.38 There are also reports of higher frequency of cells in karyorrhexis in alcoholics.39 Furthermore, ethanol also increases the number of chromosome breakage and sister chromatid exchange.40
In this study, the largest number of individuals that drink alcohol belong to Tarahumaras (50%), followed by Coras (36.7%), Tepehuanos (30%) and Huicholes (23.3%). The highest number of MN was found in Tepehuanos, which suggests that alcohol intake is not relevant for MN development in these populations.
Working with indigenous populations represents a big challenge because the accessibility to the communities is difficult, and there exist cultural and language barriers. To the best of our knowledge, this is the first report that determines the number of micronuclei and nuclear anomalies in Mexico’s indigenous population, however, some limitations must be considered: 1) the quantification of herbicides of biological samples was not performed; in addition, the time and duration of exposition to this contaminant was not recorded. 2) The number of cigarettes and the frequency of smoking were not obtained. 3) The frequency of use of alcohol and volume are not precise. These limitations should be considered to design additional studies in Mexican indigenous populations.











 nueva página del texto (beta)
nueva página del texto (beta)


