Brucellosis is one of the most common zoonotic diseases in the world.1 Human brucellosis (HB) is an infection caused by facultative intracellular, gram negative bacteria; it is transmitted through the consumption of contaminated dairy products, or by direct contact with infected animal tissues in endemic areas. Certain occupational groups-especially abattoir workers, veterinaries, and farmers-are at increased risk.2 Clinical manifestations of HB include fever, constitutive symptoms, malodorous perspiration, hepatomegaly, and splenomegaly.3,4 The wide differential diagnosis associated with this disease makes it prone to be confused with other infections in endemic areas.3,5 This is important because undiagnosed, untreated acute brucellosis may lead to subacute or chronic disease, which may be severely debilitating, especially for manual workers.2
In Mexico, HB is endemic with a reported annual incidence of 25.7 cases per 100 000 person-years.6 The largest report to date analyzed 67 982 sera of healthy individuals (one to 98 years old) obtained from the National Seroepidemiolgy Survey. This study estimated a national mean seroprevalence of 3.42%, and found an association with gender (women), but not with occupation.7 Also, two recent blood bank surveys performed in tertiary care centers reported a seroprevalence between 2.1 and 3.6% in their donated products.8,9
Bovine brucellosis (BB) is also widespread in Mexico with a reported prevalence ranging from 0.04 to 5.68%, depending on the region. The main risk factors are the introduction of diseased animals to brucellosisfree herds and inappropriate practices in birth assistance. This is true of both dairy and beef cattle.10
The economic impact of brucellosis (in terms of human and animal productivity losses) is evident.11,12 But the epidemiology of those in close contact with cattle is not well understood.13 The aim of this study was to describe the seroprevalence and associated factors for brucellosis among dairy farm workers (DFW) in a large production facility in central Mexico.
Materials and methods
We conducted a secondary analysis of a data set and sera from a previous cross-sectional study collected by our group during 2009-2011 in a dairy farm.14
Study area and population
This dairy farm facility is located in a municipality of the state of Hidalgo, Mexico; it encompasses 126 stables and one abattoir house with an average cattle population of 26 000 animals; 1 200 DFW, 200 household contacts and 11 abattoir workers. In some stables, owners provide housing for their workers. This consists in small rooms adjacent to where farming activities are performed. We approached individual owners of each stable and sought authorization to enter the stable and invite people to participate. All farm workers, their on-site living family, and workers from an abattoir house that processes cattle from these same facilities were invited to participate, provided they were ≥15 years old. Two physicians administered a standardized questionnaire and full medical exam to all consenting participants. Additionally, they obtained a blood sample from each participant.
Serological methods
In 2015, we thawed (it had been preserved at -70C°) and tested the sera for Brucella spp. antibodies by the slide agglutination test using a commercial antigen suspension of Brucella abortus (Bio-Rad, Mexico), following the manufacturer's specifications. Briefly, we marked microtiter plates with rows of 5 squares each, and placed individual patient serum in each square from left to right in the following quantities: 0.08, 0.04, 0.02, 0.01, and 0.005 mL. Then, we put a single drop of the antigen suspension in each square and mixed with a clean applicator. We then sited the plates on a horizontal mixer (Corning, New York) for three minutes and observed for positive agglutination. From left to right, the corresponding titers were 1/20, 1/40, 1/80, 1/160, and 1/320. We defined seropositivity as a titer ≥1:40 in a single specimen, and recent infection with titers ≥1:160, according to previous studies in similar populations.15,16
Statistical analysis
We compared the characteristics of seropositive and seronegative participants using the χ2 test, and Student's unpaired t-test or Mann-Whitney U test for continuous variables with normal or non-normal distribution, respectively. We included in the multivariate analysis (unconditional logistic regression) characteristics with p-values ≤0.20 in bivariate analysis or with biological plausibility. We used a hierarchical backward elimination approach, and estimated the odds ratios (OR), 95 percent confidence intervals (CI) and p-values. The statistical analysis was performed with STATA 11.0 (StataCorp, College Station, TX, USA).
Ethics
This study was conducted in full accordance with the Declaration of Helsinki. The aims of the study were communicated to the participants and a written informed consent form was obtained and signed before inclusion. The institutional review board of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (Comité Institucional de Investigación Biomédica en Humanos, reference 2051) approved this protocol. Whenever subjects were minors, they were asked for consent and the parents signed the informed consent form. Those participants who reported any signs or symptoms (irrespective of the suspected etiology) during the study period, were first examined by the medical staff of the study and then referred to their local health clinic for proper diagnosis and treatment.
Results
Among the eligible population (1 411), 442 subjects were invited to participate and 379 accepted and were included. In 2015, sera from 331 were available for testing. Each sample represented a unique individual. The seroprevalence of Brucella spp. antibodies was 18.1% (60/331; 95% CI 14.1-22.7). Among the positive cases, the median titer was 1:80 (range 1:40 - 1:320). Demographical data were available for 97.2% (322/331) of the subjects; their characteristics are shown in Table I. Eighty-eight percent (53/60) of the positive cases were male (p=0.047); the median age was 35 years (Interquartile range [IQR] 27-43). Seropositivity by occupation was highest among calf caretakers (45.4%); followed by foreman (27.5%), tractor operator (25%), milker (24.2%), and veterinary (22.7%). In bivariate analysis, people living on-site and daily hours in contact with cows were associated with seropositivity. In multivariate analysis, the characteristics: calf caretaker, daily hours in contact with cows, and living on-site remained as independently associated factors for seropositivity (Table II).
Table I Demographic characteristics of dairy farm workers tested for brucellosis, according to serological status. Hidalgo, Mexico, 2009-2011
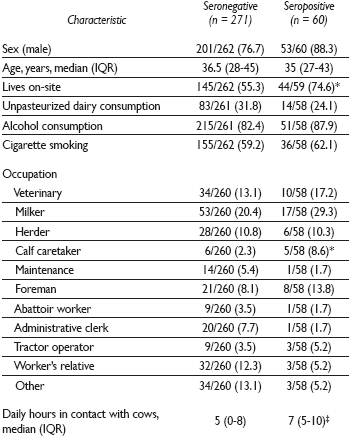
Note: data are number (%) of cases, unless otherwise indicated. n values across categories might be less than the counts in the column headings due to missing data. IQR= Interquartile range
* p<0.05 determined by the χ² test
‡ p<0.05 determined by Mann-Whitney U test
Table II Characteristics of seropositive cases among dairy farm workers, by multivariate analysis. Hidalgo, Mexico, 2009-2011

IQR= Interquartile range
* p< 0.05 by logistic regression
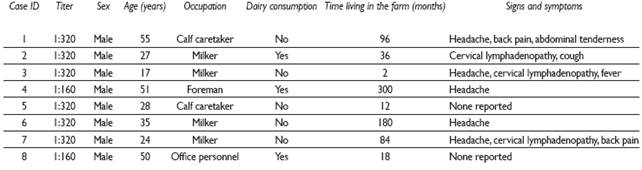
There were eight seropositive cases (8/60; 13.3%) with titer values at or above the 1:160 cut-off points. The median time living in the farm among the recent infection group was 60 months (IQR 15 -138), in contrast with the seronegative group for which it was 138 months (IQR 72 - 240; p=0.055). Symptoms reported by these subjects during clinical evaluation are described in Table III.
Discussion
The present study showed a high seroprevalence of Brucella spp. antibodies among DFW, and an association among workers with prolonged and close contact with cattle.
To our knowledge, this is the first survey conducted among people living in close contact with cattle in Mexico. The preceding seroepidemiological studies in this country, which reported a seroprevalence of HB between 2.8 and 3.4%, drew their samples from more urban and diverse populations. This may explain why they were not able to associate occupation with seropositivity.7,10 However, our report suggests that while HB has expanded from the rural to the urban setting, mainly through unpasteurized dairy products, closeness with infected cattle remains a major risk factor.
We did not observe an association between unpasteurized dairy consumption and seropositivity. This may be explained in the following manner. Firstly, consumption of unpasteurized milk is often prohibited by supervisors, and thus, is prone to be misreported by workers.14 Secondly, we can assume that both BB and raw milk consumption are widespread in the community, and therefore, no differences will be observed. Lastly, airborne transmission, although uncommon in the general population, is likely to be the principal route of contagion in this setting.2 In our study, the occupation with the strongest association was calf caretaker. This occupation is constantly in contact with cattle and even serves the function of veterinary (a known risk factor), assisting uncomplicated births.16 The livestock composition of previous, similar studies was heterogeneous, including cattle, goats, sheep, and camels, among others; and found no clear association with unpasteurized milk consumption.5,13,17,18 The complex dynamics in this kind of setting prevent studies from signaling a single source of infection. Conversely, in our study there was exclusive presence of bovine livestock, suggesting that close contact with infected cattle tissue is a strong associated factor.
This study has some limitations. The agglutination test remains the preferred serological method for epidemiological surveys of HB; and at titers of ≥1:160 for acute infection, it has a reported sensitivity and specificity of 97-100% and 88-89%, respectively.19 We defined seropositivity from a titer ≥1:40 because previous studies have demonstrated that antibody titers remain positive several months after infection.20 But crossreactivity with other bacterial antigens (such as those from Yersinia spp. and Escherichia coli O157) is possible, although unlikely in this study setting.15,21 Furthermore, culture and subsequent characterization at the species level in both human and bovine samples is needed to establish a clearer association. Although, we believe B. abortus should account for the majority of cases given that it is the main causative agent of infection in cattle.
The World Health Organization has proposed occupational hygiene as an important step in prevention of human disease. This includes wearing protective clothing, disinfection of equipment, hand washing, eye protection, use of respirator and continuous medical surveillance of workers at risk.22 Understandably, most of these measures are difficult to achieve given the financial constraints that exist. But some of the most basic actions such as the use of protective equipment and hand washing should be strictly enforced, especially for those who spend a significant amount of hours in contact with cattle, as this was associated with seropositivity in this study.
The impact of brucellosis in this setting is unknown, and perhaps this is what hinders a larger effort from across the private and public sector to eradicate the disease. However, there are many indicators that suggest that prevention strategies are not only feasible, but would positively impact human health and production.6,23 For example, a cost-effectiveness study on mass vaccination of livestock found that by allocating costs to all sectors this strategy would benefit everyone.24 Also, well-implemented programs of milk pasteurization in informally marketed products can effectively reduce human transmission.25











 nueva página del texto (beta)
nueva página del texto (beta)