Cancer is one of the primary causes of morbidity and mortality in the world, with 14.1 million new cases reported annually. In 2012, an estimated 8.2 million deaths occurred, and the estimated prevalence of cancer in the last five years included 32.6 million people. A disparity has been documented between developed countries and developing countries, with 57% of new cases (8 million) and 65% of recorded deaths (5.3 million) in 2012 occurring in developing countries.1,2 If this trend continues, the situation may grow even worse due to global population growth and the impact of aging. Given this information, the World Health Organization (WHO) estimates that 21.3 million new cases and 13 million cancer-related deaths will occur annually by 2030; 60% of these will occur in the least-developed countries.3 The situation in Latin America and the Caribbean is very similar; in 2012, 1.1 million new cases and 603 300 deaths were documented. By 2030, an estimated 1 680 000 new cases will occur, representing an 84% increase.2,4
The increase in cancer cases observed in the last few decades is partially due to the global epidemiological transition that has occurred in recent years, which is mainly due to net population growth and the impact of aging. In developing countries in particular, improvements in health indices have been observed due to decreases in infectious diseases, malnutrition, and infant mortality, which have contributed to increased life expectancy and consequently to an increase in mortality from chronic and degenerative diseases such as heart disease and cancer.3 Although cancer has a multifactorial etiology, various factors have been identified through epidemiological studies that can influence the development of malignant neoplasms such as genetic susceptibility, race or ethnicity, obesity, exposure to hormones, radiation, certain chronic infections, and tobacco and alcohol use.5-8
Definition
Given this scenario, cancer registries are a fundamental key to controlling this group of diseases. Their main function is to register, in a complete, continuous, and systematic manner, the personal characteristics of all cancer patients, as well as clinical data and the anatomical pathology of each malignant tumor, for further analysis and interpretation of the information.9 In order to function properly, a cancer registry must undergo continual analysis of three central processes: 1) identification and registration of cases; 2) systematization and analysis of information; and 3) dissemination of the findings. The information produced by cancer registries can be used in different fields, including etiological investigation; primary prevention (evaluation of cancer control programs); secondary prevention (evaluation and monitoring of screening and early detection programs); tertiary prevention (survival analysis); and service planning, in a manner that benefits individuals as well as society as a whole. For this reason, cancer registries are an essential part of a complete cancer control program, serving to establish priorities while at the same time providing necessary data to foresee future needs.10,11
Types of cancer registries
Histopathological registry. This type of registry collects information from one or more pathology laboratories and is useful for laboratory needs. It provides an incomplete and skewed cancer profile, essentially determined by the types of tissues that the laboratory can process.
Hospital registry. This type of registry collects information from all cancer patients treated at one or more hospitals. It is useful for administrative purposes because it aids in prioritizing hospital resources. In addition, it facilitates monitoring of health programs and allows the detection of patterns or frequencies of different types of cancer treated in the hospital as well as monitoring of the outcomes of treatment, survival rates, quality of life, and adverse effects of treatment. The sources of information for these registries include outpatient clinics, hematology laboratories, specialty diagnostic laboratories (nuclear medicine), anatomical pathology laboratories, autopsy services, issuers of death certificates, surgery services, oncology services, radiation therapy providers, and chemotherapy providers. This type of registry provides an incomplete and skewed cancer profile because it is determined by the population that is treated at a particular medical center.
Population registry. This type of registry systematically collects information on all new cancer cases in a particular geographic area and is determined by multiple sources. The main sources of information for these registries are a) public and private hospitals and medical centers; b) public and private outpatient surgery centers; c) public and private anatomical pathology laboratories; d) civil registry offices that issue death certificates, particularly lists of certificates of residents whose cause of death was cancer or probable tumor or those in which cancer is referenced in some manner; e) public and private specialty cancer diagnostic centers; f) public and private hospice centers; and g) public and private nursing homes. These registries provide a more reliable cancer profile for estimating population indicators of incidence, mortality, survival rates, and prevalence. The estimation of these indicators requires demographic data reported in population and housing censuses as well as mortality records. Population registries play an important role in cancer epidemiology, allowing for the estimation of incidence rates by tumor location, age, sex, and other factors. Through patient tracking, it is possible to estimate the cancer prevalence, which provides a useful indicator of the burden of this disease in the community. This method is also an affordable and efficient resource for enrolling cases for intervention, cohort, and case-control studies. Additionally, these registries can identify geographical and temporal changes through estimation of trends.
Thus, population registries play a unique role in the planning and evaluation of cancer control programs. Currently, they are considered the gold standard for cancer registries, as it is only through their use that it is possible to estimate population indicators such as cancer incidence, prevalence, survival rates, and mortality as well as the trends of these measures over time.10-13 Although the definition of an optimal population size to be covered by a cancer registry does not exist, in practice, the recommended size is between 1 and 5 million; working with larger populations can make it difficult to maintain completeness and quality of the data. In countries with large populations where it can be difficult to achieve national coverage, it may be more effective to establish self-contained regional registries that are also related with each other. This is the case with the Surveillance, Epidemiology, and End Results (SEER) program in the United States or the cancer registry networks established in Africa, India, Brazil, and Argentina.14,15 This strategy of establishing regional cancer registries stems mainly from the coverage, costs, and sustainability of the registries. Various factors that directly influence the cost and profitability of these registries have been identified such as the size of the geographic area to be covered, inclusion or exclusion of rural areas, local cost of living, quality of hospital registries, volume of cases, and whether the registry is new or well-established.16 Regarding the central processes and analysis of the registry, the process of case identification and capture uses approximately 88% of the registry budget, while the analysis accounts for the remainder (12%).17
Legal issues and confidentiality
Reporting of cancer cases to the registry can be voluntary or obligatory. Legal issues should also be considered when a cancer registry is planned because, in many countries, it is necessary to ensure a legal basis as well as preserve the confidentiality of each patient. Thus, cancer registries must always adhere to both national and international confidentiality codes or laws related to the protection of personal data18 and to matters regarding health research19 because epidemiological research based on cancer registry data is the most valid and efficient method to plan and evaluate all aspects of cancer control.
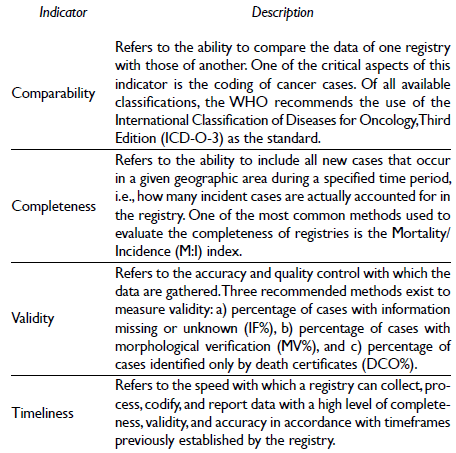
Quality indicators for cancer registries
Given that cancer registries are a fundamental part of disease control and epidemiological surveillance, it is necessary to clearly define rules for data collection and storage. Which cases can be registered should be well-defined, along with the type of coding that will be used and the types of reports that should be generated. In this context, the WHO's International Agency for Research on Cancer (IARC) and the Iberoamerican Network of Epidemiology and Information Systems on Cancer (Red Iberoamericana de Epidemiología y Sistemas de Información en Cáncer - REDEPICAN) provide the criteria for quality and systematic procedures that should be used in population-based cancer registries. In this manner, the information generated can be standardized and of optimal quality. Accordingly, of IARC has described four quality indicators for cancer registries: a) comparability, b) completeness, c) validity, and d) timeliness (table I).10,20-23 Within that framework, the IARC has developed an ad hoc system for the collection and storage of information: CanReg5 (Cancer Registry Software). With this electronic tool, the IARC aims to improve the storage and processing of data for better quality control in registries. The availability of computerized systems has revolutionized much registry work and has permitted the implementation of registries in low- and middle-income countries, as demonstrated by the network of cancer registries in Africa with its headquarters in Senegal.24
Dissemination of cancer registry findings
Data from population-based cancer registries that are qualified as acceptable according to the four quality indicators recommended by the IARC must be published in the series "Cancer Incidence in Five Continents (CI5)". This publication represents a joint effort of three institutions: the Union for International Cancer Control (UICC), the IARC, and the International Association of Cancer Registries (IACR). To date, the series comprises 10 volumes, beginning in 1960 with the publication of volume I, which includes data from 32 registries from 29 countries, while volume X includes data from 225 registries from 60 countries.25,26
The main objective of this series is to compare and to the extent possible incidence data in a wide range of geographic areas of the five continents. Additionally, the 54 years of data contained in the series have allowed epidemiological studies of the evolution of risk factors and incidence trends as well as the formation of hypotheses that may explain the observed differences between geographic areas, age groups, living areas, and possibly ethnic groups.
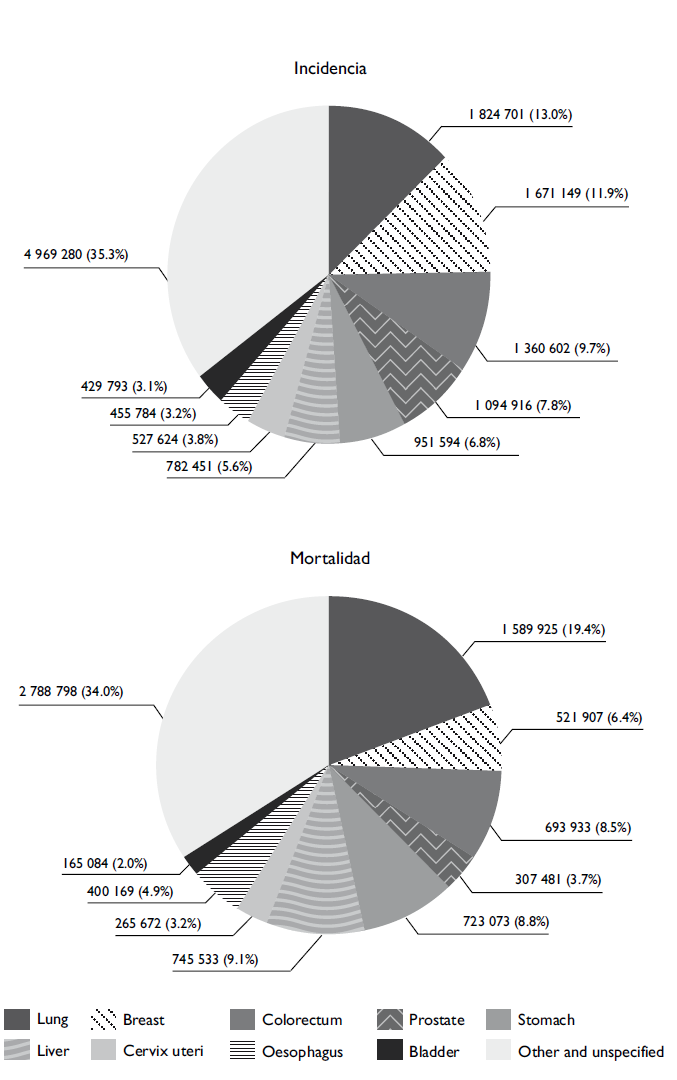
Another source of utilization and publication of the population registry data that complies with quality controls is the global estimates published on the GLOBOCAN website, which was made public in 2001. The main objective is to provide current estimates of the leading cancer types according to sex and age. The most recent publication, GLOBOCAN 2012, provides estimates of 28 types of cancer in 184 countries, offering a global overview of cancer. According to the most recent publication, the main types of cancer in the global population are lung cancer, with 1.8 million cases (corresponding to 13% of all new cases), followed by breast cancer, with 1.7 million cases (11.9%); colorectal cancer, with 1.4 million cases (9.7%); prostate cancer, with 1.1 million cases (7.9%); and stomach cancer, with 950 000 cases (6.8%). However, the most frequent causes of death due to cancer are lung cancer, with 1.6 million cases (corresponding to 19.4% of total deaths), followed by liver cancer, with 800 000 cases (9.1%); stomach cancer, with 720 000 cases (8.7%); colorectal cancer, with 690 000 cases (8.5%); and breast cancer, with 520 000 cases (6.4%) (figure 1).1
The importance of cancer registries
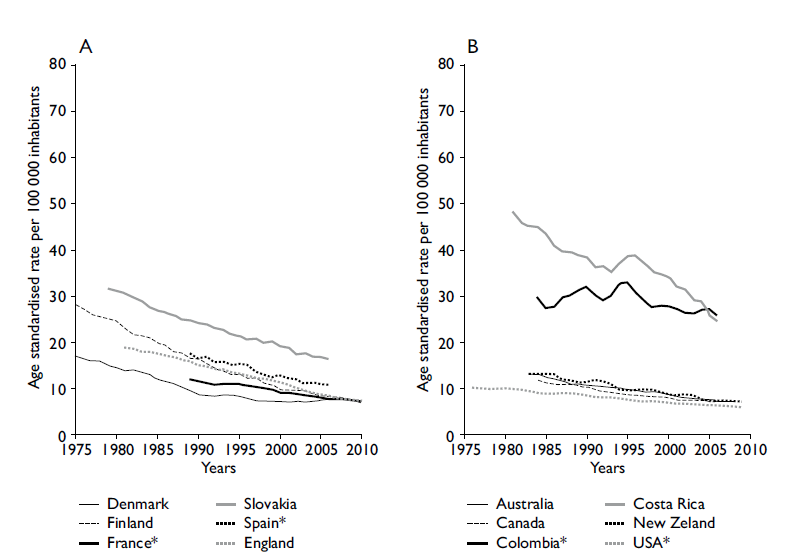
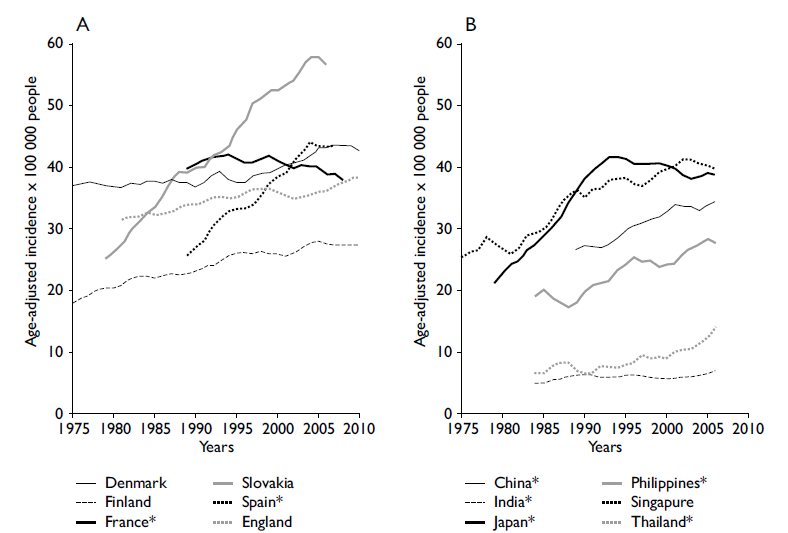
To understand the role of population-based cancer registries in planning and evaluation for control of the disease, the two most common neoplasms of the digestive system are described. a) Stomach Cancer. The epidemiological picture of stomach cancer has been formed through the use of cancer registries since 1975; significant changes in its pattern of morbidity and mortality have been observed over time. At that time, it was the most common neoplasm in the world; however, it has been displaced in frequency by other tumors such as lung, breast, colorectal, and prostate cancers.1 As seen in figures 2 A and B, the last three decades have seen a marked decrease in the incidence of stomach cancer. This suggests that strategies of controlling risk factors for this neoplasm have been successful. b) Colorectal Cancer. The epidemiological picture of colorectal cancer contrasts with the previous example. Currently, colorectal cancer is one of the most common neoplasms in both men and women, representing approximately 10% of the global cancer incidence.1 The trend of increased global incidence can be observed in figure 3. Paradoxically, it has been observed that the increase in colorectal cancer has occurred primarily in countries where there has been a marked transition toward a higher level of development such as China, the Philippines, Singapore, and Slovenia. Given this situation, it is necessary to implement control strategies in developing countries and, at the same time, to emphasize the problem of this neoplasm in low- and middle-income countries.27
Coverage of cancer registries in latin America
Unfortunately, a great disparity exists between developed countries and developing countries regarding epidemiological surveillance of cancer through population registries. It is worth noting that 65% (5.3 million) of the incident cases documented in 2012 occurred in low-to-middle-income countries.1-3 A troubling reality is that the majority of cases are diagnosed at an advanced stage of the disease, which is associated with a high mortality rate. Moreover, it is in these countries that a greater underreporting of cases is documented, mainly due to a scarcity of cancer registries endorsed by the IARC-WHO. In Central and South America, only 6% of the population is included in population-based cancer registries versus 83% in North America (United States and Canada). This 6% coverage of Latin America is in Cuba, Puerto Rico, Costa Rica, and Uruguay, each of which has a national population registry; Brazil, Argentina, Colombia, Chile, and Ecuador have 15, 7, 6, 3, and 2 regional registries, respectively (figure 4).2,10,24 It is important to mention that one of the oldest registries in Latin America is in Cali, Colombia, which has been in uninterrupted service for more than 50 years.28 In view of this, Mexico is significantly lagging, as it only has a statistical system from which the number of cancer deaths in the country can be obtained.29 For this reason, it is vital to implement a registry of this type to better gauge this public health issue. In addition, a registry would allow better planning of prevention, diagnosis, treatment, and rehabilitation of cancer and funding opportunities for improved cancer control in population.

PBCR= Population-based Cancer Registry
Source: reference 10
Figure 4 World overview of cancer registries
Conclusion
The most efficient method to address the problem of cancer is though the development and implementation of a national cancer control plan. Population-based cancer registries are a fundamental part of the operation of this plan and are necessary for achieving results, not only to estimate epidemiological frequency measures and trends for tumors, gender, and place of residence but also to evaluate the quality of cancer diagnosis and treatment of these patients. Registries are ultimately the gold standard for evaluating the results of various interventions and prevention efforts aimed at reducing the morbidity and mortality of one of the most serious public health problems of our age.











 nueva página del texto (beta)
nueva página del texto (beta)