Lymphomas comprise a heterogeneous group of haematological malignancies, classified according to their clinical and anatomic-pathological features and, lately, to their cytogenetic markers.1 Despite the therapeutic advances, nearly a third of the patients develop resistance, even with the addition of new therapeutical drugs, including monoclonal antibodies, as rituximab for B-cell malignancies. The new diagnostic tools have been the cornerstone to design recent therapy targets, which must be included in the current treatment guidelines of this sort of neoplasms by means of clinical trials and evidence-based medicine. International guidelines have been primarily focused on diagnosis and therapeutical recommendations, including all the new methodology applied to these malignancies.2,3 However, although in Mexico attention to some non- Hodgkin lymphomas is cost-free (covered by a Federal Program), treatment of most of these neoplasms is paid by the patients, which makes mandatory to review the impact of actual diagnosis, stratification and therapeutical strategies in this group of entities to identify and propose options that may have a social impact in such patients.
Definition and classification
Lymphomas constitute a broad group of malignancies that have been classified by WHO.1 The main objective of this classification is to identify different entities according with clinical, morphological, immunohistochemistry, genetic and molecular analysis. This classification divides Hodgkin lymphoma (HL) in five groups, and non- Hodgkin lymphoma (NHL) according to cell origin, belonging to B-cells 36 defined entities and six temporary entities; T-cell lymphomas are grouped in 23 defined and four temporary entities.
Epidemiology
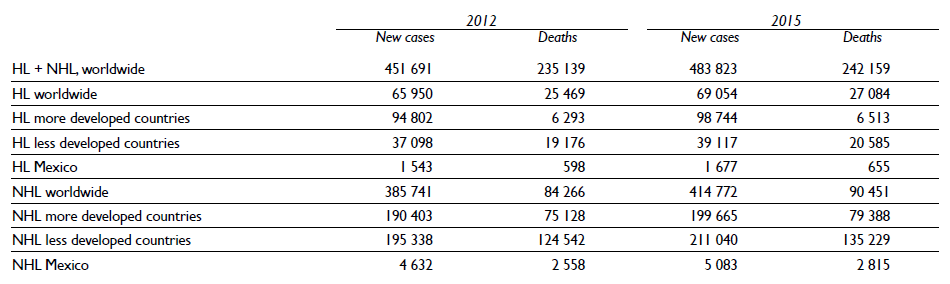
According to Globocan,4 an increase of Hodgkin and non- Hodgkin lymphoma has been documented worldwide (table I). HL has a higher incidence in more developed regions (2.0 x 100 000), in comparison with less developed regions (0.5 x 100 000); however the proportion of deaths is higher in the latter. A similar pattern has been documented also in NHL, with a global incidence of 4.3 x 100 000; but particularly higher, up to 12.8, in more developed countries compared with 2.8 in less developed countries. The higher proportion of deaths occurs in less developed countries, which may reflect either diagnosis in later stages of the disease, or lower access to optimal medical treatment.
Diagnosis
Diagnosis requires a histological analysis of lymph nodes or extranodal disease, including a panel of immunohistochemistry analysis. A Mexican guide of the minimal recommendations to evaluate NHL has been published, and is summarized in table II.5
After histological diagnosis, clinical staging is mandatory. For such purposes clinical chemistry must include liver and renal function evaluation; β2 microglobulin; LDH; blood cytology and determination of HIV status, and hepatitis B and C profile. An image method is required and Positron emission tomography (PET-CT) has been set as an ideal tool for staging disease for both NHL and HL. Sensitivity of PET-CT is particularly useful for extranodal disease. Upstaging occurs more often than downstaging, with management implications in some patients. Management change after upstaging is more common in Follicular lymphoma (FL) than other lymphomas, especially for patients with limited disease on CT.6
Bone marrow involvement must be discarded in lymphoproliferative disorders. Focal Fluordeoxyglucose (FDG) uptake within the bone or bone marrow, liver, and spleen is highly sensitive for involvement in HL and aggressive NHL and may obviate the need for bone marrow biopsy.7 In contrast, diffuse increased uptake may occur with abnormal focal uptake, but in HL, diffuse uptake without focal activity often represents reactive hyperplasia and should not be confused with lymphomatous involvement. PET-CT can miss low-volume involvement, typically 20% of the marrow and coexistent low-grade lymphoma in Diffuse Large B Cell Lymphoma (DLBCL), although this rarely affects management. The sensitivity of PET for diffuse marrow involvement is limited in FL, mantle-cell lymphoma, and most indolent lymphomas, where biopsy is required for staging.
If PET-CT is not available, computed tomography is recommended, and bone marrow aspiration is indicated. Finally, evaluation of ejection fraction of left ventricle is indicated in all patients that may require treatment with anthracyclines.
Additional invasive procedures, such as lumbar punction may be required in patients with nasal or centrofacial involvement or patients with HIV infection. Finally, if Waldeyer ring is involved, gastrointestinal endoscopy is recommended.3
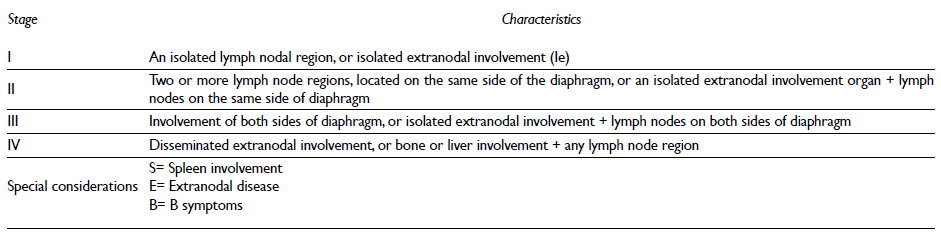
Clinical stage has been considered with Ann Arbor classification (table III). The presence of B symptoms (fever, profuse diaphoresis or loss weight > 10 %) requires to be considered within clinical stage.
Treatment
Local treatment of lymphomas with radiotherapy is indicated for low grade lymphomas, or as consolidation after systemic therapy for initially bulky disease.
Systemic treatment is indicated according to clinical entity, stage, number or previous treatment, as well as prognostic factors, as has been considered within international and Mexican guidelines.2,3,5
New approaches have been developed, including immunotherapy, monoclonal antibodies, iMIDs (immunomodulatory drugs), as well as targeted therapy, such as tyrosine kinase inhibitors:
1) Monoclonal antibodies (mAb): mAbs represent the cornerstone of passive immuno-therapy, which involves engineering of B or T cell receptors targeting a desired antigen and infusion into patients with disease.8 The CD20-directed monoclonal antibody rituximab established a new era in lymphoma therapy.9,10
Thereafter, obinutuzumab (GA101) which represents a type II mAb increased ADCC and direct apoptosis both in vitro and in vivo,11 and in 2013 was approved by FDA for untreated Chronic lymphocytic leukemia, however this antibody has been tested for aggressive B-cell NHL, as well as DLBCL and MCL, with promising results.8,12 Other epitopes in CD20 antigen have also been targeted and ofatumumab has been tested in indolent and agressive NHL as single agent or combined with chemotherapy.13 Veltuzumab, which differs from rituximab only in one aminoacid, has demonstrated response rates of 44% in patients previously treated with rituximab.(14) Other anti CD20 monoclonal antibodies, like ocrelyzumab and LY2469298 are currently in clinical trials.
Epratuzumab, an IgG1 humanized anti CD22 mAb, has been evaluated as single agent in indolent and agressive NHL and DLBCL.15 Its response rate increased significantly when added to rituximab in FL (64%) and up to 96% in DLBCL.16
CD19 acts as a co-stimulatory molecule for B-cell receptor signaling.(8) MEDI-551 is an afucosylated anti-human CD19 mAb with a response rate of 24% in heavily pre-treated DLBCL an FL in phase I-II trials.17
CD40 is expressed in more than 90% of B-cell malignancies. Lucatumumab and dacetuzumab, both anti CD40 mAb have shown response rates of about 33% in pretreated rituximab patients.18,19
Immunotoxins composed of antibodies and cytotoxic plants or bacterial toxins were evaluated in clinical trials in patients with relapsed HL and Anaplastic Large Cell Lymphoma (ALCL).10 Brentuximab vedoietin has shown significant activity in heavily pretreated HL, including relapse after autologous stem cell transplantation and is approved in this indication.
The chemokine receptor CCR4 is expressed on a subset of Type 2 helper (TH) and regulatory T-cells (Treg) and is involved in lymphocyte trafficking. Many adult Peripheral T Cell Lymphoma (PTCL) express both CCR4 and its ligands. CCR4 (+) T-cell lymphomas are associated with a poorer prognosis, possibly because of downregulation of T-cell mediated antitumor host response.20 Mogamulizumab has shown preliminary results in T-cell leukemia/lymphoma (ATLL) with response of 50% and a median overall survival of 13.7, which lead to its approval in Japan for this indication.21
Programmed cell death 1 (PD-1) is a negative costimulatory receptor critical for the suppression of T-cell activation. Induction of T-cell tolerance via PD1-PD1L interaction is associated with survival of malignant B-cell. PD1 inhibitors, including pidilizumab (CT-011), pembrolizumab (MK-3475) and nivolumab (BMS936558) have shown encouraging results in solid tumors,22 and have also been tested in hematological malignancies, with particularly promising results in Hodgkin lymphoma, where a response rate over 50% in heavily pretreated cHL patients, including 67% failing a prior autologous stem cell transplant and all having disease progression after brentuximab vedotin.23,24
Similarly, nivolumab showed an overall response rate of 87% among 23 heavily pretreated patients and independent of prior brentuximab vedotin exposure; early follow-up data shows 86% PFS at 24 weeks.23 PD1 blockade may also be important across a range of lymphoid malignancies as reflected by a phase II trial of nivolumab, showing responses in DLBCL, FL, and T-NHL.25 PD1 inhibitors seem very promising in preliminary results of clinical trials underway.
Pidilizumab, a humanized IgG-1κ recombinant mAb that targets PD1, demonstrated in combination with rituximab a 66% of objective response.(8)
Bispecific T-cell engagers (BiTE) molecules contain the variable domains of two antibodies joined together: one binds CD19 and the second binds the CD3 antigen of T-cells. This complex activates T-cells to destroy the tumor cell via perforin-mediated apoptosis. Blinatumumab, a BITE mAb, was used as single agent in NHL, where it showed an objective response of 82%, lasting up to 32 months.(26)
2) Proteasome inhibitors: The ubiquitin-proteasome pathway controls protein content and function through the degradation of polyubiquitinated intracellular proteins.
Bortezomib was the first drug of this group and was approved for the treatment of mantle cell lymphoma after chemotherapy failure. Other entities with encouraging results are refractory cutaneous T-cell lymphoma, non-germinal center subtype of diffuse large B cell lymphoma, follicular lymphoma, marginal zone lymphoma and Waldenstrom's. Thereafter, carfilzomib, an irreversible inhibitor of the catalytic activity of proteasomes has also been tested in lymphomas.27,28
3) Histone deacetylase inhibitors: HDAC inhibitors (HDACis) are potent inducers of growth arrest, differentiation, and apoptosis of tumor cells. There are three HDACi approved in North America for use in lymphomas: vorinostat, romidepsin, and belinostat. Vorinostat is active in cutaneous T-cell lymphomas (CTCL). Romidepsin is approved for use in both CTCL and PTCL. Most recently, belinostat was approved as a single agent for relapsed and refractory PTCL.29-32
Overall, HDACi appear more active in T-cell malignancies and combination trials with chemotherapy are underway. There are currently two ongoing trials of CHOP plus either romidepsin or belinostat.
4) Immunomodulatory drugs (iMIDs): These agents have pleiotropic effects, including decreased IL-6. VEGF and TNF alpha thalidomide is approved for treatment of multiple myeloma and has been used in mantle cell lymphoma (MCL) with limited efficacy. Thereafter, lenalidomide was approved for relapsed MCL, 32 and also has single agent activity in several types of relapsed lymphoma, including FL and MCL. 33 Additionally, this drug has improved the response rate, when added to first line chemotherapy in the sub-type specific activated B cell DLBCL.
5) BTK inhibitors: BTK is inhibited by ibrutinib,34 which is approved in the United States for use in chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL). In heavily pretreated patients with B-cell malignancies, a response rate of up to 60% has been achieved in phase I-II trials. 35 In activated B-cell (ABC) subtype of diffuse large B-cell lymphoma (DLBCL), it has increased responses in 40%.
6) PI3K inhibitors: Further downstream of BTK is PI3K.36 The delta isoform of PI3K is specifically expressed in hematopoietic cells, and it has been targeted with idelalisib (formerly CAL-101, GS-1101), which is now approved by DFA in refractory indolent lymphoma and in CLL. Idelalisib has also activity in CLL, and is synergistic with rituximab,37 with improvement in OS (92% for the combination versus 80% for rituximab alone, HR =0.28 with P=0.02).
IPI-145 is an oral dual inhibitor of both the delta and the gamma isoform, and is currently in active investigation.38,39
7) BCR signaling inhibitors: Several components of BCR signaling are potential targets, including LYN and SYK. Fostamatinib disodium is an oral agent that is highly specific for SYK, with responses ranging from 10% in FL patients to 55% in CLL patients.40
8) Apoptosis: BCL2 overexpression confers a drug resistant phenotype in different lymphomas. Initial drugs targetting BCL2 had only modest activity.
Currently, the most promising agents are direct inhibitors of anti-apoptotic family members, including BCL2, BCLXL, BCLw and MCL.8 Currently, ABT-199, with a higher specificity for BCL2 and BCLXL, showed activity in relapsed/refractory lymphomas.25
Conclusion
The number of agents available to manage lymphomas has increased in the last years. Nevertheless, their cost and toxicity will impact the duration of treatment and compliance. Understanding the biology of lymphomas will be a tool to individualize treatment, according with predictive factors or biologic stratification.











 text new page (beta)
text new page (beta)