In Latin American countries, five year survival from breast cancer varies from 66 to 87%.1-3 There is absence of survival data at population level, due to the limited number of population-based cancer registries.4 A study held in Mexico General Hospital from 1990 to 1999, showed an overall 5-year survival of 59.9%. Women in clinical stage I had higher survival (82%) compared to those in clinical stage IV (15%), (p <0.01).5
It has been suggested that the delay in initiating treatment, if it leads to progression in the clinical stage of the disease, could reduce the survival of women with breast cancer.6 There are two principal types of health care delays: patient's delay, which arises from a delay in seeking medical care after the self-discovery of a possible breast cancer symptom, and system delay, which includes:time in obtaining appointments, scheduling diagnostic tests, receiving a definitive diagnosis and initiating treatment.6,7 Both, patient- and system-delays could worsen the prognosis for women with breast cancer by affecting disease progression and initiation of treatment.7
A meta-analysis of 87 studies showed convincing evidence that women who initiated treatment 3 to 6 months after the appearance of symptoms had a significantly lower five-year survival than those who waited <3 months.8 Recent studies suggest that care delay does not adequately explain breast cancer survival,9,10 because sicker patients could receive quicker medical care.6,7,10 A meta-analysis published in 2013 showed that for every four weeks of delay between surgery and the initiation of chemotherapy, a statistically significant reduction of 15% in overall survival was observed.11 The so-called wait time paradox is in discussion, and it proposes that various factors (e.g., tumor biology, health infrastructure and patient behavior) aside from time influence survival.12,13
Income, education, insurance, age and ethnicity could affect access to health care services and thus influence health care delay, the clinical stage at the time of diagnosis14 and survival.14-17 In the United States, women without insurance or with Medicaid showed lower survival rates.15,18,19
In addition, lower education, greater distance between the patient's home and the hospital where women receive health care and delay in starting treatment have been, also associated with larger tumors.20,21 This study assess the effect of health care delays: from the first symptoms to the first contact with a doctor, from receipt of mammography results to receipt of diagnostic biopsy results, and from diagnosis to the initiation of treatment, on five-year survival. We evaluated these delays in a sample of women with breast cancer histopatologically confirmed who attended second- and third-tier hospitals in six states nationwide.
Materials and methods
Study population
The present study uses data from a study, whose primary aim was to analyze the integral care processes for women who sought radiology and oncology services in Mexico. The present study includes 854 women from 35 to 69 years of age who were histopathologically diagnosed with breast cancer between January 2007 and December 2009. These cases were recruited from 11 medical departments in hospitals of principal health institutions, including the Mexican Social Security Institute (Instituto Mexicano del Seguro Social, IMSS) (n= 101), the State's Employees' Social Security and Social Services Institute (Instituto de Seguridad Social al Servicio de los Trabajadores del Estado, ISSSTE) (n= 61), the Secretariat of Health (Secretaría de Salud, SS) (n= 655) and the Secretariat of National Defense (Secretaría de la Defensa Nacional, SEDENA) (n= 37) from five states in Mexico and Mexico City. Women were selected from the waiting rooms of breast cancer clinics. All were in treatment at the time of the interview (surgery, radiotherapy, or chemotherapy). Follow-up continued until December 31, 2013. Nurses were trained to administer face-to-face interviews in all medical departments. The interviews were carried out in a space where the patients' privacy could be respected. The study was approved by the ethical research committee of the National Institute of Public Health of Mexico and by the participating hospitals. Every participant gave her consent.
Study variables
The survival time in months starting from the initiation of treatment until death or until the end of follow-up was estimated. Initiation of treatment was defined as the date that the surgery, chemotherapy or radiotherapy was carried out, whichever occurred first.
Time variables were constructed as follows: a) total time in natural days from first symptom to the initiation of treatment; b) time in natural days from first symptom to consultation with a doctor about symptoms; c) time in natural days from receipt of the mammography results to diagnostic biopsy results, and d) time in natural days from biopsy to the initiation of treatment.
Other variables included: age, age at diagnosis (tertiles), socioeconomic status (low, middle, high), clinical stage (I, II, III and IV), insurance system (SS, IMSS, ISSSTE and SEDENA), use of preventive health services (smear test, blood pressure, and blood sugar and cholesterol screening tests - at some point in life - Yes/No), family history of breast cancer, report of breast self-exam (at some point during life - Yes/No), and breast signs or symptoms, i.e., lumps, pain, and other (thickening of the skin, nipple retraction, secretion from the nipples, orange peel skin texture, changes in the size and shape of the breast or dimpling).
Sources of information
Patient's interviews
The dates of interest were obtained from interviews that were carried out with the women diagnosed with breast cancer who attended clinics included in the study. We inquired as to the mammography date, the date of diagnostic evaluation, the date of biopsy, the date that the result was received, and the date that treatment was initiated. If the woman received surgical treatment, radiotherapy, chemotherapy, or hormone therapy, the initiation and termination dates of the procedures were solicited. To corroborate the results, each woman was asked to show the medical care card on which these dates were written.
Medical records
In addition, the participants' records were reviewed to corroborate dates of interest, and to obtain information regarding the size of the tumor, clinical stage and exams to determine the extent of the disease.
Mortality data base
Mortality information was obtained from the database on mortality kept by the Epidemiological and Statistical Mortality System (Sistema Epidemiológico y Estadístico de las Defunciones, SEED). The use of the SEED database was made possible by an agreement established between the Secretary of Health and the National Institute of Public Health of Mexico. Additionally, a letter of commitment to confidentiality was signed with regard to the use and disclosure of information from the analysis of the mortality database. To identify the cause of death, the Guide for completing death certificates and fetal deaths of the Mexican Center for the Classification of Diseases (Centro Mexicano para la Clasificación de Enfermedades, CEMECE) was used.22 The following data were collected: 1) death data, i.e., date of death and 2) cause of death, according to the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) (C500 to C509).
Databases linking
The software link Plus [Beta Version 3.0] was used to link the databases and identify women who had died. This software is a probabilistic cancer registry-linking program developed by the United States' Centers for Disease Control (CDC) Division of Cancer Prevention and Control in support of the CDC's National Program of Cancer Registries (NPCR). The common variables in the two databases were identified (name, paternal last name, maternal last name, and date of birth).
Statistical analysis
The time variables were categorized in quartiles. The medians of the different times estimated as functions of certain sociodemographic and clinical characteristics were analyzed utilizing Kruskall-Wallis tests.
The present study is a retrospective follow-up of women from initiation of treatment to death or the end of follow-up, with death being the event of interest. The dependent variable corresponded to the women's survival time, i.e., the time measured in months from initiation of treatment (initial event) to death/the end of follow-up (final event). Right and administrative censors were those women who died from another cause or those who survived the study follow-up time, respectively. Initially, a non-parametric analysis was carried out (i.e., Kaplan Meier)23 to estimate the probability of dying at five years from initiation of treatment. The estimated probability was stratified by clinical stage (I-IIA and IIB-IV) using the log-rank test to evaluate the similarity between survival functions. To evaluate the effect of the different times between medical care on the instantaneous risk of dying, a Cox proportional hazards model was used. Additionally, the trends in the time were evaluated by placing the variables into the Cox model in a continuous manner. The following variables were considered as potential confounders: age, age at diagnosis, socioeconomic status, insurance system, use of preventive health services (smear test, blood pressure, and blood sugar and cholesterol screening tests) , clinical stage, breast self-exam, family history of breast cancer, tests to determine the extent of disease (chest teleradiography, liver ultrasound, bone scan, metastatic bone series imaging, and positron emission tomography), and signs and symptoms for which they went to the doctor to have their breasts examined. Variables that had a p<0.20 in the bivariate analysis and those considered by the literature were taken into account in the multiple analysis.7,9,12,24-29
The life table was used to calculate the percentage of women who survived at five years, as a function of certain sociodemographic and clinical variables as well as clinical stage (I-IIA vs. IIB-IV). All of the statistical analyses were carried out with STATA version 14.0 software.*
Results
In total, 854 cases of histopathologically confirmed breast cancer were included in the survival analysis. During the five years follow-up in 100% of women, 193 of them died, 166 (19.4%) were due to breast cancer and 27 were due to other causes. By clinical stage, the deaths were distributed in the following manner: stage I (5/88, 5.7%), stage II (27/324, 8.3%), stage III (94/235, 27.5%) and stage IV (31/62 , 50%). The related proportion of women with in situ tumors was 2.9% (24 women), and these were not included in the analysis.
The sample characteristics are described in table I. The median age of the women was 52 years (IQR=44.2-60.3). The proportion of women with breast cancer by clinical stage was 10.5% in stage I, 38.6% in stage II, 40.7% in stage III, and 7.4% in stage IV. The median health care times estimated in the study were the following: total time = 139 natural days (IQR=82.5-258), time from when a woman felt a symptom to when she consulted about a doctor about it = 30 natural days (IQR=6-150), time from the receipt of the suspicious mammography results to diagnostic biopsy = 31 natural days (IQR=14-56) and time from diagnostic biopsy to initiation of treatment = 37 natural days (IQR=18-63).
Table I Sociodemographic and clinical characteristics in histologically diagnosed women with breast cancer in Mexico. 2007-2009
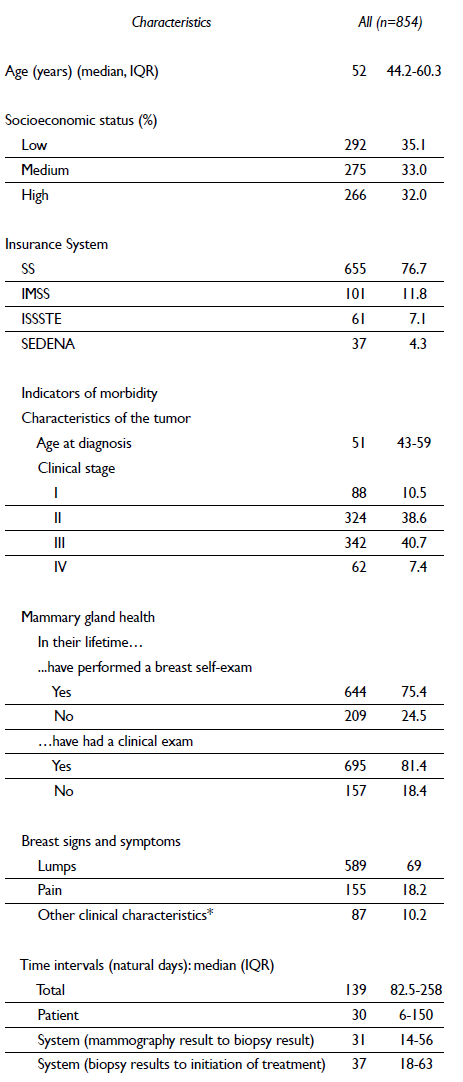
* Thickening of the skin, nipple retraction, nipple secretions, orange pee skin texture, changes in the size and shape of the breast or dimples. Some percentages do not add to 100% because of missing data
Information regarding the medians of the estimated times is presented in table II. When evaluating the medians of the distinct times by clinical stage, statistically significant differences were observed (p<0.001). With regard to the socioeconomic level, women of a lower socioeconomic status reported a greater time in seeking medical care (time from first symptoms to consultation with a doctor), median of 60 days, IQR=7-185, compared with those with a high socioeconomic level, median of 30 days, IQR=3-90 (p <0.05). Women of a high socioeconomic status generally reported shorter times from receipt of the suspicious mammography results to diagnostic biopsy. When analyzing the time that the patient took to contact their doctor -to initiation of treatment, longer times were consistently observed in the more advanced clinical stages: stage III, 152 days (IQR=86-275), and stage IV, 201 days (IQR=95-410); p=0.002 (table II). However, 35% of women received the result of the diagnostic biopsy 20 days after they received the suspicious mammography results. In addition, 42.6% reported having initiated treatment 30 days after the diagnostic biopsy, and 30.8% initiated treatment 60 days after the receipt of biopsy results. The majority of the women (68.6%) received surgery as a first treatment; however, the percentage of women who received surgery differed by clinical stage: 93.2, 90.1, 42.5 and 57.1% for stages I, II, II and IV, respectively (p<0.001) (data not shown).
Table II Medians and interquartile ranges by times in health care and their relationships with certain sociodemographic and clinical variables in women histopathologically diagnosed with breast cancer in Mexico, 2007-2009
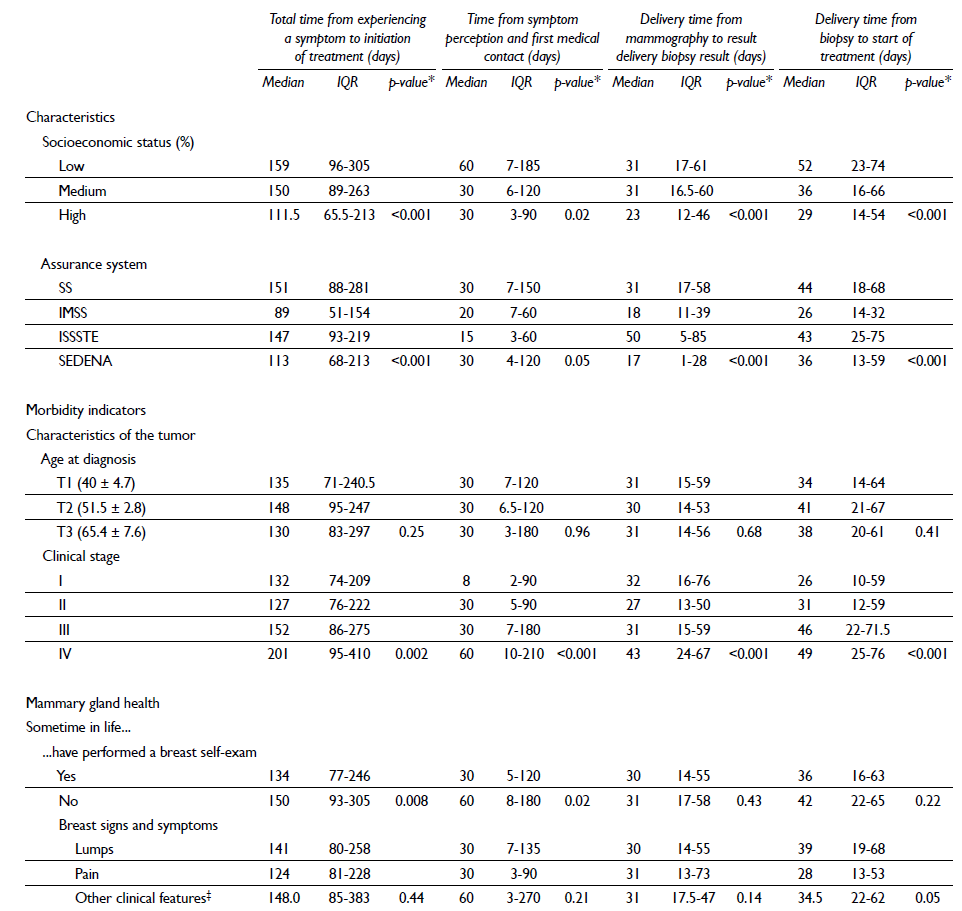
* Kruskall Wallis
‡ Thickening of the skin, nipple retraction, nipple secretions, orange peel skin texture, changes in the size and shape of the breast or dimples
Figure 1 shows the Kaplan-Meier estimations by quartiles (Q1-Q4) from the time of receipt of suspicious mammography results to diagnostic biopsy results (in months), by clinical stage (I-IIA vs. IIB-IV) and statistically significant log-rank test (p<0.001). Deaths among women in stages IIB-IV increased with increasing wait time.

Figure I Kaplan-Meier estimators for five-year survival, by quartile of delay between receipt of mammography results and receipt of diagnostic biopsy results, by clinical stage (I-IIA vs IIB-IV). A. Survival in the first quartile of care delays among women with breast cancer, by clinical stage (log-rank, P = .001). B. Survival in the second quartile of care delays among women with breast cancer, by clinical stage (log-rank, P = .001). C. Survival in the third quartile of care delays among women with breast cancer, by clinical stage (log-rank, P = .001). D. Survival in the fourth quartile of care delays among women with breast cancer, by clinical stage (log-rank, P = .001)
The life table of women as a function of certain sociodemographic and clinical variables and clinical stage (I-IIA vs. IIB-IV) is shown in table III. Women in clinical stage IIB-IV (advanced) showed, in general, a lower five-year survival.
Table III. Cumulative five-year survival (life table) by certain sociodemographic and clinical variables and clinical stage (I-IIA vs IIB-IV) in women histopathologically iagnosed with breast cancer in Mexico, 2007-2009
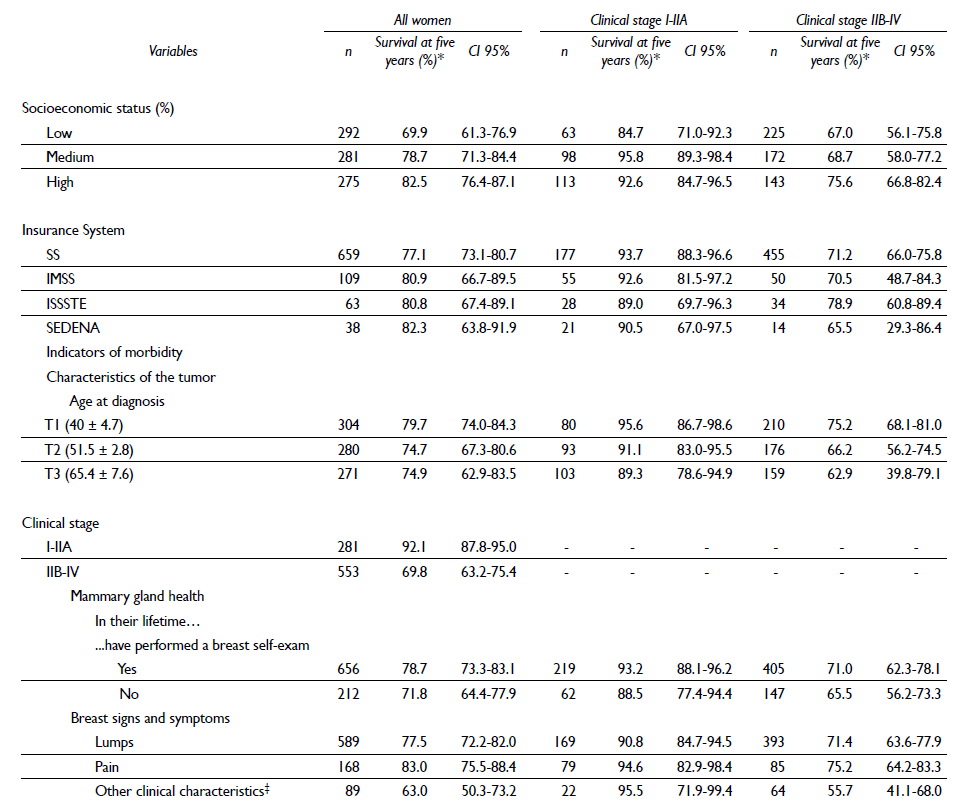
* The probability of survival at five years is presented in each estimation, given that they survived the previous year
‡ Thickening of the skin, nipple retraction, nipple secretions, orange peel skin texture, changes in the size and shape of the breast or dimples
Finally, the results of the bivariate and multiple analyses are shown in table IV. Results from the multivariate analysis showed that the principal variables that were associated statistically significant with lower survival were increased time in days from receipt of mammography results to diagnostic biopsy results, advanced clinical stage, history of not having done a self-exam and perception of the following symptoms: thickening of the skin, nipple retraction, nipple secretions, orange peel skin texture, changes in the size and shape of the breast or dimples.
Table IV Bivariate and multiple analysis of sociodemographic and clinical variables associated with survival in women histopathologically diagnosed with breast cancer in Mexico, 2007-2009
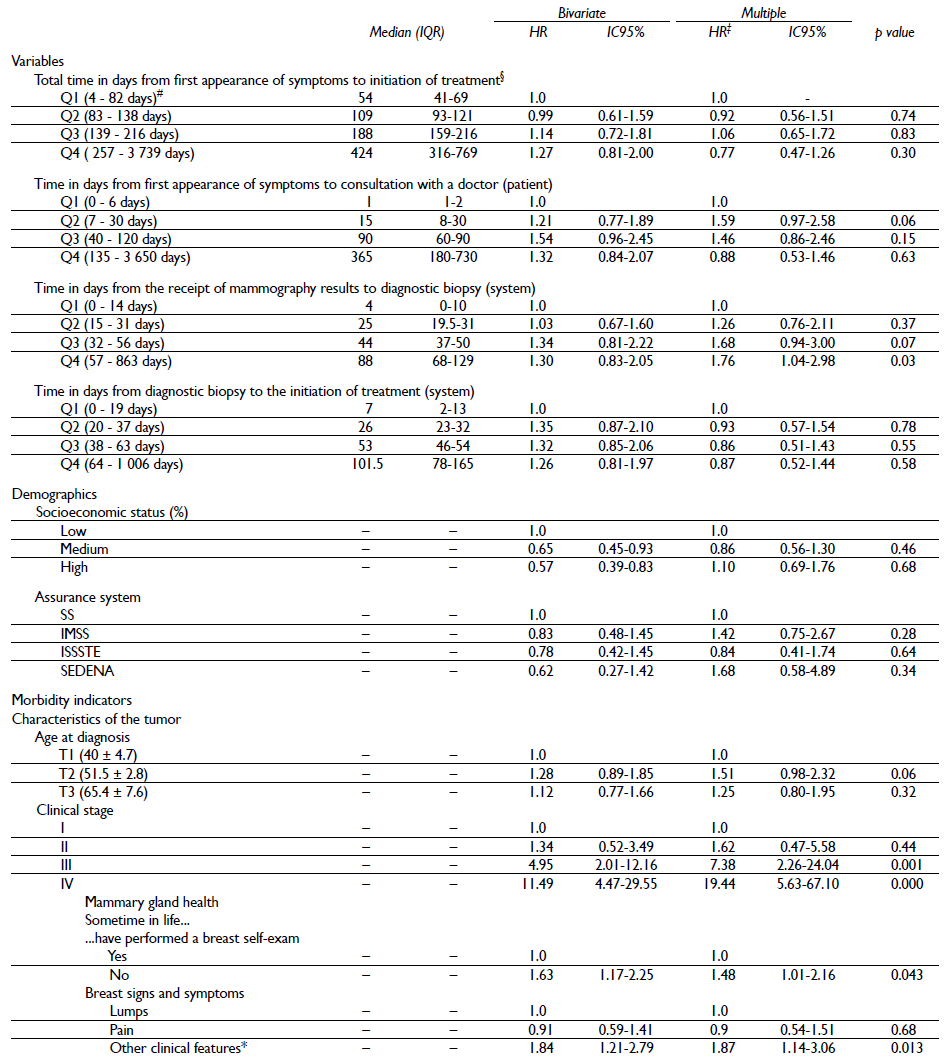
* Thickening of the skin, nipple retraction, nipple secretions, orange peel skin texture, changes in the size and shape of the breast or dimples. Some percentages do not add to 100% due to "missing" data
‡ Instantaneous risk model, adjusted by all the variables included in the table
§ Instantaneous risk model, adjusted by the variables included in the table, except for patient and system times
# Range (minimum - maximum)
Regarding the time in days from receipt of mammography results to diagnostic biopsy results, compared to women in quartile 1 (Q1), survival was lower among women in Q3 and Q4 (HR =1.68, 95% CI 0.94-3.00; HR = 1.76, 95% CI 1.04-2.98, respectively),adjusted for socioeconomic status, insurance system , age at diagnosis, clinical stage, history of having ever done a self-exam and perception of symptoms in the breast (table III). No statistically significant association was observed for other time indicators.
Discussion
We observed that the delay in the time from the receipt of mammography results to the receipt of diagnostic biopsy results was related to an increased risk of disease-related mortality. The times from symptom perception to seeking medical care and from receipt of diagnostic biopsy to initiating treatment were not statistically significantly associated. Advanced clinical stages (III and IV), not having a history of self-examination and the presence of signs and symptoms apart from feeling a "lump" or pain were also associated with an increased risk of mortality. With regard to the clinical stage, 10.5% of the women were in clinical stage I, similar to the findings of López-Carrillo and colleagues30 and in contrast to findings in the United Kingdom, where 37.4% of women belonged to this group,31 in Germany (48.4% of tumors classified as localized)24 and in Canada (46.9%).32
Total time
No association was observed between the total time from initial signs and symptoms to the initiation of treatment and the risk of dying from breast cancer. Richards and colleagues (1999) showed that marked delays between the onset of symptoms and the initiation of treatment were associated with lower survival rates.33 Consistent with our results, Smith and colleagues found that the association between total time and survival was not statistically significant (HR=1.00; 95%CI 0.99-1.00).9
In Mexico, two studies have estimated this delay but not its association with survival. Bright and colleagues (2011) documented that on average, the time from onset of symptoms to treatment was 8.4 months: 8.6 months for early clinical stages and 7.5 months for advanced clinical stages.34 Unger and colleagues (2015) reported that the median time between identification of the problem and initiation of treatment was seven months. They also found that the broadest interval was that between the first medical appointment and diagnosis (median of four months) and that almost half of women were diagnosed in clinical stages III and IV.29
Time between the first symptom and seeking medical care
In this study, we divided the time into quartiles to determine whether length of time between first symptoms and consultation with a doctor were associated with the risk of death.35 The only statistically significant association with risk was found for the second quartile compared to women in the first quartile, and the association was marginal (HR=1.59 95%CI 0.97-2.58, p=0.06). The median patient wait time when seeking medical care was 30 days (IQR=6-150 days). Similarly, a study carried out in a hospital in Mexico showed that women waited an average of 38 days from the onset of symptoms to the first medical contact.34 Unger and colleagues reported that the median delay in the patients analyzed was 10 days, IQR=0-60 days.29 One study showed that women with greater delays in seeking medical care were diagnosed in advanced stages of the disease (OR=6.37, 95% CI: 2.84-14.30).36 Another study revealed a median patient delay of 16 days, a shorter interval than that reported in our study. Another finding was that in women with well-differentiated tumors, the proportion of advanced-stage tumors did not change when the delay increased (p-trend=0.83), whereas in women with poorly differentiated tumors, this trend was monotonic (p-trend=0.03).24 Studies that focused on patient delay consistently found that this delay is shorter for patients who reported monthly breast self-exams compared to those who carried out self-exams less frequently.24 We similarly found that those who carried out self-exams experienced shorter delays (30 days IQR=5-120) than those who did not carry out practice these exams (60 days IQR=8-180) (p=0.02). Huguley and colleagues found that women who performed self-exams had smaller primary tumors and fewer lymphatic axillary nodules compared to those who did not perform exams. Five-year survival was better in women who practiced self-exams (76.7%) compared to those who did not (60.9%) (p=0.0001).37 In our survival analysis, a significant association was observed between not performing breast self-exams and the risk of dying (HR=1.48 95%CI 1.01-2.16, p=0.043). Other studies have shown similar results.25,38,39 Burguess and colleagues reported that a greater delay on the patient's end was correlated with the size of the tumor (p=0.0002) and with the clinical stage (p=0.01), but not with the self-exam (p=0.4).40 Similar results were observed by Auvinen and colleagues.41 In contrast, a study that reported factors associated with patient delay found that self-examination was not associated with this delay (OR=0.73 95% CI 0.31-1.74).42 However, these studies did not associate self-examination with survival.
Time between receipt of mammography results and diagnostic biopsy results
Greater time between the mammography and the diagnostic biopsy results was associated with a 76% increase in risk (HR=1.76; 95%CI 1.04-2.98). The median delay was 31 natural days (IQR=14-56). Studies carried out in countries with extensive resources suggest that patient delay can be linked to presentation with larger tumors and worse survival.8,43 However, less evidence is available with regard to system-attributed delays.8,27 In contrast to our study, Smith and colleagues revealed that diagnostic delay (time interval from the date of the first screening exam with abnormal data -clinical exam or mammography- to the date of diagnosis) was not significantly associated with the risk of dying (HR=1.00, 95%CI 0.99-1.00) in 314 patients from the South Carolina breast and cervical-uterine cancer early-detection program.9 A greater delay in diagnosis could be associated with greater mortality in urinary tract, colon and breast cancers,33,44,45 though other studies have shown no such association for breast, colorectal, lung and gastroesophageal cancers.26,44-47 Still other studies found elevated mortality with short diagnostic intervals for lung cancer48,49 or high mortality with short and long diagnostic intervals for colorectal cancer (i.e., a U-shaped relationship).12 These variations in the associations between the diagnostic interval and survival have been explained by differences in the diagnostic mechanisms, the behavior of the doctor and patient, health care system performance and the biological behavior of the tumor.12,26 McPhail and colleagues showed that one-year survival is affected by the clinical diagnostic stage. In breast, prostate and colorectal cancers, patients in stage IV have a greater effect on survival.31 In our study, clinical stages III and IV were associated with a greater risk of dying compared to stage I (HR=7.38 95%CI 2.26-24.04, HR=19.44 95%CI 5.63-67.10, respectively). Delay affects progression; Knaul and colleagues indicated that of patients who were in stage II in 2002, 55% were still in the same stage by 2006, while 22% and 18% progressed to stages III and IV, respectively, and only 5% died. Of the patients who were in stage III in 2002, only 22% were still in the same stage, while 70% progressed to stage IV and 9% died. Of the patients diagnosed in stage IV, 86% perished during the study period.50 Although progression was not measured in terms of clinical stage in this study, patient and system delays could have an effect.
Time between the receipt of diagnostic biopsy results and initiation of treatment
The time between receipt of diagnostic biopsy results and the initiation of treatment was not associated with the risk of dying. The median time in natural days was 37 (IQR=18-63). Sainsbury and colleagues examined information on 36 222 patients with breast cancer from 1976 to 1995. The results showed that provider delays at treatment initiation increased from 10 to 12 days between 1976 and 1995 and that the median delay from the first hospital visit to treatment initiation increased from seven to 13 days in the same period. These times are shorter than those obtained in our study. In addition, the patients who experienced delays of <30 days between the doctor's referral and treatment had lower survival compared to women who experienced longer delays (p<0.001),51 which could be explained in part by the low percentage of patients who experienced delays >4 months (2.6%). Additionally, patients who sought health care and received treatment within the first 30 days had lower survival than those with greater delays. Potentially, rapid growth of the tumor could prompt the patient to seek earlier medical care, but these tumors could also have more aggressive phenotypes.51 In our study, 40.4% of women experienced a delay >30 days between the receipt of the mammography results and the receipt of the biopsy results, and 49.5% of women experienced a delay >30 days between the receipt of biopsy results and the initiation of treatment. Additionally, 82.1% of women experienced delays >67 days between the initial symptom and the initiation of treatment. McLaughlin and colleagues (2012) carried out an analysis of a retrospective cohort to estimate the effect of the time between the diagnosis and treatment on survival in adult women with breast cancer in the United States. Their results showed that even if the time between diagnosis and the initiation of treatment did not affect survival among women in early clinical stages, it did affect the survival of women in advanced stages, particularly when the time between diagnosis and treatment was greater than 60 days (HR=1.66 95%CI 1.00-2.77).6 Some authors suggest that in some cases, delays can be due to the lack of specificity in the detection of alarming signals or "red flags". Some patients have reported seeking referral to a specialist three or more times before diagnosis.52 One study showed that the number of procedures undertaken before initiation of treatment, specifically surgery, was associated with a greater waiting time. Women who were referred directly after their initial procedure had a median wait time of 24 days, compared to 32 and 48 days in those who underwent one and two procedures, respectively. Potentially, a greater number of complex diagnostic procedures may offer advantages despite the associated delay in accessing surgery.53 We were unable to estimate the modifying effect of the number of biopsies. In England, to improve survival of patients with cancer, strategies that focus on an early presentation (reducing delay to improve the clinical stage at diagnosis) have been established through health care-quality assurance and guarantees of equity in services.54
In Mexico, the lack of resources (training of professionals) and sub-optimal infrastructure for providing opportune care to women who seek health services could partially explain the delays in diagnosis and initiation of treatment,55,56 as can be observed even on the first level of care,57 as well as in the lack of quality monitoring of mammography and the insufficient number of radio-oncologists.58 In this way, regarding the standards established by the Official Mexican Standard (NOM-041-SSA-2011), delays in health care are far removed from the indicators of access and effectiveness59 as well as from international standards.60
However, the relationship between diagnostic interval and grade of tumor aggressiveness may be counterintuitive; patients with wider diagnostic intervals show greater survival compared to those with shorter diagnosis intervals (waiting-time paradox). This correlation is confounded by the differential indications for a diagnostic test according to clinical characteristics.21 For example, women in clinical stage IV are seen faster than those in clinical stage I. In this way, it is possible that the more aggressive tumors cause a more urgent need for care as well as accelerated dissemination, which implies a worse prognosis. These patients might also face, for example, toxicity due to the treatments or surgical complications.9 In contrast, slow-growing tumors produce non-conclusive symptoms that result in more prolonged diagnostic intervals12,61 but that leave time to initiate treatment.45
Age at diagnosis
In this study, age at diagnosis was marginally associated with the risk of dying. Compared to women in the first tertile, women in the second tertile (51.5 years SD 2.8) were at a greater risk of dying (HR 1.51 95%CI 0.98-2.32, p=0.06). The largest care delay was that between receipt of mammography results and receipt of diagnostic biopsy results (Tertile 1, age at diagnosis, 34 natural days, IQR=14-64; Tertile 2, 41, natural days, IQR=21-67; Tertile 3, 38 days, IQR= 20-61; p=0.41). Age is a risk factor for breast cancer, and age at diagnosis is related to survival.62 In contrast to our findings, Brandt and colleagues showed that women younger than 40 years had a worse prognosis (RR=1.40 95%CI 1.04-1.88) compared to women aged 40 to 49 years. In women aged 50 to 59 years, the risk of dying was not statistically significant (RR=1.29 95%CI 0.98-1.70), particularly in women with positive axillary nodules.63 Other studies have shown that increasing age is associated with worse survival,28,64,65 while others have not found this association.66,67
Signs and symptoms
Compared to feeling a nodule in the mammary gland, symptoms like orange peel skin texture, nipples retraction, secretion from the nipples, changes in the size and shape of the breast or dimples are associated with 87% higher risk (HR=1.87 95%CI: 1.14-3.06, p=0.013). Similarly, Redondo and colleagues showed a statistically significant association between delays to initiating treatment greater than 30 days and the ductal type, as well as signs or symptoms apart from feeling a nodule in the breast (p= <0.05).10 The literature has shown an association between skin lesions and discharge from the nipple and a nine-fold increased risk of breast cancer, with a two-fold increased risk when pain is reported. It is important that health professionals identify and communicate possible risks associated with cancer to swiftly refer any patient with suspected disease to ensure continuity of care.68 In Mexico, López-Carrillo showed that 90% of women surveyed in three hospitals nationwide had identified the presence of a lump in their breast on their own.30
The time to being re-called for biopsy is an essential component of a program's success.69 Performing a biopsy as soon as possible is particularly important to health outcomes,70 initiation of treatment and subsequent survival. Diagnosis requires resources, time, technology, and personnel, among other factors.71-73 Thus, completion of follow-up with women with abnormal mammography findings is an important goal. In accordance with Bairati and colleagues, continuity of care is the mediator through which services are received as coordinated and uninterrupted events consistent with the medical needs of the patients.74-76 A fundamental trait associated with continuity of care is the preservation of information from past findings, clinical assessments and decisions and the use of these elements in the management of patients in a way that gives stability to clinical assessment objectives and methods and the organized and reasonable development of care. According to Donabedian, providers would ideally share this information to construct a coherent management plan. Having only one provider in charge would facilitate functioning of these constructs; however, given the characteristics of the health system, an organized and appropriate system for transferring patients is needed if more than one provider or information source exists.77
Strengths
This study is the first in Mexico to analyze delays in care associated with survival. This study also provides information relevant to decision-makers and ultimately provides a basis for improving quality of care from the perspective of the users and analyzing improvements in the quality of healthcare services with respect to medical care delays.
Limitations
Although this study does not represent the general population, it may represent the population of women with breast cancer who attend secondary- and tertiary-level medical care centers and oncological centers. Although dates of interest were corroborated in the medical care card and some in the medical records, this strategy was only used in women who did not remember a specific date. Other limitations are related to the use of a death registry as source of mortality data. As well as being a limitation, the use of a mortality registry can also be considered an advantage, given that these types of studies are very costly, which will make it necessary to implement population cancer registries at a regional level in Mexico to evaluate survival, access to diagnostics and quality of health care. Another limitation was the lack of information related to the variables necessary to construct the Nottingham Prognostic index. The histological indicators of tumors that were systemic since their beginnings (triple negative) or those that show slow tumoral biology could better explain the effect of time, as may be the case for other types of tumors that are affected by time. In addition, some patients were excluded because of a lack of data, including clinical stage and diagnostic and treatment initiation dates.
Recommendations
This study provides information on distinct health care systems that is relevant to decision-makers. In addition, it provides a basis for analyzing improvements in medical care from the perspective of the users, which is useful for analyzing improvements in the quality of health services with respect to delays in medical care; however, various factors associated with delays in medical care must be addressed using quali-quantitative research: organizational factors, including access to medical care, and geographical factors, including cultural patterns and other factors. In systems that administer health information in Mexico, histoprognostic indicators should be used to better explain the biology of the tumor, such as the tumor size, number of ganglia and grade of differentiation.
Conclusions
This is the first study in Mexico to analyze access to medical care in association with survival of women with breast cancer. The results of this study suggest that a high percentage of women are found outside of the health-access indicators for Mexico. For this reason, strategies should be implemented to reduce the time from receipt of mammography results to diagnostic biopsy results to increase survival.











 nueva página del texto (beta)
nueva página del texto (beta)


