The activation of peroxisome proliferator-activated receptor gamma (PPARγ) consistently induces apoptosis, inhibits cellular proliferation, and promotes cellular differentiation in both hormone-dependent and hormone-independent mammary tumors.1-5PPARγ is a ligand-dependent transcription factor that belongs to the superfamily of nuclear hormone receptors (estrogen, progesterone). Ligands modulate PPARγ functions, thereby releasing receptor corepressors and permitting binding of coactivators, which facilitates its transactivation.6 An important gene in said transactivation of PPARγ is PPARγ coactivator 1 beta (PPARGC1B), which can also bind to and activate the α and β estrogen receptors (ERs) in vitro in a ligand-independent manner, with greater affinity for ERβ.7
Genetic variants of these genes, Pro12Ala of the PPARγ gene and Ala203Pro of the PPARGC1B gene, have been evaluated in relation to the risk of breast cancer (BC) in previous epidemiological studies. Evidence that the Pro12Ala polymorphism of the PPARγ gene is related to BC is contradictory. In two studies conducted, one in Taiwanese women and the other in the cohort of the Nurses' Health Study, no significant associations between the Pro12Ala variant and BC were found.3,8 In contrast, in a prospective study in the USA, a marginally significant increased risk of BC was detected in homozygous carriers of the G allele.9 For its part, the Ala203Pro polymorphism has scarcely been studied in relation to BC. Wirtenberger and colleagues reported an increased risk of familial BC for the carriers of the GC and CC genotypes of Ala203Pro in a German cohort.7 Li and colleagues, although not evaluating Ala203Pro, found an increased risk of ER-positive BC in Swedish and Finnish women who carried risk alleles of several polymorphisms of PPARGC1B.10 Our research group confirmed the absence of a significant association between the Pro12Ala variant of the PPARγ gene and BC and detected a marginally significant negative association in women who were heterozygous for the Ala203Pro polymorphism of the PPARGC1B gene.11
Recently, our research group has also identified that the adult female residents in northern Mexico, who are exposed to inorganic arsenic (iAs) principally through drinking water12 and food,13 with less capacity for urinary elimination, evaluated through the percentage of elimination of monomethyl arsenic (%MMA), presented an increased risk of BC (Odd Ratio [OR]%MMAQ5vs.Q1= 2.63; 95% confidence interval [95%CI] 1.89-3.66; p for trend < 0.001).14,15 While the mechanisms by which iAs could be a cofactor of BC have not been clearly elucidated, it has been observed that sodium arsenite mimics the effects of estradiol in the BC cell line MCF-7, inducing cellular proliferation, which, in turn, is a risk factor for this type of cancer.16 Additionally, during in vitro tests, iAs interferes with adipogenesis signaling by reducing the expression of PPARγ17,18 and consequently affects the balance of cellular proliferation and differentiation in breast, colon, and prostate tumors.17 Additionally, iAs can act as an endocrine disruptor and can thus be considered related to BC by means of the overexpression of aromatase, an enzyme that limits the rate of estrogen synthesis.19 The expression of this enzyme can be inhibited by PPARγ activation via the overexpression of BRCA1 (tumor suppressor gene) and the inhibition of PGE2 (proinflammatory prostaglandin E2).20
In accordance with the preceding findings, the objective of this study is to evaluate whether the previously observed association between the methylation capacity of iAs and BC is modified according to the presence of the Pro12Ala polymorphism of the PPARγ gene and the Ala203Pro polymorphism of the PPARGC1B gene in Mexican women, considering that BC is a major problem not only in Mexico but also in Latin America.21
Materials and methods
Study population
During the period from 2007 to 2011, a case-control study was conducted in five northern Mexico states (Chihuahua, Coahuila, Sonora, Durango, and Nuevo León) where the incidence of BC is higher than elsewhere in the country.14 For the purposes of this report, the first 197 cases and 220 recruited controls were included.
The eligibility criteria of the cases were the following: women with histologically confirmed BC, a minimum age of 18 years, no history of any other type of cancer, and a minimum of one year of residence in the study area. The patients were identified in hospital units (n = 17) of the Mexican Institute of Social Security (Instituto Mexicano del Seguro Social - IMSS), Secretary of Health, University Hospitals, and the Institute of Security and Social Services for State Workers (Instituto de Seguridad y Servicios Sociales de los Trabajadores del Estado - ISSSTE) located in those states. The response rate was 94.8%.
The controls were healthy women without a history of cancer who were matched with the index case by age (±5 years) and place of residence. Controls were identified in the five aforementioned states, by means of the master sampling frame of the System of National Health Surveys of Mexico, composed of basic geostatistical areas (BGAs) with square-shaped (20-80) regions in urban areas and tracts of approximately 10 000 hectares with identifiable natural limits in rural areas. The BGAs were randomly selected and, within them, the squares, in which the domiciles were located systematically to identify eligible women. When an eligible woman was not found in the domicile or was not available to participate, a new one was located; however, if there was more than one eligible woman, one of them was selected randomly. The study was approved by the ethics committee of the National Institute of Public Health.
Interview
After signing the informed consent, participants were directly interviewed regarding their reproductive characteristics and their consumption of tobacco and alcohol, among other variables of interest in the original study. Anthropometric measurements were also obtained for calculating the body mass index [BMI = body weight (kg)/height (m2)].
Biological samples
The women donated a venous blood sample and a first morning urine sample (prior to any treatment for cancer). Urine samples were collected in sterile, latex-free, polypropylene containers and were stored at -20°C in the Department of Toxicology of the Center for Research and Advanced Studies (Centro de Investigaciones y Estudios Avanzados - CINVESTAV) of the National Polytechnic Institute until dispatched for analysis at the University of Arizona. The blood samples were stored at -70°C in the Genetic Epidemiology laboratory of the Center for Research on Infectious Diseases (Centro de Investigación Sobre Enfermedades Infecciosas -CISEI) of the National Institute of Public Health (Instituto Nacional de Salud Pública- INSP) until their analysis.
Determination of arsenic concentrations
The concentrations (µg/L) of the urinary species arsenite (As3+), arsenate (As5+), monomethylarsenate (MMA5+), dimethylarsenate (DMA5+), and arsenobetaine (AsB), were determined by high-performance liquid chromatography with inductively coupled plasma mass spectrometry (HPLC-ICP-MS) in the Analytical Section of the Center for Risk Identification at the University of Arizona, according to the methodology described by Gilbert-Diamond and colleagues.22 The measurements that were below the limit of detection (LOD) were attributed with the corresponding LODs (AsB: 0.08; As3+: 0.15; As5+: 0.41; MMA5+: 0.12; DMA5+: 0.12) divided by two (LOD/2), in accordance with the suggestion of Barr and colleagues.23 These proportions were similar between the cases and the controls (table I). The urinary creatinine concentration (mg/dL) was determined with a commercial kit with 1 mg/dL as the detection limit, according to the methodology described by the manufacturer.24
Table I Characteristics of the study population
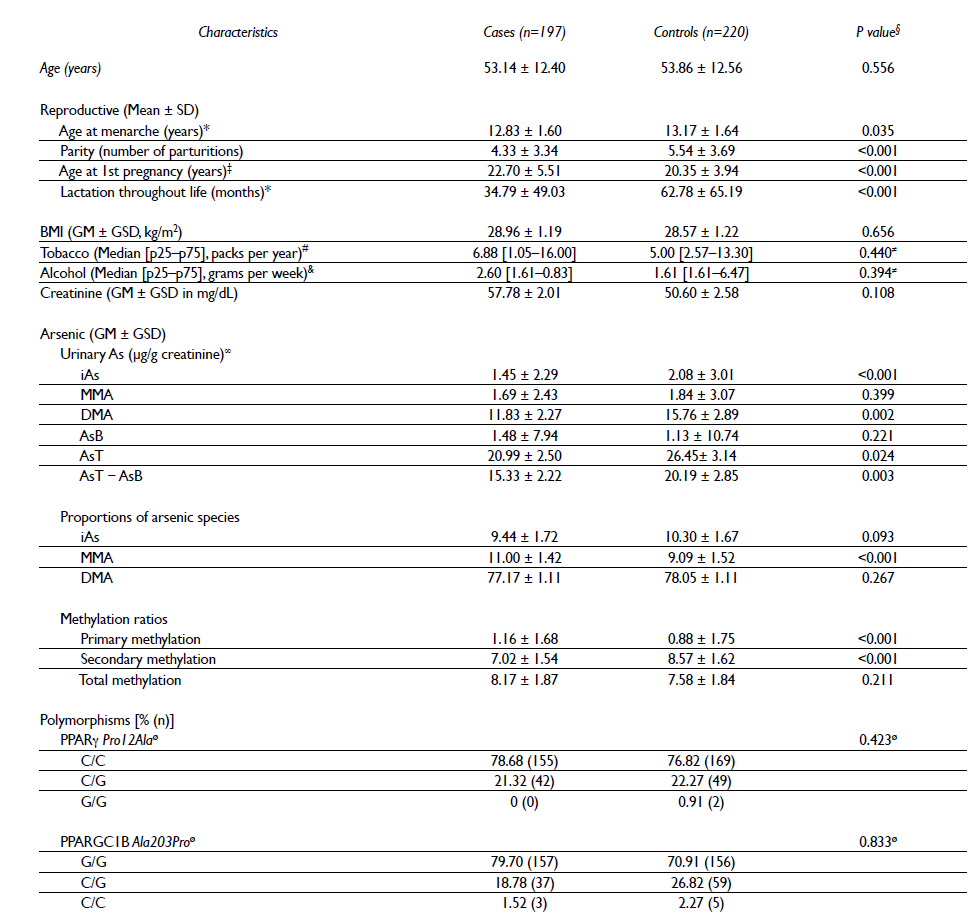
SD= standard deviation, BMI= body mass index, GM= geometric mean, GSD= geometric standard deviation, M= median, p25= 25th percentile, p75= 75th percentile, iAs= inorganic arsenic, MMA= urinary monomethyl arsenic, DMA= urinary dimethyl arsenic, AsB= arsenobetaine, AsT= total arsenic, AsT−AsB= total arsenic minus arsenobetaine
* Age at menarche n= 416, lactation throughout life n= 410, for missing values
‡ Age at first pregnancy n= 395 (22 nulliparous women)
§ P value of Student's t-test for continuous variables
# Only the women who smoked tobacco were considered. The n (cases/controls) was 52/61
& Only the women who consumed alcohol were considered. The n (cases/controls) was 38/22
≠ P value of the Mann-Whitney U test < 0.05
∞ The percentages of samples with no detectable levels of AsB, As3+, As5+, MMA5+, DMA5+ were respectively 31.36%, 20.45%, 66.82%, 4.09%, and 0% among the controls and 20.30%, 33.00%, 71.07%, 1.02%, and 0% among the cases
ø P value of the Hardy-Weinberg test > 0.05
The iAs was obtained from the sum of the concentrations of the urinary species As3+ and As5+, while the total arsenic (AsT) was estimated based on the sum of the concentrations of iAs, MMA5+, DMA5+, and AsB. The proportions of the urinary iAs species (%iAs, %MMA, and %DMA) were obtained by dividing the concentration of each species by the difference between AsT and AsB (AsT-AsB). The following methylation ratios were calculated: primary methylation= MMA/iAs, secondary methylation= DMA/MMA, and total methylation= DMA/iAs.
Determination of the Pro12Ala polymorphism of the PPARγ gene and the Ala203Pro polymorphism of the PPARGC1B gene
DNA was extracted from the blood samples by means of a semi-automated method using an ABI PRISM 6100 Nucleic Acid PrepStation. The purity and integrity of the samples were evaluated using UV spectrophotometry and 0.8% agarose gel electrophoresis.11
The selected polymorphisms, rs1801282 (Pro12Ala) and rs7732671 (Ala203Pro), were analyzed by allelic discrimination by means of end-point PCR with TaqMan probes in an Applied Biosystems 7900 Real-Time PCR System. All of the assays were performed in duplicate and were determined and analyzed using <<Sequencing Detection System>> software (SDS 2.3, Applied Biosystems).
Statistical analysis
The age at menarche, parity, age at first pregnancy, and lactation throughout life presented normal distributions and were compared between the cases and controls with Student's t-test, while the BMI, the distributions of the arsenic species, the methylation ratios and proportions were normalized with a logarithmic transformation. The consumption of tobacco and alcohol could not be normalized, so the medians that were compared with the Mann-Whitney U test are presented. The genotype frequencies of interest were evaluated using the Hardy-Weinberg test.
By means of unconditional logistic regression models, an odds ratio (OR) was calculated with a confidence interval of 95% (95%CI) for each of the urinary arsenic species proportions and methylation ratios with the BC. These variables were categorized into tertiles according to the distribution in the controls. Two multiple models were considered: 1) adjusted for AsT-AsB, AsB, and normalized creatinine and the original variables of age (years), age at 1st pregnancy (years, categorized), lactation throughout life (months), BMI (kg/m2), tobacco (packs per year), and alcohol (g per week); and 2) further adjusted by the polymorphisms of interest. Included as confounding factors were the covariables that modified the OR between the iAs metabolites and the BC.14
The multiple model, adjusted for the covariables mentioned, was stratified by genotypes grouped according to the dominant model of the genes of interest (GG+GC vs. CC and GC+CC vs. GG for PPARγ Pro12Ala and PPARGC1B Ala203Pro, respectively). It was not possible to evaluate the co-dominant and recessive models due to the reduced numbers of women homozygous for mutations in both genes. Additionally, to evaluate the interactions between the polymorphisms and the arsenic variables, the multiplicative term, genxAs, which consisted of the genotypes in the dominant model (AB+BB vs. AA) and the tertiles of each arsenic species, was added to the multiple models. A value ≤ 0.20 was established as the level of statistical significance. The data analysis was performed using the statistical package Stata 12.0 (StataCorp, College Station, TX, USA).
Results
Table I shows that by the design of the study, the mean age was similar between cases and controls (53.14 vs. 53.86 years). As for the reproductive characteristics, the cases had significantly lower ages at menarche, parity, and lactation throughout life and older age at first pregnancy compared with the controls. With respect to BMI, consumption of tobacco and of alcohol, no statistically significant differences between the cases and the controls were observed.
Significant differences in the concentrations of iAs, DMA, AsT, and AsT-AsB adjusted for creatinine were observed between the cases and the controls but not for MMA and AsB. Compared with the controls, the %MMA (11.00 vs 9.09) and the primary methylation (1.16 vs 0.88) were significantly higher in the cases. Contrary to this finding, the secondary methylation was significantly lower in the cases than in the controls (7.02 vs 8.57). No significant differences in %iAs, %DMA, the total methylation ratio, and the urinary creatinine levels were observed. The observed distributions of the genotype frequencies were determined to be in Hardy-Weinberg equilibrium for both polymorphisms.
Table II shows that women with high urinary %MMA showed increased risk of BC that remained after adjusting for the polymorphisms of interest (ORT3vs.T1= 3.57, 95%CI 1.99-6.38; p for trend < 0.001), which was consistent with the OR observed for primary methylation (ORT3vs.T1= 3.51, 95%CI 1.96-6.28; p for trend <0.001). In contrast, a decreased risk of BC was observed for those women who had high urinary %iAs (ORT3vs.T1= 0.50, 95%CI 0.28-0.88; p for trend = 0.012) and higher values of secondary methylation (ORT3vs.T1= 0.33, 95%CI 0.19-0.59; p for trend <0.001). All of the aforementioned results were similar when considering only the observations with detectable levels of the respective arsenic variables (data not shown).
Table II Arsenic variables and risk of breast cancer in women of northern Mexico

Model 1: adjusted for the variables AsT−AsB, AsB, and natural log-transformed creatinine, in addition to age (years), age at 1st pregnancy (years, categories: <18, 19-21, 22-38, nulliparous), lactation throughout life (months), BMI (kg/m2), tobacco (packs per year), and alcohol consumption (g per week)
Model 2: also adjusted for the polymorphisms of PPARγ Pro12Ala and of PPARGC1B Ala203Pro, in two categories
* T1 reference category; iAs, T1= (1.47-8.97), T2= (9.01-12.66), T3= (12.73-51.11); MMA, T1= (1.11-8.14), T2= (8.15-10.87), T3= (10.87-22.60); DMA, T1= (42.99-75.78), T2= (75.95-82.06), T3= (82.15-96.66); Primary methylation, T1= (0.12-0.77), T2= (0.79-1.15), T3= (1.15-2.62); Secondary methylation, T1= (2.78-6.93), T2= (7.02-10.04), T3= (10.08-86.93); Total methylation, T1= (0.84-6.06), T2= (6.09-9.00), T3= (9.07-65.25)
Table III shows that no statistically significant interactions were obtained between the polymorphisms of interest as well as the arsenic variables and the risk of BC.
Table III Arsenic variables and risk of breast cancer stratified by polymorphisms of PPAR γ and PPAR GC1B in women of northern Mexico

* T1 reference category; iAs, T1=(1.47-8.97), T2=(9.01-12.66), T3=(12.73-51.11); MMA, T1=(1.11-8.14), T2=(8.15-10.87), T3=(10.87-22.60); DMA, T1=(42.99-75.78), T2= (75.95-82.06), T3= (82.15-96.66); Primary methylation, T1= (0.12-0.77), T2= (0.79-1.15), T3= (1.15-2.62); Secondary methylation, T1= (2.78-6.93), T2= (7.02-10.04), T3= (10.08-86.93); Total methylation, T1= (0.84-6.06), T2= (6.09-9.00), T3= (9.07-65.25)
‡ Models adjusted for the variable AsT−AsB, AsB, and natural log-transformed creatinine, in addition to age (years), age at 1st pregnancy (years, categories: <18, 19-21, 22-38, nulliparous), lactation throughout life (months), BMI (kg/m2), tobacco (packs per year), and alcohol consumption (g per week)
Discussion
According to our results, polymorphisms of the gene PPARγ Pro12Ala and of its coactivator PPARGC1B Ala203Pro do not modify the association between methylation capacity of iAs and BC, demonstrating that the association between the methylation capacity of iAs and BC (reported in a previous study)14 was maintained after adjusting for these polymorphisms.
The association between polymorphism Pro12Ala of PPARγ and BC is inconclusive. The results of a recent meta-analysis suggest that the carriers of the GG+CG genotype may be less susceptible to developing this tumor (OR = 0.85, 95%CI 0.73-0.98).25 This finding is consistent with previous observations in Danish women that carriers of the G allele exhibited a decreased risk of BC.26 In contrast, an increased risk of BC was identified in North American women homozygous for the G allele (OR = 2.91, 95%CI 1.05-8.04).9 In our study, we observed a proportion (11.64%) of carriers of the G allele in the control group, close to the frequency reported in the Mexican population,27 and, as in other studies, we did not find an association between these polymorphisms and BC, even after adjusting for exposure to iAs (ORCG+GGvs.CC = 0.90, 95%CI 0.56-1.43) (data not included in the tables).
Regarding polymorphism Ala203Pro of the coactivator PPARGC1B, very few studies have evaluated its connection to the risk of BC, and the results have been inconsistent. In a population of German women with mutations negative for BRCA1 and BRCA2 and with familial BC, an increased risk of BC was observed in the carriers of the CC and CG genotypes.7 While these women are a highly selected study population, as the frequency of BC by mutations in BRCA1 and BRCA2 is only 5 to 10% at the population level, and are not necessarily comparable with the women studied,28 the results are opposite to those observed in this study, even after adjusting for the methylation capacity of iAs.
In the stratified models, according to the dominant model of the genotypes of interest, non-significant differences were observed between the ORs of the arsenic variables with BC and the heterozygous and homozygous mutations compared with the ancestral homozygotes. This finding may suggest that the risk of BC is modified in some genetic subgroups, especially at high levels of exposure to iAs.
Moreover, our results show that women with high %MMA and higher values of the primary methylation ratio presented an increased risk of BC that remained after adjusting for the polymorphisms of interest, in contrast with the negative association observed for those women who had higher values of secondary methylation. This finding confirms the evidence regarding the monomethylated metabolite of iAs (MMA) that has been consistently associated with increased risks of various types of cancer, in contrast to the dimethylated forms (expressed in higher values of secondary methylation), which have shown inverse relations.29-36
Our findings should be interpreted with caution, given the strengths and limitations of this study, which include the following: the blind measurement of biomarkers, which decreases the probability of error of differential measurement; the high response rates between cases and controls, which reduce the probability of selection bias; and the small sample size, which reduces the statistical power of the study.











 nueva página del texto (beta)
nueva página del texto (beta)


