Although human papillomavirus (HPV) vaccination is the current primary prevention method for cervical cancer, the impact of vaccination will not be observed for another two to three decades. Consequently, secondary prevention must be strengthened, particularly in developing nations where cervical cancer is among the leading causes of cancer-related mortality for women.1,2 New technological advances have the potential to increase the effectiveness of programs for the secondary prevention of cancer. These new tools include the detection of high-risk HPV types (hrHPV).3 Transient hrHPV infections are, however, very common, and only a small proportion persist and lead to the development of cervical neoplasia.4-6 As a result, a high proportion of hrHPV-positive women do not require colposcopy. Therefore, the introduction of hrHPV-based screening is forcing public health planners to seek triage alternatives for hrHPV-positive women.7 Various triage alternatives may help identify those women requiring further diagnostic testing and confirmation. To date, there is no consensus on the most efficient and reliable triage strategy for hrHPV-positive women.3,8-10 These different triage methods must be further evaluated to determine which is most cost-effective in settings such as Mexico. There are various testing strategies that show great promise as potential triage tests, which could be performed on a single cervical sample. These strategies include: a) hrHPV genotyping for HPV16/18 given that 70% of cervical lesions are attributable to these two types. Together HPV16 and HPV18 along with HPV45 account for 75% of squamous cell carcinoma cases and 94% of adenocarcinomas;11 b) expression of the onco-protein E6 as a marker of neoplastic progression;12 c) cytology for the detection of morphological changes; d) immunocytochemistry for the detection of markers of neoplastic progression like p16INK4a/Ki67, a test with high specificity in the diagnosis of cervical intraepithelial neoplasia of grade 2 or higher (CIN2+).13,14 The available triage testing alternatives may help identify women who require further diagnostic testing and confirmation, cause less anxiety, and help to avoid unnecessary treatment and expenses in women who have a low risk of progressing to cancer.1,15,16 In addition, beyond screening and triage, there is no agreement regarding the best method for the diagnostic confirmation of hrHPV-positive women. Recent studies have documented that colposcopy has poor sensitivity and poses logistical difficulties, particularly in low-resource settings.17,18
To formally address these issues, a large population-based study called the Forwarding Research for Improved Detection and Access for Cervical Cancer Screening and Triage (FRIDA Study) was initiated. This project seeks to determine the most effective screening and triage program to reduce the number of patient visits, costs, and patient anxiety associated with screening. A formal cost-effectiveness evaluation will be conducted for the various screening and triage methods.
The target population for this study is over 100 000 women aged 30 to 64 years who use the health services of Sanitary Jurisdiction No. 1 in the state of Tlaxcala, Mexico. This is the first population-based study to be conducted under real conditions to determine the performance of a cervical cancer screening program. Although certain aspects of the study may be site-specific, the results of this analysis will help to guide the development of cervical cancer screening programs in developing countries such as Mexico.
This article describes the study design and methodology of all the FRIDA Study activities: recruitment, hrHPV testing and triage procedures, colposcopy and histological evaluations, case management and quality control measures. This manuscript also reports the baseline characteristics of the first 30 829 women who enrolled in the study.
Materials and methods
FRIDA Study objectives
The main study objective is to evaluate the performance and cost-effectiveness of different triage tests to detect cervical intraepithelial neoplasia of grade 2 or higher (CIN2+) in hrHPV-positive women between 30 and 64 years of age (figure 1).
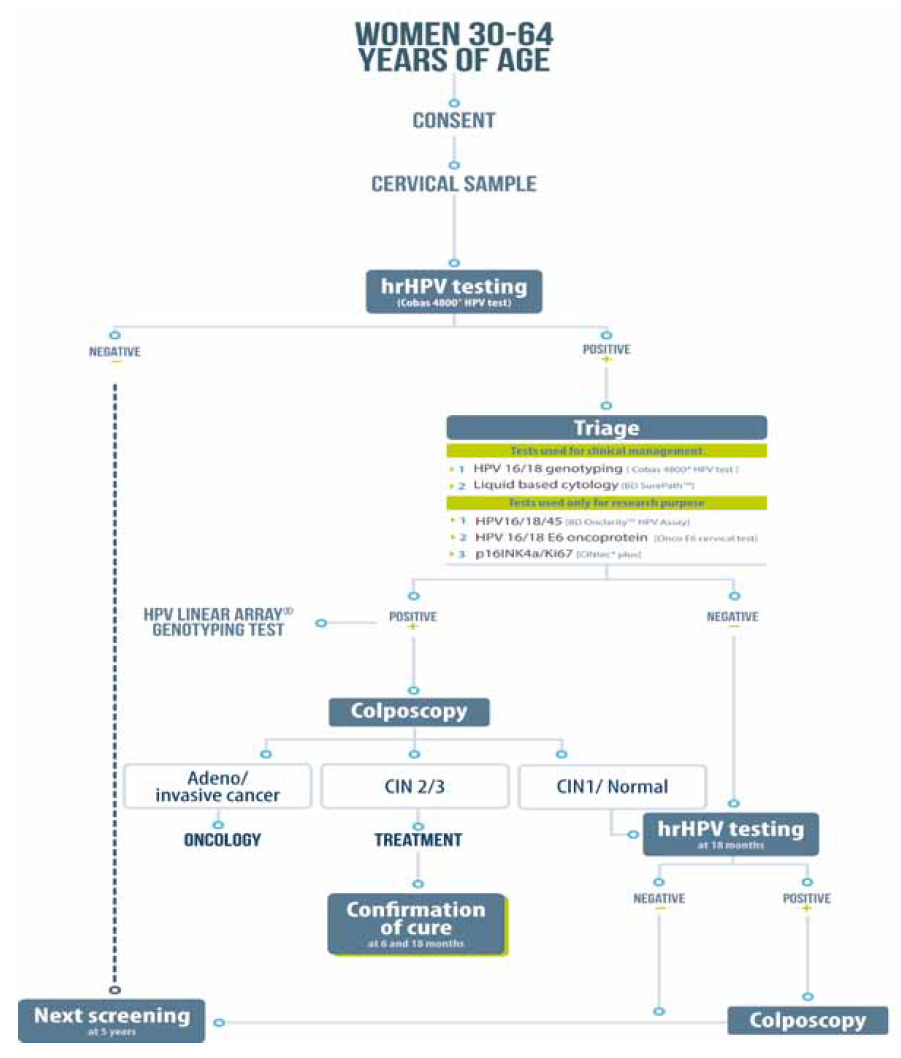
* Forwarding Research for Improved Detection and Access for cervical cancer screening and triage
Figure 1 Overview of study design for the primary objective. FRIDA Study,* Tlaxcala, Mexico, 2013-onwards
A second objective is to compare the performance of different tests for hrHPV detection with self-collected and clinician collected samples: the Cobas 4800 System [Roche Molecular Diagnostics, Pleasanton, CA, USA], and the BD Onclarity HPV Assay [Becton, Dickinson and Company (BD) Diagnostics, Sparks, MD, USA].
Study population
This is an ongoing population-based study targeting all women ages 30 to 64 years who reside in any of the 32 municipalities in Tlaxcala State that are covered by the Sanitary Jurisdiction No. 1 of Tlaxcala Health Services. This includes 100 primary health care centers that offer cervical cancer screening services. Trained staff at the health care facilities involved in this study verify that the women who are invited to participate meet the study inclusion criteria: aged 30 to 64 and users of the health services of the Sanitary Jurisdiction No. 1 of Tlaxcala. Women who are pregnant at recruitment or have had a hysterectomy are excluded from the study. Legally disabled women who are unable to give verbal informed consent as required by the study protocol are also excluded from participating.
The study was approved by the Institutional Review Boards (IRBs) of the participating institutions: National Public Health Institute (INSP) [1094]; Tlaxcala State Ministry of Health (SSET) [SS.DECI-OI-13/12]; and IMSS [R-2013-785-070]; as well as the Mexican regulatory agency COFEPRIS [CAS/OR/01/CAS/123300410C0044-3578/2012]. The FRIDA Study is registered in ClinicalTrials.gov, number NCT02510027.
The state of Tlaxcala is located in Central Mexico, approximately 120 km east of Mexico City. Tlaxcala is the smallest state in Mexico and encompasses approximately 1.0% of the national population. Tlaxcala has 60 municipalities and a population of 604 161 females, which accounts for 51.6% of the total population. According to the 2010 census, 20% of the population lives in rural locations.19 Illiteracy is relatively low (5%), half of the population lives in poverty, and 10% of the state's population lives in extreme poverty. Tlaxcala has a stable population, with a net migration rate of only 1.2%.20 The Tlaxcala State Health Service is divided into three administrative districts or Sanitary Jurisdictions. This study is being performed within the Sanitary Jurisdiction No. 1, which includes 32 of Tlaxcala's 60 municipalities.
Within Sanitary Jurisdiction No. 1, the target population for the hrHPV testing-based Cervical Cancer Screening Program is over 100 000 women between the ages of 30 and 64. We anticipate enrolling approximately 80 000 women to achieve a coverage rate of 80%.
Sanitary Jurisdiction No. 1 health services infrastructure
This study will include all of the cervical cancer prevention programs of the different health institutions in our target jurisdiction. For logistical reasons, we will gradually incorporate the different health institutions across the jurisdiction. We have started recruitment within the Tlaxcala Health Services (THS) system. THS serves women insured by the Seguro Popular, a federally-run, safety-net insurance provider. Seguro Popular covers close to 80% of the target population. After the program is fully implemented within the THS, we will begin to incorporate the remaining health care institutions of our target jurisdiction, including the Mexican Social Security Institute (IMSS) and the Institute of Social Security and Services for Government Workers (ISSSTE). At the time of this manuscript was written, we were only enrolling women with Seguro Popular insurance.
The Sanitary Jurisdiction No. 1 of THS has approximately 100 primary health care facilities where cervical cancer screening is offered. Primary health care services are provided by a medical-staff-unit that consists of one doctor, two nurses and some health promoters. This primary unit is responsible for preventive and primary health care activities. In Tlaxcala, the cervical cancer screening services have the necessary infrastructure, human resources and materials required to conduct the FRIDA Study protocol, with the exception of an HPV laboratory. Cervical samples for HPV testing are processed at the HPV laboratory of the INSP in Morelos state.
All nurses and physicians at the 100 THS facilities offering cervical cancer screening services were trained before the beginning of the study. During the training process, study group members visited the field sites to explain the study design, recruitment strategies, and procedures for the verbal consent process. The study group members trained the participating nurses and clinicians to collect proper cervical samples (for cytology as well as hrHPV testing), label and manage the samples, and complete the screening formats and report results. A supervision process was established at the beginning of the study to ensure the quality of recruitment procedures, as well as proper cervical sample collection methods.
All cytology procedures are centralized at the Tlaxcala State cytology laboratory at Laboratorio Estatal de Salud Pública de Tlaxcala (LESP). This laboratory receives all cervical cytology specimens from the THS in the entire state. All staff members have been trained and certified for liquid-based cytology interpretation.
Women requiring colposcopic evaluation are referred to two colposcopy clinics within the THS (at the Tlaxcala General Hospital and the Women's Hospital in Tlaxcala). These clinics perform all colposcopic evaluations for Sanitary Jurisdiction No. 1. Colposcopists received training by a group of senior colposcopists and medical oncologists who regularly visit the colposcopy clinic about every three months to ensure the quality control of colposcopic procedures. The pathology laboratory at Tlaxcala General Hospital examines all study samples as well as the histologic diagnosis of the gynecological and surgical specimens collected within the state. Histologic evaluation of all samples (biopsies and endocervical samples) is performed by a panel of three experienced pathologists (certified by the Consejo Mexicano de Anatomopatólogos A.C.). Women diagnosed with invasive cervical cancer are referred to the National Cancer Institute in Mexico City for management and treatment.
Recruitment
The recruitment period started in August 2013 and is expected to continue until the first trimester of 2017. As part of the regular care offered by the Tlaxcala cervical cancer screening program, all women attending any of the 100 THS health care centers are informed about the study and invited to participate. The potential participants receive an explanation of the benefits of the additional diagnostic resources offered as well as, the potential discomfort from the sample collection, as a result of participating in the FRIDA Study. Prior to beginning any study activities, verbal consent is obtained by health personnel at the study sites and documented as part of the intake form for the screening program. During the recruitment visit, participants provide demographic data as well as obstetric and gynecologic history in a private office. The information provided by the participants is recorded on a pre-printed registration form that was developed for the FRIDA Study, in conjunction with the screening program in Tlaxcala. Exclusion criteria for the FRIDA Study are included on this form. The data from the registration forms is entered into a computer-based information system for the Cervical Cancer Screening Program in Tlaxcala (SICET). The information system is available through the internet, ensuring the accuracy of the data in real time. All hard copies are scanned and digital forms are stored for any audit procedure, if necessary.
Cervical sample collection and management
Prior to sample collection, the screening registration forms and vials for transporting the cervical samples are labeled with a unique barcode corresponding to each participant. Trained physicians or nurses perform a pelvic exam and collect two cervical samples using a Cervex-Brush (Rovers). The first collected sample is placed in a vial containing the BD CytoRich preservative (BD Diagnostics, Burlington, NC), and the second sample is placed in a ThinPrep vial (Hologic, Inc., Bedford, MA). Both samples are temporarily stored at room temperature at the health center until they are delivered to the HPV laboratory facilities. The ThinPrep vials are delivered on a weekly basis to the HPV laboratory at the INSP in Cuernavaca, Morelos, México, and stored at 2-8°C until hrHPV testing can be performed. The SurePath vials are delivered on a weekly basis to the cytology laboratory in Tlaxcala and stored at 2-8°C until analysis. These samples are used for reflex cytology in women who test positive for hrHPV. For quality control, reflex cytology is also performed on a random sample of 2% of the hrHPV-negative specimens.
hrHPV testing
All laboratory procedures are performed at the INSP HPV laboratory, where the samples are processed in strict accordance with the manufacturer's instructions. All cervical and/or vaginal specimens are tested for hrHPV using the Cobas 4800 HPV test (Roche Molecular Systems, Pleasanton, CA), a qualitative in vitro assay for the detection of 14 hrHPV types. This automated system simultaneously extracts cellular (including β-globin) and viral HPV DNA. The target DNA is then amplified by PCR using primers specific to HPV and β-globin. The PCR master mix contains primers and probes specific for 14 hrHPV genotypes: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68. HPV16 and HPV18 are then identified individually.**)
Triage procedures for clinical management
The following triage tests are performed as reflex testing on all participants with a positive hrHPV test.
HPV16/18 genotyping, Cobas 4800 HPV test (Roche Molecular Systems, Pleasanton, CA): This test identifies genotypes HPV16 and HPV18 individually.
Cytological triage (Papanicolaou stain) is performed on all hrHPV-positive specimens. The cytology sample is processed using the PrepStain System according to the manufacturer's instructions (TriPath Imaging, Inc., Burlington, NC, USA) at Laboratorio Estatal de Salud Pública de Tlaxcala. To produce the Papanicolaou-stained slides, an aliquot of centrifuged cell pellets is placed directly onto the PrepStain(r) instrument for automated processing.
Cytology interpretation is performed according to the Bethesda 2001 criteria.21,22 All slides are reviewed by two cytotechnicians who are blinded to the other's interpretation. Slides are randomly distributed among the cytotechnicians to balance the reading of each staining. If there is a disagreement between two readings, a cytopathologist will be responsible for determining the final diagnosis. The cytopathologist also re-reads 5% of the normal slides and all of the atypical squamous cells of undetermined significance or worse (ASCUS+) slides as a means of quality control in compliance with the current Mexican Cervical Cancer Screening Program.23,24 Both the cytotechnicians and the cytopathologist are aware of the hrHPV status for all samples. Trained cytotechnicians work under the supervision of an experienced and certified cytopathologist.
Interpretation of triage results
All women with a positive triage result for HPV16 or HPV18 or ASCUS+ are referred to colposcopy for further evaluation and treatment, if necessary. All hrHPV-positive but triage-negative women are re-screened 18 months later for hrHPV. Those with negative hrHPV results at the 18 month re-screen will be re-evaluated in five years as part of the regular Cervical Cancer Screening Program.23,24 Alternatively, women who remain hrHPV-positive after 18 months undergo colposcopy.
Triage procedures for research purposes and not for clinical decision making:
HPV16/18/45 genotyping, BD Onclarity HPV Assay [BD Diagnostics, Sparks, MD, USA]: This assay amplifies an HPV DNA target by real-time PCR and identifies HPV types 16, 18, 31, 45, 51, 52, and 59 individually while concurrently detecting the other six hrHPV types in two pools (33, 56, 58, 66) and (35, 39, 68).25
OncoE6 Cervical Test (ArborVita Corporation, Fremont, CA, USA): this is an immunochromatographic test that uses a lateral flow format, based on detection of the HPV-E6 oncoprotein found in HPV16 and HPV18 infections from cervical samples collected in ThinPrep vial, using the same principle as previously described.12,26 Elevated E6 oncoprotein expression in HPV 16/18 infected cervical cells is required for epithelial cell transformation to occur, therefore the presence of E6 protein represents an attractive, disease-specific viral biomarker for cervical precancerous progression.
p16INK4a/ Ki-67 CINtec PLUS (REF. 9531 Roche mtm laboratories, Mannheim, Germany). This staining is processed from the residual pellet of the cytology preserved in 2 mL of SurePath Preservative Fluid, according to the manufacturer's instructions. The residual cell pellet is stored at 2-8°C until future immunocytochemical staining. The interpretation of the biomarkers is performed according to manufacturer instructions.***)
Colposcopy
All women referred for colposcopic evaluation undergo a complete evaluation by a trained provider. The colposcopic findings are reported according to the 2011 International Federation of Cervical Pathology and Colposcopy guidelines,27 and recorded in the colposcopy report form. The colposcopists are aware of all triage results as well as the complete medical background of each referred patient. The colposcopists are asked to document their clinical impression of each cervical quadrant using Reid's Colposcopic Index. Before biopsy collection, endocervical sampling is performed using an Endocervex Brush. A biopsy is collected from each quadrant from the most abnormal, acetowhite area of the squamocolumnar junction (using baby Tischler cervical biopsy forceps).
Histological confirmation
Histological evaluation of all samples (biopsies and/or endocervical samples) is performed by a panel of three pathologists. The four biopsies are embedded in a single paraffin block. Two levels of the biopsies are sectioned and stained with hematoxylin & eosin (H&E). A pellet of the endocervical sample is created and treated as a cervical biopsy. Two pathologists review all biopsy slides (and the cone biopsies from loop electrosurgical excision procedure (LEEP) of the women who have CIN2+, as described below) and report the diagnosis according to Mexico's Cervical Cancer Screening Program's criteria (table I). If the diagnoses of the two pathologists agree, that is the final diagnosis. If the diagnoses are discordant, an additional interpretation is made by a third pathologist.
As a part of the study protocol, an immunohistochemistry assay is performed for the qualitative detection of the p16INK4a antigen on formalin-fixed, paraffin-embedded tissue sections prepared from the cervical biopsies. Two sets of slides are prepared and read by the same two pathologists: one for H&E staining and another for CINtec histology (CINtec Histology Kit, Roche mtm laboratories AG). The interpretation of the H&E-stained slides is performed by the same pathology panel, who are blinded to the first interpretation. The results of the CINtec histology preparation are not used for case management.
Case management and follow-up
The participants receive appropriate treatment based on their histologic results. A loop electrosurgical excision procedure (LEEP) is performed if the histologic results confirm CIN2/3. If positive margins are noted, a repeat LEEP is performed. All women treated with LEEP and found to have negative margins are scheduled for repeat hrHPV testing and cytology after six months. If hrHPV infection or cytological abnormalities are still present, they are referred for a new colposcopy. Participants who test negative for hrHPV and have a normal cytological evaluation undergo hrHPV testing 18 months after treatment. If they are still negative for both tests at 18 months, they are not tested again for hrHPV for another five years. By contrast, those with abnormal cytology or a positive hrHPV test undergo colposcopy.
Women diagnosed with cancer are referred to a tertiary care hospital, the National Cancer Institute in Mexico City, where they are managed according to the current medical standard of care.
All participants with histology-confirmed CIN1/normal are re-screened using hrHPV testing after 18 months. If the participant is still positive, she is referred for colposcopy using the study algorithm for biopsy collection (figure 1). Participants who test negative for hrHPV after the 18 month screening are tested again in five years.
With a minimum anticipated sample size of 80 000 participants, to the FRIDA Study will be able to evaluate the performance of different triage tests among a predicted 9 000 hrHPV-positive women with considerable precision; sensitivities of 60% may be estimated with 95% confidence intervals of 58-65%, with a Beta of 80% and an alpha of 0.05.
Cost-effectiveness analysis
A cost-effectiveness analysis (CEA) will be performed from the perspective of the following health care institutions in the city of Tlaxcala: IMSS, ISSSTE, Tlaxcala Health Services (THS), and Tlaxcala-based private health services. The aim of this CEA is to determine the incremental costs and health outcomes of the various molecular and cytological triage tests as techniques for the detection of cervical cancer in hrHPV-positive women. These tests include the HPV-16/18 (Cobas 4800), HPV-E6 oncoprotein (Arbor Vita), HPV16/18/45 genotyping (BD Onclarity HPV Assay) and cytological triage (with and without immunostaining). The costs and health outcomes of using the individual HPV-based interventions will be compared to cytology. The costs associated with using the various triage strategies will be identified and quantified. The health outcomes associated with these triage strategies will be the number of CIN2+ cases detected. A decision tree model will be used to estimate the cost-effectiveness of the various triage strategies. The incremental benefit of performing multiple-quadrant biopsies relative to a (colposcopy-guided) directed biopsy will also be assessed via a sensitivity analysis.
Secondary objective: Comparison of different hrHPV assays
To address the secondary objective of this study, a subsample of 10 000 women will provide both a vaginal self-sample and a clinician-collected cervical sample (figure 2). The women first self-collect a vaginal sample for hrHPV testing at the healthcare facility, using the Rovers Viba-Brush (Rovers Medical Devices B.V., Oss, Netherlands).****) Nurses instruct women how to properly collect the samples and provide brochures with illustrated instructions. These women then receive a pelvic exam during in which a clinician collects a cervical sample using the Cervex-Brush. The Rovers Viba-Brush and the Cervex-Brush (Rovers) are rinsed in ThinPrep medium (Hologic, Marlborough, MA) immediately after sample collection. The samples are kept at room temperature until delivered on a weekly basis to the INSP HPV laboratory, where they are stored at 2-8°C until analysis. Both the self-sample and clinican collected sample are tested simultaneously using two different hrHPV testing assays: the Cobas 4800 [Roche] as described above and the BD Onclarity HPV Assay [BD] as described above.
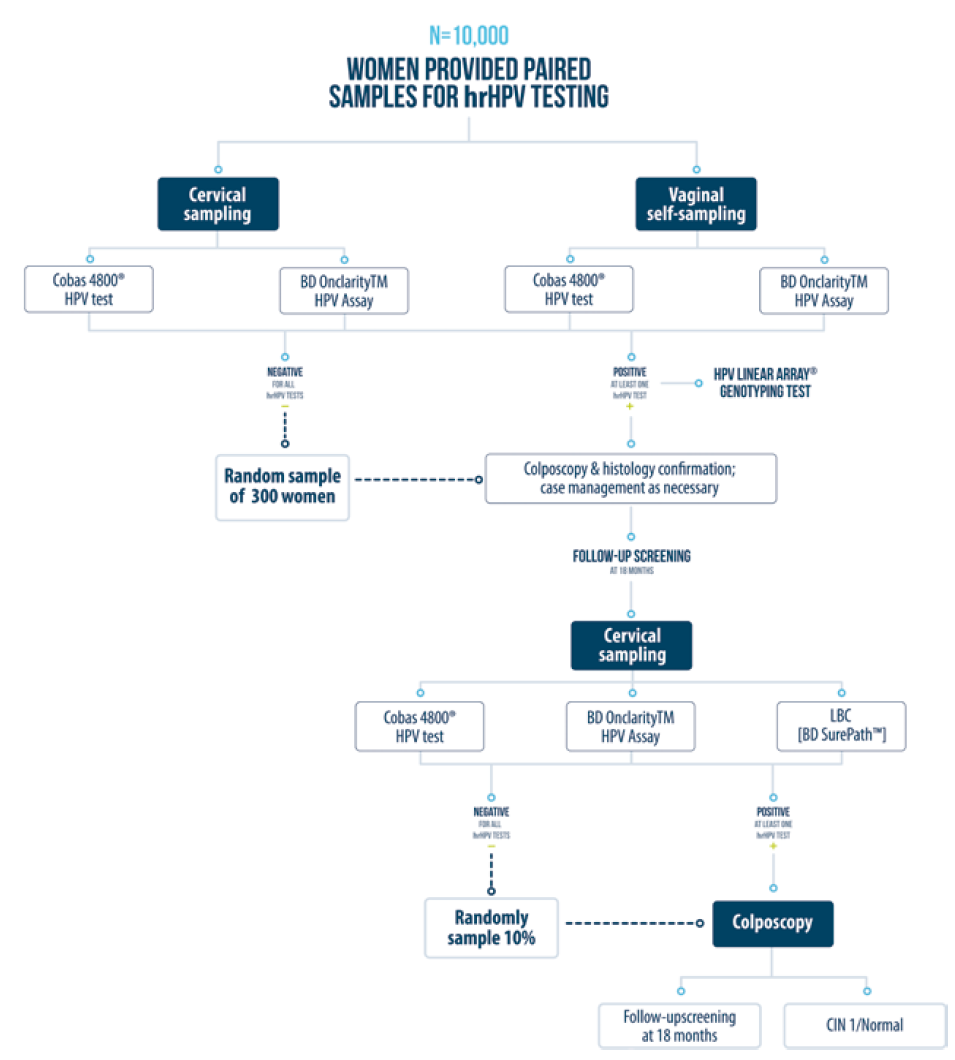
Figure 2 Overview of study design for the secondary objective. Comparison of different hrHPV assays. FRIDA Study. Tlaxcala, Mexico, 2013-onwards
Participants with at least one positive hrHPV result are referred for colposcopic evaluation. For quality control purposes, a group of 300 women from the paired self-collected and clinician-collected samples group who test negative for hrHPV will also be referred for colposcopic evaluation. All detected CIN2+ lesions are treated accordingly. Women found to be free of disease are evaluated again using the same hrHPV assays (Cobas 4800 and HPV Assay BD) and liquid-based cytology (SurePath [BD]) after 18 months. Participants who are positive for any of these tests are referred for colposcopy. All colposcopy and diagnosis confirmation activities are identical to the rest of the aforementioned FRIDA Study procedures.
Results
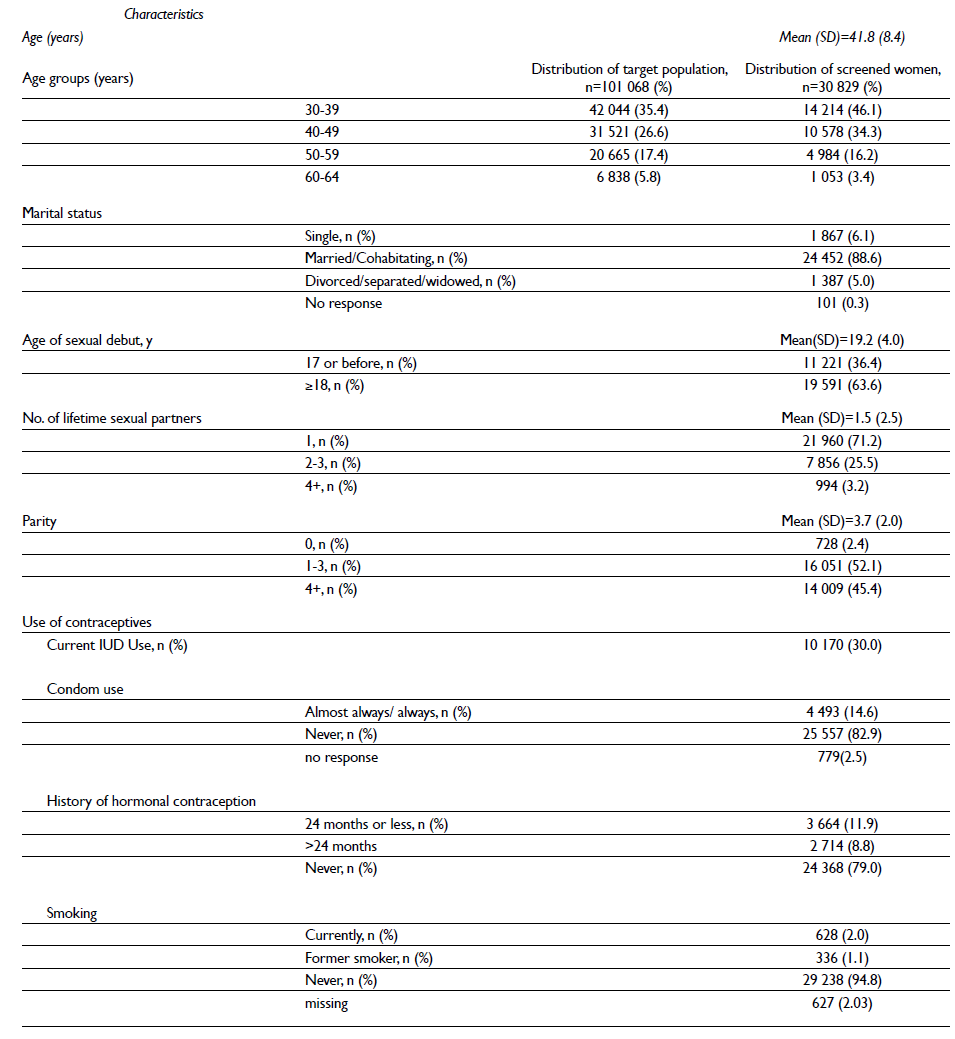
Of the 31 629 women who were invited to participate in the Tlaxcala cervical cancer screening program by the end of August 2015, 1.5% (n=483) were excluded because they did not meet the study inclusion criteria for the following reasons: hysterectomy (n=465) and pregnancy (n=18). The participation rates were slightly higher among younger women (30-49 years old) than older women (50-64 years old). The mean age of the participants at the time of recruitment was 41.8 years (SD 8.4). The majority of participants reported having had one lifetime sexual partner and never having used condoms; nearly all have never smoked (table II).
Table II Sociodemographic and clinical characteristics of the FRIDA population (the first 30 829 women screened) Tlaxcala, Mexico, 2013-onwards
A total of 30 829 cervical specimens were tested for hrHPV. The overall prevalence of hrHPV (14 types) was 11.0%. The prevalence of hrHPV infection across age groups has a U-shaped distribution with two peaks of hrHPV prevalence, the first among women aged 30-39 years and the second among women over the age of 50 (table III). The hrHPV-negative women who had a cervical sample collected will be re-evaluated in five years as part of the regular Cervical Cancer Screening Program. For internal quality control purposes, 1% of the participants with a positive hrHPV result, independent of the triage result, are also directly referred for colposcopic evaluation. All remaining hrHPV-positive samples and a group of hrHPV-negative samples (2%) will be stored for future analysis.
Table III hrHPV results and type-specific prevalence by age group among the 30 829 women in the FRIDA Study. Tlaxcala, Mexico, 2013-onwards
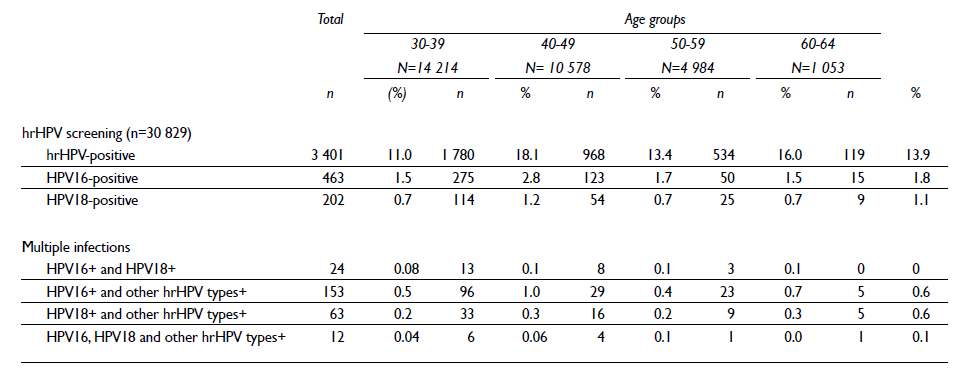
To date, 3 401 participants with a positive hrHPV result have undergone triage procedures. The overall prevalence of HPV16 and HPV18 was 1.5% and 0.7%, respectively (table II). Similar to the aforementioned hrHPV pattern, both HPV16 and HPV18 exhibit a bimodal infection pattern with peaks among the youngest and oldest age groups (table II).
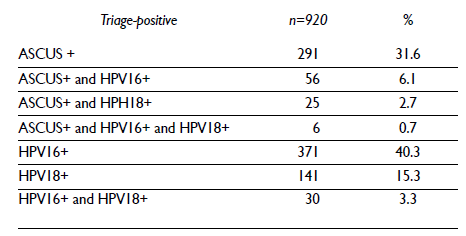
Among the hrHPV-positive women with available cytology results (n=3 158), 11.8% had an ASCUS+ result (table IV). Among the ASCUS+ women, 23.9% have high-grade squamous or glandular intraepithelial lesion result (95/372). Among the hrHPV-positive women, the prevalence of at least one positive triage test (triage positive) was 27.0% (920/3401). Among the triage-positive women, more than half were positive for HPV16 and/or 18 but had a negative for intraepithelial lesion or malignancy (NILM) cytology result (53.6%) and only 9.5% of triage positive women were positive to both triage tests (ASCUS+/HPV 16/18 positive) (table V).
Table IV Cytological triage results among hrHPV-positive women. FRIDA Study. Tlaxcala, Mexico, 2013-onwards
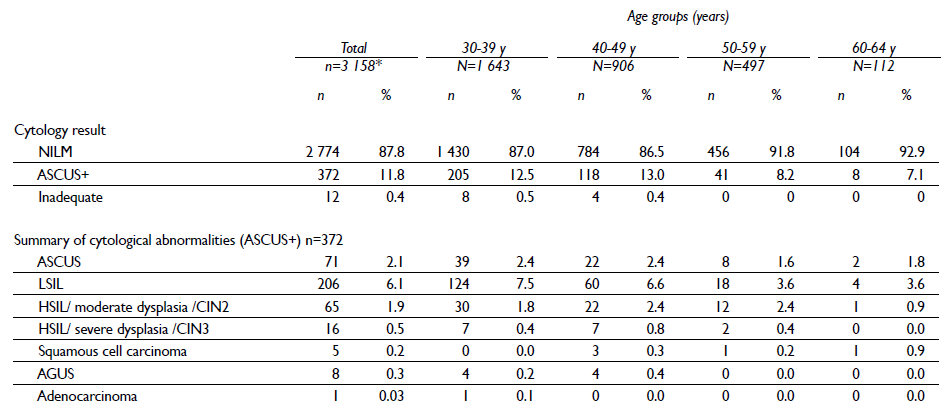
*From the 3 401 hrHPV-positive women, cytology results are available for only 3 158. Results are pending for 243 hrHPV-positive women.
Abbreviations: NILM= negative for intraepithelial lesion or malignancy; ASCUS= atypical squamous cells of undetermined significance; CIN=cervical intraepithelial neoplasia, AGUS=atypical glandular cells of undetermined significance
Table V Summary of triage results among hrHPV-positive women. FRIDA Study. Tlaxcala, Mexico 2013-onwards
A total of 920 triage-positive women have been referred to colposcopy, 616 (67%) women have attended, and valid biopsy results are available for a total of 590 women. Of the 304 women who have not attended the colposcopy clinic, 17 declined to continue participation in the study, 27 moved away, and 50 were evaluated and treated at another medical institution. The remaining 209 participants were scheduled for the first or second time (if they failed to attend the first time). In addition, nine women became pregnant and will be rescheduled after their deliveries. On average, it has taken approximately 3 months from the time a participant receives a positive triage result until they receive colposcopic evaluation.
Discussion
FRIDA is an ongoing population-based demonstration study that was designed to assess the performance and cost-effectiveness of different tests to triage hrHPV-positive women within the Cervical Cancer Screening Program in Mexico. This paper provides an extensive account of the methodology and initial enrollment activities of the FRIDA Study.
The high sensitivity of the hrHPV test, if used as a primary screening test is offset by a higher proportion of false-positive results for women with transient hrHPV infections. Our study seeks to evaluate different triage alternatives to better manage women with hrHPV infections by helping to reduce the number of clinic visits, remove unnecessary steps in the diagnostic confirmation process, and reduce program costs.
The overall baseline prevalence of hrHPV (using the Cobas 4800 PCR methodology for primary hrHPV-based screening hrHPV) to date is 11.0%, which is consistent with previous studies in Mexican women screened for hrHPV.28-31),*****)
One of the main strengths of the FRIDA Study will be the head-to head comparison of triage strategies in a population-based hrHPV screening program, in which all women who test hrHPV-positive receive all triage tests. There is no other study that has evaluated triage strategies in an HPV-based screening program in real-life conditions. While the HPV ATHENA study was the first to explore different triage methods by incorporating HPV cytology screening algorithms as well as genotyping, the results of the ATHENA trial are representative of women undergoing cervical cancer screening in the United States, where the epidemiological pattern of HPV is different than in Mexico.3,7 For example, in Latin American countries, the prevalence of hrHPV is greater among the older age groups, relative to that in North America, and this pattern is observed not only for the hrHPV types overall but also for the HPV-16/18 types.32-34
To cater to the specific needs of the Mexican population, this study has lowered the age of HPV screening to age 30 instead of 35. This extension of screening may help to reduce the burden of cervical disease in women aged 30 to 35 years, compared to cytology screening alone, as suggested in previous studies.3,35
We furthermore seek to improve upon the current cytologic screening methods. We are using monolayer cytology and an automated process to ensure quality control of the cytological material and eliminate residues (blood, mucus) that hinder reading. This advantage may help to improve the limitations of conventional cytology in countries such as Mexico.36
Recent studies have documented that colposcopy has a poor rate of disease detection because 30% of lesions are not visible during colposcopy even by highly experienced colposcopists. In addition, Mexican providers traditionally have a very low rate of biopsy collection, due to a lack of skill (this may vary depending on experience and practice). As a result, colposcopists are unable to recognize the lesions that are not clinically visible.28 Therefore, instead of using directed biopsies, we are collecting biopsies systematically by quadrant. Several studies support this decision by showing that the sensitivity of colposcopy could be improved by collecting more than one biopsy. These studies report an improvement in detection of CIN2+ with increased by biopsy collection can be increased by as much as 37%.18,37-39 The involvement of colposcopists in the systematic collection of biopsies will be an important step to ensure the detection of CIN2+ cases that require prompt treatment. The FRIDA Study will also document the benefit of adopting a systematic collection of cervical biopsies as routine colposcopy practice.
We realize that the Tlaxcala population may not represent all women in Mexico; however, the results of the performances and cost-effectiveness analysis may be very informative for other states in Mexico. The FRIDA Study results may be applicable to urban and semi-urban areas in the country, representing close to 70% of the population in Mexico.
The enrollment phase of this study has generated important information on the prevalence of HPV infection and the various stages of cervical cytological abnormalities in Tlaxcala, Mexico. The sensitivity and specificity of the different triage strategies and the cost-effectiveness evaluation will be reported in future publications.
The FRIDA Study Group
Cosette Wheeler, Patti Gravitt, Mark H. Stoler, Enrique Carmona, Héctor Figueroa, Kevin Ault, Kathleen M Schmeler, Philipe Castle, Victor Granados, David Bishai, Paula Ramírez, Pilar Hernández, Leith León, Daniel Alvarez, Elizabeth Barrios, Rubí Hernández, Indira Mendiola, Vicente González, Mauricio Hernández-Ávila, Leticia Torres-Ibarra, Eduardo Lazcano, Eduardo Franco, Jack Cuzick, Attila Lorincz, Thomas C. Wright, Anna Barbara Moscicki, Yvonne N. Flores, Joacim Meneses, Pablo Méndez, Berenice Rivera, Samantha E. Rudolph and Jorge Salmerón.











 nueva página del texto (beta)
nueva página del texto (beta)



