Cancer caused over 8 million deaths worldwide in 2010 and has emerged from the third leading cause of death in 1990 to the second leading cause in 2013 only behind cardiovascular disease. Findings from the current Global Burden of Disease study (GBD)1 estimate more than 14 million new cancer cases and more than 8 million cancer deaths in 2013 from which 56% of new cancer cases, 62% of cancer deaths, and 70% of cancer related Disability Adjusted Life Years (DALYs) occurred in developing countries. By 2020, the world population will increase to 7.5 billion people and approximately 15 million new cancer cases will be diagnosed with predicted 12 million cancer deaths.2-4 Most cases will occur in low and middle-income countries (LMIC). In 1970 these countries were responsible for 15% of the global cancer burden, which increased to 58% by 2008. By 2030 the world estimates are 27 million new cases and 17 million deaths and LMIC are expected to contribute to 70% of all cancer deaths.5-6
Differences in cancer incidence between populations can be explained by environmental, behavioral, dietary, occupational and infectious exposures. It has been estimated that almost 25-30% of cancer deaths are related to tobacco, 30-35% are linked to diet, about 15-20% are due to infections, 4-6% to alcohol consumption, and a lower percentage are related to other factors like radiation, stress, physical inactivity, environmental pollutants, etc.7 Despite the substantial progress made in prevention, early detection, and more effective treatment options (surgery, chemo-, targeted-, and radiotherapy), cancer burden is increasing due to population growth and aging as well as risk factors like smoking, obesity, and dietary patterns.2,8-10
In this study we provide an overview of the burden of 28 cancers for the 32 states in Mexico, for 20 age groups and both sexes from 1990 to 2013. We performed a decomposition analysis to discern whether the changes in mortality and incidence were due to population growth, changes in age structure or changes in incidence rates.
Data sources and methods
The general methods used in the GBD study have been published elsewhere.11-15 The analytical strategy to determine cancer incidence and mortality in Mexico can be described in four steps. The first step involved extraction of mortality and incidence data from data sources collected for the GBD study that included data on cancer incidence and cancer mortality collected from cancer registries, literature reviews, and the Cancer Incidence in Five Continents (CI5) series. No data for cancer incidence was available from Mexico. Mortality data were extracted at the most detailed cause- and age-specific level with International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes. The ICD-9 and ICD-10 codes were then mapped to each GBD cancer group. GBD cancer groups include all ICD codes pertaining to neoplasms (ICD-9, 140-239; ICD-10, C00-D49) except for Kaposi sarcoma (KS) (C46) and non-melanoma skin cancer (NMSC) (C44). In the second step, mortality incidence (MI) ratios were estimated for each cancer site, country, age, sex, and year. In the third step, cancer mortality was estimated using the GBD CoD (cause of death) database. The methods used to generate the CoD database are reported in detail elsewhere.1 Undefined codes or codes referring to causes that cannot be an underlying cause of death were redistributed according to the method described in Ahern and colleagues.16 For Mexico sources for cancer mortality include vital registration system data, verbal autopsy studies, and other sources (table I).17 The CoD database mortality data were used as input into the Cause of Death Ensemble Model (CODEm) to estimate the number of deaths attributable to each cancer assessed in the analysis. The CODEm results were adjusted using CoDcorrect, an algorithm that uses uncertainty distributions around cause fraction estimates for each GBD cause of death to scale estimates to all-cause mortality estimates in each country, year, age, and sex group. These death estimates were used to calculate years of life lost (YLLs). The fourth step was to apply MI ratios to CoDcorrect death estimates to obtain cancer incidence estimates for each year, age, and sex group.
Table I Data source list of child and adult mortality data sources used in the GBD 2013

VR/SRS/DSP: Vital registration, sample registration system, and disease surveillance points
HH: Household deaths
CBH: Complete birth history
SBH: Summary birth history
SIBS: Sibling survival
Decomposition analysis of cancer trends
There are three factors that determine the number of cases or deaths over time: population growth, population age and sex structures, and age- and sex-specific rates. To estimate the effect of population growth, two scenarios were considered: In scenario (1) the population size of 2013 was used combined with the rate, sex, and age structure of 1990. The difference between the 1990 numbers and the numbers estimated by applying the 2013 population size to the 1990's rate, age, and sex structures therefore represents the increase in cancer incidence/deaths that can be explained by population growth. In scenario (2) the effect of aging was estimated by applying the 1990 age-sex specific rates to the 2013 age-sex specific population. The difference in incident cases reported herein shows the proportion of the change in incident cases between 1990 and 2013 attributed to the changing age structure of the population. To estimate the effect of changing incidence rates on the incident cases, we applied the incidence rates for 1990 to the population size and age structure of 2013. The difference between scenario (2) and scenario (1) is due to aging of the population. The difference between 2013 numbers and scenario (2) is due to a change in age-sex specific rates. The effects were calculated as the percent change in the number of the cases or deaths for each factor compared to 2013.
Deprivation status
As part of the study of poverty and social progress a deprivation index was developed that includes several dimensions of economic, social, and quality of living conditions. These deficit indicators explore different dimensions like educational attainment (proportion of illiterate population and under 15 years with incomplete primary education), housing conditions (proportion of households without electricity, potable water, uncovered floors, overcrowding); development indexes (proportion living in localities smaller than 5 000 individuals), and economic dimension (proportion of working population with less than two minimum salaries).18 These nine indicators are estimated from the National Census of Population and Housing 2010 by the National Population Council (Conapo, in Spanish).19 The states and municipalities are classified in five categories (very high (VH), high (H), medium (M), low (L) and very low (VL) levels) according to the index distribution. The states with very high deprivation status are Guerrero, Chiapas and Oaxaca, which cover a population of 12 million people. States with a high deprivation index include Veracruz, Puebla, Hidalgo, San Luis Potosí, Michoacán, Tabasco, Campeche and Yucatán, which cover a population of 28 million people (25% of the total Mexican population). Nayarit, Zacatecas, Guanajuato, Durango, Tlaxcala, Sinaloa, Querétaro, Morelos and Quintana Roo achieve a medium deprivation index and cover 18.6 million people (16.5% of the Mexican population). Nine states have low deprivation status and are Baja California Sur, Chihuahua, Sonora y Tamaulipas, Aguascalientes, Colima, Jalisco and the state of Mexico where 34.3 million people live (30.6% of the total population). Finally, the states with very low level of deprivation are Coahuila, Baja California, Nuevo Leon and the capital city in the Federal District covering 19.4 million people (17% of the population).
Results
The estimates for the Mexican Burden of Disease study (MBD-2013) reveal notable differences in rates and trends between cancers in men and women and between states. On the national level, in 2013 a total of 195 925 cancer cases occurred with 102 241 in females and 93 683 in males. The age standardized incidence rate (ASIR) per 100 000 was the highest for prostate (35.5) followed by breast (23.6), colorectal (14.6), stomach (13.5), cervical (12.0) and lung cancer (10.9). Among women, breast cancer incidence was twice as high as cervical cancer. For men, prostate cancer was the most commonly diagnosed cancer. A total of 84172 deaths occurred in males (41281) and females (42891). The age standardized death rate (ASDR) per 100 000 was the highest for lung cancer (10.3) followed by stomach (9.7), prostate (8.3), liver (7.8), colorectal (7.2), cervical (6.2) and breast cancers (6.2) (table II).
Table II Incident cases and deaths for all cancers and 28 cancer groups in Mexico, 2013
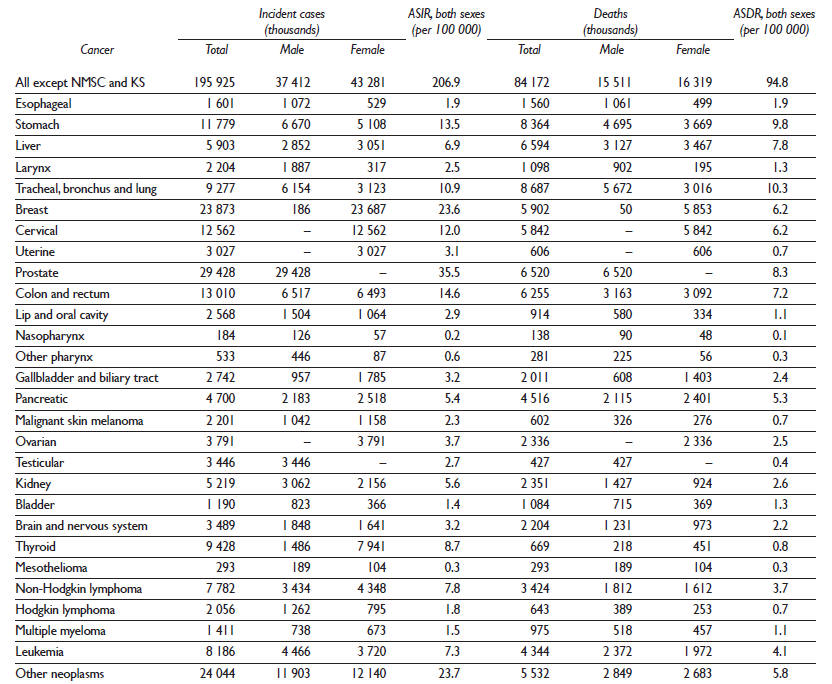
ASDR: age-standardized death rate
ASIR: age-standardized incidence rate
ICD-9: International Classification of Diseases, ninth revision
ICD-10: International Statistical Classification of Diseases and Related Health Problems, tenth revision
KS: Kaposi sarcoma
NMSC: non-melanoma skin cancer
YLDs: years lived with disability
YLLs: years of life lost
Cancer groups are defined based on ICD codes and include all codes pertaining to neoplasms (ICD-9 140-239; ICD-10 C00-D49) except for NMSC and KS
The age specific contributions of the different types of cancer show a very distinctive pattern with leukemia and the combined "other neoplasms" group (containing rare cancers like malignant neoplasm of bone and articular cartilage of limbs, malignant neoplasm of thymus, and others) being the main contributors to cancer incidence in children and adolescents (age <20 years) and accounting for nearly 70% of the total burden of cancer in these age groups. As age advances, different cancer types predominate. For example, breast and cervical cancer contribute with nearly 40% of the total cases of cancer in women aged 30 to 50 years while prostate, stomach, and tracheal, bronchus and lung cancers predominate in older age groups (figure 1A). Cancer deaths distribution by age showed also a distinctive pattern where leukemia, and non-Hodgkin lymphoma contribute with almost 50% of the overall causes of cancer deaths in the younger age groups (5 to 19 years old). In the reproductive ages among women aged 30 to 49 years cervical, breast and ovarian cancers predominate and are responsible for 25 to 30% of total cancer deaths in this age group. The death pattern among the population older than 49 years changes towards the prominence of tracheal, bronchus and lung, prostate, stomach and liver cancer, which contribute to around 50% of all cancer deaths for this age group in 2013 (figure 1B).
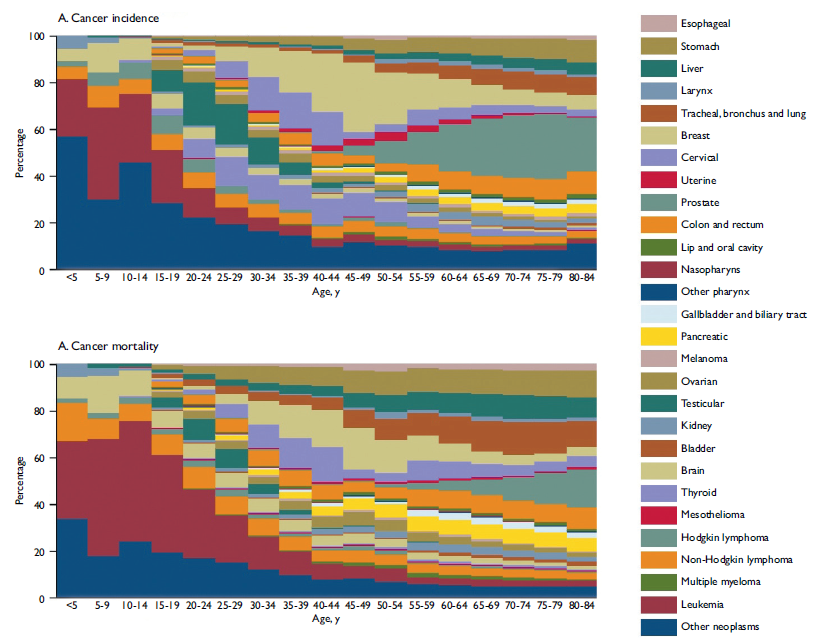
Figure I Age-specific contributions of cancer types to total cancer incidence and mortality, both sexes. Mexico, 2013
Population growth contributed to the increase in cancer deaths between 1990 and 2013 with 36%. If the population structure and size had remained the same between 1990 and 2013 there would be 3.2% fewer cancer deaths. This translates into fewer malignant skin melanoma deaths, as well as fewer cervical (98.3%), gallbladder (94%), larynx (56.5 %), tracheal, bronchus and lung (47.5%), Hodgkin lymphoma (42%), stomach (40.5%), other pharynx (23.6%), uterine (13.4%), fever lip and oral cavity (7%) cancer deaths. On the other hand we observe an increase in the number of deaths from kidney cancer (99.6%), non-Hodgkin lymphoma (99%), colon and rectum (92.8%), ovarian (91.4%) mesothelioma (67.6%), brain (60%), prostate (45.8%), breast (42.4 %), pancreatic (27.1%), testicular (14.1%), nasopharynx (13.4%), leukemia (12.7%), bladder (12.6%), thyroid (10.9%), liver (10.3%), and esophageal (5.4%) cancers (table III).
Table III Decomposition analysis of cancer trends in national mortality, both sexes. Mexico, 1990 to 2013

GBD: Global Burden of Disease
ICD-9: International Classification of Diseases, ninth revision
ICD-10: International Statistical Classification of Diseases and Related Health Problems, tenth revision
KS: Kaposi sarcoma
NMSC: non-melanoma skin cancer
Cancer groups are defined based on ICD codes and include all codes pertaining to neoplasms (ICD-9 140-239; ICD-10 C00-D49) except for NMSC and KS
To estimate the effect of population growth we applied the population size of 2013 onto the rate, sex, and age structure of 1990. Since the global population grew by 36% between 1990 and 2013; death rates and age structure remained the same as in 1990, death due to all cancers increased by 36% in this counterfactual scenario.
To estimate the effect of aging on number of death we applied the age structure of 2013 onto the rate, sex distribution, and population size of 1990. The change in deaths reported herein shows the proportion of the change in deaths between 1990 and 2013 that can be attributed to the changing age structure of the population
To estimate the effect of changing deaths we applied the death rates for 1990 onto the population size and age structure of 2013. The change in deaths reported herein shows the proportion of the change in number of deaths
The different geographical patterns that can be observed in the incidence and mortality rates throughout Mexico are evidence of a complex epidemiological transition. Incidence rates from 1990 to 2013 increased in northern Mexico in the states of Baja California Norte, Sonora, Chihuahua and Tamaulipas from 60 to 90% with more modest increases of 45% to 60% in the states of Coahuila, Sinaloa, Durango, Nuevo Leon as well as Chiapas, which is one of the poorest states in Mexico. The same geographical patterns were seen for mortality rates, although with a different magnitude. Death rates increased from 15 to 45% in the northern states as well as in the southern state of Chiapas, smaller increases can be observed in the coastal areas; and interestingly, decreases up to 30% are clearly observed in the central region surrounding Mexico City (figure 2).
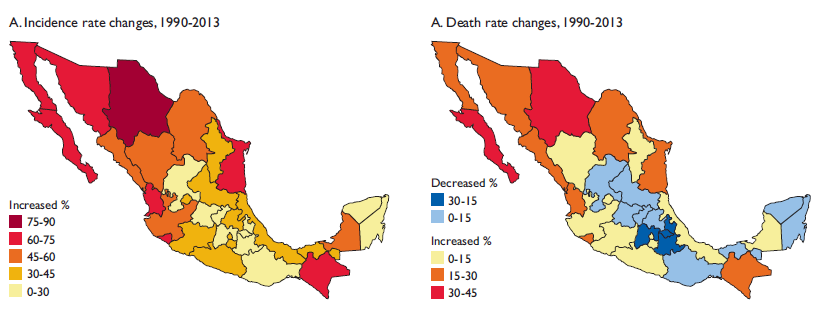
Figure 2 Relative changes in age-standardized incidence and death rates, in both sexes, for all cancers. Mexico, 1990 to 2013
The ranking of cancers by age standardized incidence rates for both sexes show that prostate cancer ranks first across the country, followed by breast cancer in the states with a low deprivation status, and cervical cancer in the states with high deprivation. Additionally, stomach and colorectal cancers rank highly throughout the country. Tracheal, bronchus and lung cancer were in a median position in states with low deprivation and ranked lower in states with a high deprivation status such as Hidalgo (11th), Oaxaca (12th), Tlaxcala (13th), and Puebla (14th) (figure 3).
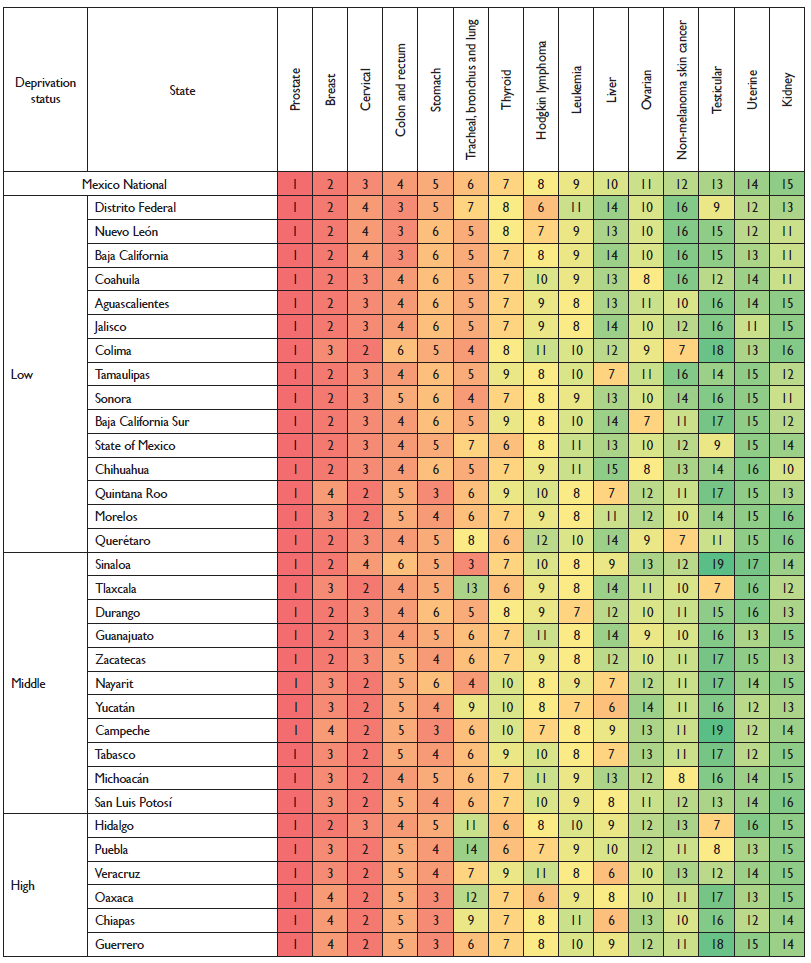
Colors correspond to the ranking, with dark red as the cancer with the most incident rates and dark green as the cancer with the least incident rates for the location indicated. Rankings do not include the "other cancer" group. The numbers inside each box indicate the ranking.
Figure 3 Cancers ranked by age standardized incidence rate in both sexes by deprivation status at the state level. Mexico 2013
Breast and cervical cancer ranked first and second respectively for ASIRs in women regardless of the state's deprivation status with the exception of the states Oaxaca and Chiapas, where cervical cancer is ranked first. Rankings for thyroid cancer, colon and rectum cancer and stomach cancer show a homogenous distribution throughout the country. Among men, prostate cancer had the highest age standardized incidence rate in every state followed by lung cancer among the states with a low deprivation and stomach cancer among the states with a high deprivation status. Liver cancer ranking differed according to the deprivation status and was ranked fifth among the states with high deprivation, and in the 8th to 10th position among states with a lower deprivation status.
The ranking of age standardized mortality rates (ASMR) among females showed that cervical cancer was ranked first in the states with high deprivation while breast cancer has the highest ASMR in states with a low deprivation. Liver cancer for women who live in the states with the highest deprivation status ranked second or third for ASMR while lung cancer takes this position for women in states with a lower deprivation (figure 4).

Colors correspond to the ranking, with dark red as the cancer with the most incident rates and dark green as the cancer with the least incident rates for the location indicated. Rankings do not include the "other cancer" group. The numbers inside each box indicate the ranking.
Figure 4 Cancers ranked by Age Standardized Death Rate per 100 000 in females by deprivation status at the state level. Mexico 2013
In men, prostate and lung cancer are in the first and second position according to their ASMR but in the states with a high deprivation, lung cancer is displaced by stomach and liver cancers. Leukemia and Hodgkin lymphoma also have a more prominent position in the poorest states of Oaxaca, Chiapas, Puebla and Hidalgo along with the southern states of Yucatan, Campeche and Tabasco (figure 5).
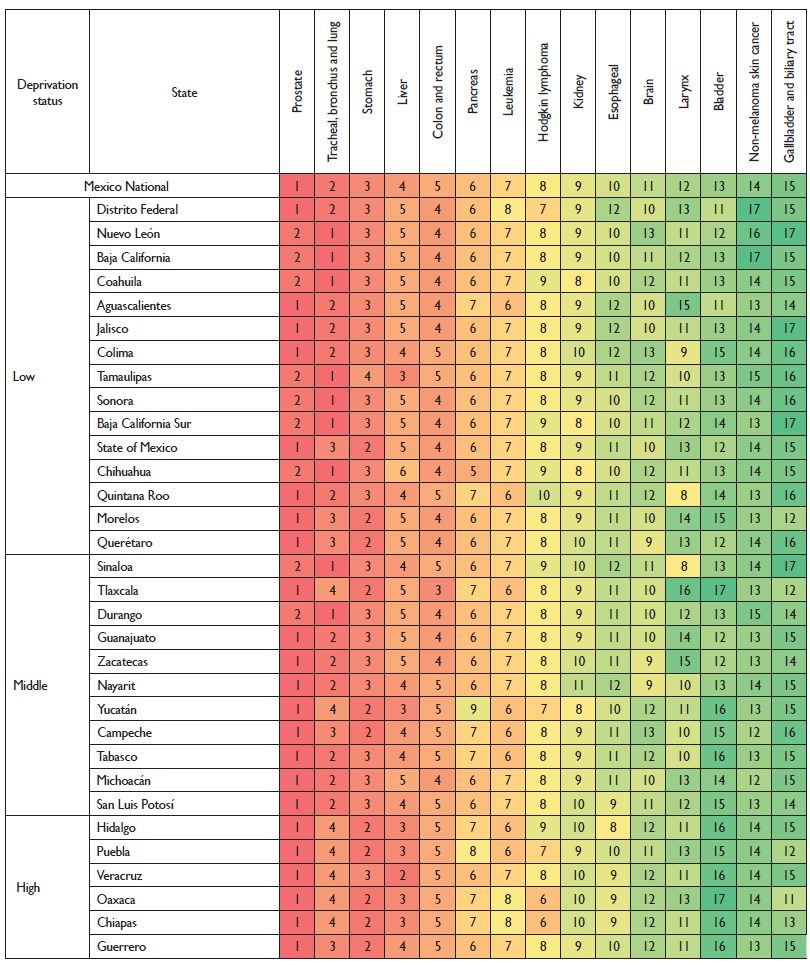
Colors correspond to the ranking, with dark red as the cancer with the most incident rates and dark green as the cancer with the least incident rates for the location indicated. Rankings do not include the "other cancer" group. the numbers inside each box indicate the ranking.
Figure 5 Cancers ranked by Age Standardized Death Rate (per 100 000) in males by deprivation status at the state level. Mexico 2013
Discussion
Previous publications describing the burden of cancer in Mexico have been limited in scope because of the analysis of only short time periods, inclusion of a limited number of cancers,20-27 incomplete or too broad geographical coverage,28-31 or special settings like the population covered by the social security system.32-34 It has therefore been difficult to assess cancer trends across the country. The GBD 2013 study provides a unique opportunity to analyze trends in cancer incidence and mortality at the state level for Mexico, which can help inform health policy and decisions regarding resource allocation.
This is the first report analyzing incidence and mortality for 28 groups of cancers at the state level by deprivation status, for both sexes and different age groups for the period from 1990 to 2013.
The comparison of the estimates from the GBD 2013 study for Mexico (MBD-2013) and the data published in the online mortality database by the National Institute for Statistics and Geography (INEGI, in Spanish)35 demonstrates the need to improve the quality of the cause-of-death data specifically for cancer. As part of the GBD methodological framework "garbage codes", which are either undefined codes on death certificates or codes that cannot be the underlying cause of death, are being redistributed to improve the quality of cause of death data. For Mexico redistribution of garbage codes in the GBD study increased the total number of cancer deaths by 12 691 (18%) compared to the official numbers published in the INEGI database. The specific causes that showed a higher number of deaths (Institute of Health Metrics and Evaluation, IHME vs INEGI) were stomach cancer (2736, +39%), cervical cancer (2055, +54%), and lung cancer (1747, +18%). The problem with garbage codes in the Mexican vital statistics has been addressed by other researchers although their approach has been to treat the unspecified and garbage codes as separated entities.28 Another difference is that if a cause of death is coded as being due to a benign neoplasms it is being redistributed to the complementary malignant neoplasm in the GBD 2013 study. The overall increase in the GBD mortality estimates after redistribution of different categories of garbage codes was the highest for uterine (37% increase) and cervical (33% increase) cancers. In the case of cervical cancer increase due to ill-defined codes was 3.3%, for indeterminate causes of death it was 1%, for death assigned to symptoms it was 0.3%, and for deaths assigned to unspecified cancer sites it was 28.8%.36 These issues point out the importance of improving the diagnostic process as well as coding practices by medical personnel certifying causes of deaths in Mexico.
Until these quality improvements can be implemented the MBD-2013 study provides a comprehensive overview of cancer incidence and mortality in men and women. The results demonstrate the complexity that the Mexican health system faces. The number of cancer deaths between 1990 and 2013 more than doubled from 77 294 to 195 925 with some cancers like prostate, colorectal, kidney, breast cancer and malignant skin melanoma showing dramatic increases in incidence rates. Because of the aging Mexican population it has been estimated that cancer incidence will rapidly increase with an expected additional 107 000 new cases per year by 2030 compared to 2012.37
Because of this expected trend cancers that are frequent in an older population deserve special attention. The MBD-2013 study highlights the importance of prostate cancer in Mexico, which is increasing in incidence and mortality throughout the country and regardless of the deprivation status. Further research is needed to determine why mortality due to prostate cancer is increasing despite the availability of early detection and improved treatment. Benchmarking with other countries that have achieved reductions in prostate cancer mortality can be helpful.38,39 While pointed out by other studies in Mexico our study demonstrates that prostate cancer deserves special attention with further efforts to improve early detection given the suffering related to a late diagnosis.40,41
Breast cancer is another example where early detection is available through breast awareness and mammography. However, coverage of mammography is still limited and most women are diagnosed in a late stage leading to suboptimal survival.42-44 It is agreed upon that incidence and mortality due to cervical cancer has decreased even though estimates differ in terms of absolute numbers.45,46 Cervical cancer but also breast cancer are paradigm examples of complex regional cancer patterns, which are clearly linked to the level of deprivation.47,48 In more developed areas breast cancer is more important than cervical while the opposite occurs in the more marginalized states of the country.49-52 While the former is influenced by life style and nutritional risk factors the latter has an infectious background and lack of access to health services determinants.
Liver cancer is another example with higher rates among more marginalized states possibly linked to infections with hepatitis B and C infection53 and higher exposure to contaminated blood transfusions in past.54,55 Higher lung cancer mortality rates were observed in the northern region with women experiencing higher mortality in the central states of Mexico,56,57 although trends are predicted to increase in the southern and poorer regions of the country.58 The decreasing mortality rates in the central regions surrounding Mexico City suggest that access to early detection programs, state of the art treatment and specialized medical services increases survival of cancer patients.
Study limitations
The benefit of estimating incidence and mortality within the GBD framework is that it ensured that cancer estimates are adjusted to be consistent with the all-cause mortality estimates, preventing inflation or underestimation of cause-specific estimates. While quite advanced, these methodologies still result in estimates, which should be used as placeholders until high-quality data become available. The cancer analysis for the MBD-2013 study relies on cause-of-death data, which is ideally supplemented by cancer registry incidence data for improved quality of the estimates. However, cancer incidence data is not available in Mexico.
The implementation of a national cancer registry program in Mexico is of utmost importance to understand the detailed burden of cancer. Cancer registry data includes more detailed information like stage of disease, treatment, and ideally survival data. If incidence estimates rely exclusively on mortality data there is the risk of over- or underestimation of incidence in the setting of rapidly changing cancer incidence (for example in the case of new screening programs) or rapidly changing mortality due to improved treatment or earlier detection.
Another limitation to using exclusively cause-of-death data is miscoding, which is a well-known problem and can lead to biased estimates.59,60 Miscoding is especially common in countries with limited diagnostic resources and arises for example when metastatic lesions are coded as primary cancers, which can lead to overestimation of cancers in anatomic sites where metastases are often found (e.g., liver, lung, bone or brain). Changing classification systems (e.g., from ICD-9 Basic Tabulation List to ICD-9 detail) can also lead to substantial changes in estimates over time. In the MBD-2013 study to improve the quality of the data sources and to ensure comparability, garbage codes or undefined cancer codes were redistributed, and different coding systems were mapped to a set of uniform GBD causes.
Conclusions
To our knowledge, this is the first effort to report national and state level cancer incidence and mortality estimates for Mexico from 1990 to 2013.61 Although substantial progress has been achieved regarding early diagnosis and treatment options including surgery, chemo-, targeted-, and radiotherapy for several cancers, the burden due to cancer in the population is increasing. This is mainly related to aging of the population as well as increased exposure to risk factors like smoking, obesity, and dietary factors. To determine an appropriate response to the effect of the demographic transition on health systems like Mexico, up to date estimates on the burden of cancer has to be available at the most granular level. These estimates will provide the information required to implement and evaluate prevention, screening, diagnosis, treatment and palliative care services needed.











 nueva página del texto (beta)
nueva página del texto (beta)


