Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Salud Pública de México
versión impresa ISSN 0036-3634
Salud pública Méx vol.57 no.5 Cuernavaca sep./oct. 2015
Artículo original
Anemia and iron deficiency in Mexican elderly population. Results from the Ensanut 2012
Anemia y deficiencia de hierro en adultos mayores mexicanos. Resultados de la Ensanut 2012
Alejandra Contreras-Manzano, Sc,(1) Vanessa de la Cruz, Sc,(1) Salvador Villalpando, MD, PhD,(1) Rosario Rebollar, Tec Lab,(1) Teresa Shamah-Levy, PhD.(1)
(1) Health and Nutrition Division, Instituto Nacional de Salud Pública, Cuernavaca Morelos, Mexico.
Abstract
Objective. To describe de prevalence of iron deficiency (ID) and anemia in a sample of Mexican elderly population from the National Health and Nutrition Survey (Ensanut) 2012.
Materials and methods. 1 920 subjects ≥60 years of age were included. Hemoglobin, serum concentrations of ferritin and CRP were measured. The risk for ID and anemia adjusted for potential confounders was assessed in logistic regression models.
Results. The overall prevalence of anemia was 13.9%, 15.2% in males and 12.8% females. For ID, overall it was 4.2%, males 4.0% and females 4.3%. The greatest prevalence of ID was found in males and females over 80 years old (6.9 and 7.0%, respectively). ID was present in 1.5 of 10 Mexican elders with anemia.
Conclusion. The prevalence of anemia was high in the elderly, however the prevalence of ID was low; there is a need to further investigate the causes of anemia in this age group.
Key words: iron deficiency; anemia; elderly.
Resumen
Objetivo. Describir la prevalencia de deficiencia de hierro (DH) y anemia en adultos mayores (AM) mexicanos participantes de la Encuesta Nacional de Salud y Nutrición 2012.
Material y métodos. 1 920 sujetos ≥60 años fueron incluidos. Se midió hemoglobina, concentraciones séricas de ferritina y PCR. El riesgo de DH y anemia ajustada por confusores fueron evaluados por medio de modelos de regresión logística.
Resultados. La prevalencia de anemia fue 13.9% (15.2% hombres, 12.8% mujeres) y de DH 4.2%, (4.0% hombres, 4.3% mujeres). La mayor prevalencia de ID se encontró en mayores de 80 años (6.9% hombres, 7.0% mujeres). 1.5 de 10 adultos mayores mexicanos con anemia presentaron DH.
Conclusión. La prevalencia de anemia continua siendo alta en los adultos mayores, mientras que la prevalencia de DH es baja. Es necesario investigar las causas de anemia en este grupo de edad.
Palabras clave: deficiencia de hierro; anemia; adultos mayores.
Anemia is a common condition in the elderly, its prevalence increases with age and it is associated with higher risk of morbidity, disabilities, low quality of life, cardiovascular or neurological diseases and risk of death.1-5 Anemia was 17.1% in elder population in 2006 and 16.5% in 2012, in national representative surveys of Mexico.6
Smith and colleages in a cohort study found that in the elderly the most frequent cause of anemia were chronic diseases (30-45%), followed by iron deficiency (ID) (15-30%).7 ID is defined as a negative balance between body requirements and iron supply.8 Insufficient dietary iron intake, malabsorption of iron and blood losses due to chronic gastrointestinal diseases or typical conditions for advanced age are common causes of ID.9,10 In 2006, ID (categorized as serum ferritin <12 µg/L) represented 9.4% of Mexicans older than 65 years, while the prevalence of inadequate iron intake (<16 mg/day EAR) was 88.2% for women and 76.6% for males.11
The objective of this study is to describe the prevalence and predictors for ID and anemia in elderly Mexican population participating in the National Health and Nutrition Survey (Ensanut) 2012, by sex, age, rural or urban dwelling, and geographic region, affiliation to social programs and health services.
Materials and methods
Study population. Information for the present analysis was extracted from the Ensanut 2012 dataset. This is a probabilistic survey, representative at the national, regional, and urban and rural levels, stratified by clusters and survey design. 1 920 adults older than 60 years (900 males, 1 020 females) have a complete registry of personal data and hemoglobin, C reactive protein (CRP) and ferritin data. Demographic and socioeconomic information was collected using specific questionnaires. Ethnicity was classified as indigenous when an indigenous language was spoken by a member of the family. Localities with less than 2 500 habitants were considered as rural and otherwise urban.6
A household wealth index (HWI) was constructed based on the household characteristics and family assets by a principal component analysis, the index was divided into tertiles to stratify the population into low, middle and high HWI categories. The country was divided in three geographic regions: Northern, Center-Mexico City, and Southern. Weight and height was collected using validated and standardized methods.12,13 Body Mass Index (BMI) was computed based on height and weight.14 Information of affiliation to social programs Liconsa (distributes fortified milk at subsidized prices) Prospera (cash transfer program that provides fortified baby food to children younger than 2 years and a drink for pregnant and lactating mothers) or Adultos mayores (older adults social program, a cash transference program for persons older than 65 years of age) were obtained through questionnaires in the survey.
Ferritin and hemoglobin determinations. Blood samples were drawn from an antecubital vein, centrifuged at 3000 g, in situ. Serum was separated and stored in coded cryovials, preserved in liquid nitrogen until delivery to the central laboratory in Cuernavaca, Mexico where stored at -70 °C until determination.
Serum ferritin concentrations were measured by chemiluminescent microparticle immunoassay method and CRP was measured by immunoassay, using ultra-sensitive monoclonal antibodies; Abbott commercial kits were used for the determination. The measurements were performed in an automatic autoanalyzer (Architect i2000, Abbott Diagnostic, Wiesbaden, Germany).
The intra assay variability for ferritin was 3.35% and for CRP 4.4%. Serum ferritin concentrations were adjusted for inflammation when CRP was >5 mg/L using the equation of Thurnham and colleages.15
Hemoglobin concentrations were measured with a fingerprick in capillary blood using a portable photometer (Hemocue, Angelholm, Sweden) and concentrations were adjusted by altitude using the equation of Haas.16
Low iron stores was defined when serum concentrations of ferritin was<15 µg/L. Anemia was defined when adjusted Hb concentrations was <120 g/L for females or <130 g/L for males. Iron deficiency anemia (IDA) was defined when an abnormally low Hb value coexisted with values of ferritin <15 µg/L.17
Data from the Ensanut 2006, a probabilistic, multistage, stratified, clustered survey were used to compare anemia, ID and IDA prevalences of 2012 survey. Ensanut 2006 methodology is described elsewhere in detail.18,19
Statistical analysis. The characteristics of the sample and prevalence of anemia and ID are described as frequencies and 95% confidence intervals, stratified by sex. Differences in characteristics and prevalence were tested through simple logistic regression adjusted by sex and group of age. We constructed to test the risks for anemia, ID and IDA, and heterogeneity of effects by sex and age, through multivariate logistic regression models adjusting by HWI status, BMI, CRP, dwelling, geographical region, ethnicity and affiliation of households to social programs.
Data from Ensanut 2006 were reanalyzed with the same criteria than for Ensanut 2012. Statistical significance was set at a=0.05 and a=0.10 for interactions. All analyses were adjusted for the sampling design of the survey, using STATA v 13.
Ethical aspects. The protocol was approved by the Research, Ethics and Biosafety Committees of Instituto Nacional de Salud Pública, Mexico. Individual informed consent letters were obtained from all participants after explaining the nature, goals and methods of the survey.
Results
This analysis includes 2 328 adults older than 60 years. The characteristics of the sample are presented in table I. Briefly, more than half of the sample was 60-69 years, 32.8% 70-79 years and 15.5% ≥80 years old, with a proportion male/female of 44.9/55.2%. Most of them live in urban (77.5%) and about 8.5% were of indigenous ethnicity; 26.3% belonged to the tertile 1, and 42.7% to the tertile 3 of HWI. A great majority presented overweight (40.9%) or obesity (27.8%). The households affiliated to Social programs were: to Prospera, 28.7%, to Liconsa, 9.2%, and to Adultos mayores, 26.1% (table I).
Prevalence of anemia, ID and IDA
Anemia. The overall prevalence of anemia was 13.9%, males 15.2%, females 12.8%. There was an increment of anemia with increasing age, i.e. it went from 8.7% to 23.6% in the three groups of age. The anemia gradient was affected by sex so that the younger group of males had a lower prevalence than the other two groups of males.
In rural areas, Center and Southern regions of the Country and in low and middle tertiles of HWI anemia was more prevalent than in urban areas, North region or high tertile of HWI. It was observed an inverse tendency for the BMI and anemia, ranking from 44.9% in the low weight category to 10.6% in obese elders. There were no differences by ethnicity.
In subjects affiliated to Prospera or Adultos mayores, the prevalence of anemia was higher, around 9.5 pp and 7.4 pp, respectively than in non-affiliated, disparities that were more evident in males than in females. Liconsa program affiliation was associated with almost half the prevalence of anemia than in non-affiliated (14.8%). These differences were observed between males affiliated and non-affiliated to Liconsa, but not in females. In iron deficient, anemia rose to 51.5%, fourfold the prevalence in non-iron deficient. No differences by sex were found (table II).
Iron deficiency (ID). The overall prevalence of ID was 4.2%, 4.0% in males and 4.3% in females. The higher prevalence of ID was observed in 70-79 y old males (6.9%) and females (7.0%) compared with the other two age categories.
In elders affiliated to Prospera ID was higher (5.2%) than in non-affiliated (3.7%); this was significantly higher in males (7.1 vs 2.9%) but not in females. The prevalence of ID was not different by dwelling, region, ethnicity, HWI or inflammation. The overall prevalence of anemia was very high in iron deficient (15.3%), being in males 11.8% and in females 18.1% (table II).
Risk of anemia, ID and IDA
In the multiple logistic regression, we found a different effect by sex and group of age for risk of anemia, being higher in males of 70-79 y (OR: 1.80) and in >80 y (OR: 2.49) than in younger males of 60-69 y. Characteristics associated with risk to present anemia were: living in the Southern region, low and middle tertile of HWI, and low BMI. On the other side, overweight and obesity were protective factors for anemia. The risk for anemia was not different by dwelling, ethnicity or affiliation to social programs. CRP >5 mg/L was associated with 1.85 times the risk for anemia than normal CRP, while ID was associated with 6.9 times the risk for anemia than non-iron deficiency (table III).
ID. The risk of ID suggests a different effect by sex and group of age, being higher for women ≥80 y old (OR: 7.9). Residents of the rural dwelling and indigenous presented higher risk factors of ID than their counterparts. Living in the Southern region was associated with less risk of ID, contrary to what was observed in anemia. (table III).
IDA. Risk of IDA was higher in males aged 70-79 y (OR: 3.7) than in 60-69 y. The higher risk of IDA was observed in females >80 y. The risk for IDA was not different by dwelling, region, ethnicity, HWI status or affiliation to social programs (table III).
Changes in the prevalence of anemia, ID and IDA between Ensanut 2006 and 2012
The overall prevalence of anemia between 2006 and 2012 did not change, remaining in 14% (figure 1A). In both surveys elderly ≥80 y old showed the highest prevalence of anemia.
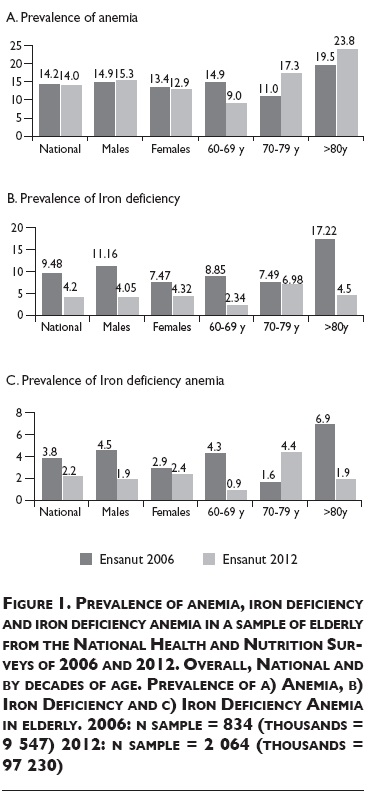
The prevalence of ID significantly decreased 5.3 pp (figure 1B). In males the prevalence reduced 7.1 pp and in females 3.1 pp between surveys. The highest reduction of ID through time was found in elderly ≥80 y old from 17.22% in 2006 to 4.5% in 2012.
Overall IDA prevalence in elderly was 3.8% in 2006 and 2.2% in 2012. We found a significant reduction of 3.4 pp of IDA in the 60-69 y old group (figure 1C).
Discussion
Anemia in Mexican elderly is similar as that of older adults from Brazil (7.7%)20 and Mexican-Americans (10.4%)21 from the NHANES III, being more noticeable in elders over 70 y, and more prevalent in males than females. These differences between sex are consistent with other studies.22-24
The magnitude of anemia due to ID is expected to be less in elderly than in other age groups (2.5 times the prevalence of anemia, OMS 2002).17 In our study ID is one contributing factor for anemia, but not the most important, since less than a third part of the prevalence of anemia was associated with ID. The low prevalence of ID in our study is in line with those reported in other countries as Ecuador (1.6%),25 Netherlands (11% males 5% females 50-79 y),24 Taiwan (ferritin <12 µg/L; males 2.6% and females 1.9% >65 y),27 USA (4% for males 7% for females >70 y),28 Singapore (0.4% in males and 2.6% in females 50-60 y),29 and Denmark (ferritin <16 µg/L; 1.8% males and 5.4% females >80 y);30 these prevalence are different by sex as we observed in our sample. From 2006 to 2012 Mexico seems to have had a significant reduction in the prevalence of ID, but it is important to note that the high prevalence observed in 2006 was probably associated to an oversampling in the Southern region in order to have a more representative sample of poor and indigenous people. There is no evidence that other factors could be associated to this reduction, such as: an improvement in the health services, a better socioeconomic status, a better health care distribution and utilization, or a positive effect of the social programs on this population. In some subgroups of elders, data suggested that being affiliated to Prospera or Adultos mayores was associated with higher risk for anemia or ID, probably evidencing a good targeting of the social programs to the most vulnerable population to undernutrition and inequities. Liconsa is a program which, according Ensanut 2012, provided fortified milk to 5.8% of rural and 10.8% of urban dwelling poor households of Mexico, and has a national coverage of 9.7%. That is why probably we did not found differences in risk for anemia among social programs and also because adulthood iron deficiency plays a minor role in producing anemia in this particular age group. Otherwise, sociodemographic characteristics of elder population as rural dwelling or low income showed significant evidence of risk for ID and anemia in elders. Nevertheless, this cross-sectional study does not allow making causal inferences.
Anemia was consistently higher in elders with low weight, probably due to malnutrition of essential micronutrients in these ages, as vitamin B12, folate, zinc, vitamin D, among others.10 In contrast, ID was more prevalent in overweight and obese subjects than in those with normal weight. It is possible that obesity underlies subclinical inflammation which induced iron sequestration, affecting iron availability to cells for its utilization; showing as a consequence an iron deficiency status-iron refractory and facing obese subjects at higher risk for ID.31.32
In our study, we observed that the increment of CRP >5 mg/L was a risk factor for anemia and seems to be a protective factor for ID, nevertheless a high CRP concentration conditions an elevation of ferritin during acute infections. Countries like Ecuador or Taiwan have reported in their surveys high prevalence of abnormally high ferritin concentrations (61.7% >100 µg/L),25 (15.7% males and 9.8% females >300 µg/L).27 In our population, 31.8% of the elderly presented serum ferritin concentrations higher than 100 µg/L and 3.8% had concentration >300 µg/L, these cut off points indicate inflammation, hemochromatosis or decompensated cirrhosis.33 This high prevalence of hyperferritinemia in the elderly may cause an underestimation of the prevalence of ID due to increases in serum ferritin associated to chronic or acute inflammation and an incorrect diagnosis of ID, even after making the correction of Turnham et al.15 using serum CRP concentrations. One limitation of this study is that we were not able to determine anemia for inflammatory response properly or determine other causes of anemia, such as folate and cobalamin deficiencies as well as a complete evaluation of iron status.
Our results on the prevalence of ID in anemic Mexican elders are comparable with those of NHANES III (16.6%).21 The higher rate of anemia not explained by ID suggests that other nutritional deficiencies, systemic inflammation or chronic renal insufficiency may be playing an important role in the pathogenesis of anemia.21,34 Structural factors, such as low HWI conditions, being Southerner, affiliated to any social programs or having a low weight, may contribute to understand the higher risk for anemia and ID in Mexican older adults.
This study is the first work to document the prevalence of anemia, ID and IDA in Mexican elder population. The main strength of this work is its probabilistic design, that provides a sampling that furnishes a national sample, with representativeness of the rural and urban dwelling and geographic regions.
Conclusion
Anemia is a major health problem in the elderly in Mexico, being the risk more severe in adults over 70 years. The prevalence of ID found in this study was low for men and women. Further research is required to identify the main causes of anemia at population-based studies in older adults to maintain and improve their health conditions.
References
1. Benoist B, McLean E, Egli I, Cogswell M. Worldwide prevalence of anaemia 1993-2005 . WHO global database on anaemia. España: WHO Library Cataloguing-in-Publication Data, 2008 [accessed 2015 July 09]. Available at: http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. [ Links ]
2. Eisensteaedt, R, Penninx BW, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev 2006;20:213-s26. [ Links ]
3. Izaks GJ, Westendorp, RG, Knook DL. The definition of anemia in older persons. JAMA 1999;281:1714-1717. [ Links ]
4. Bach V, Schruckmayer G, Sam I, Kemmler G, Stauder R. Prevalence and possible causes of anemia in the elderly: a cross-sectional analysis of a large European university hospital cohort. Clinical Interv in Aging 2014:9.1187-1196. [ Links ]
5. Denny SD, Kuchibhatla MN, Cohen HJ. Impact of anemia on mortality, cognition, and function in community-dwelling elderly. Am J Med 2006;119:327-334. [ Links ]
6. Gutiérrez JP, Rivera-Dommarco J, Shamah-Levy T, Villalpando-Hernández S, Franco A, Cuevas-Nasu L, et al. Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales. Cuernavaca, México: Instituto Nacional de Salud Pública, 2012. [ Links ]
7. Smith D. Anemia in the elderly. Am Fam Physician 2000;62(7):1565-1572. [ Links ]
8. Christopher V. Charles (2012). Iron deficiency anemia: a public health problem of global proportions. In: Jay Maddock, ed. Public Health - Methodology, Environmental and Systems Issues [accessed 2015 July 09]. Available in: http://www.intechopen.com/books/public-health-methodology-environmental-andsystems-issues/iron-deficiency-anemia-a-public-health-problem-of-global-proportions [ Links ]
9. Joosten E, Ghesquiere B, Linthoudt H, Krekelberghs F, Dejaeger E, Boonen S, et al. Upper and lower gastrointestinal evaluation of elderly inpatients who are iron deficient. Am J Med 1999;107:24-29. [ Links ]
10. Busti F, Campostrini N, Martinelli, N, Girelli, D. Iron deficiency in the elderly population, revisited in the hepcidin era. Frontiers in Pharmacology. Front Pharmacol 2014;5:83. [ Links ]
11. Mejía R, Shamah-Levy T, Villalpando S, García A, Méndez-Gómez I. Distribution of iron, zinc, cooper and magnesium deficiencies in a probabilistic sample of Mexican adults from the National Health and Nutrition Survey, 2006. Salud Publica Mex 2013;55:275-284. [ Links ]
12. Lohman T, Roche A, Martorell R. Anthropometric standarization reference manual. Champlaign, IL: Human Kinetics,1988. [ Links ]
13. Habicht J. Standardization of anthropometric methods in the field. PAHO Bull 1974;76:375-384. [ Links ]
14. World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. WHO Technical Report Series 854. Geneva: WHO, 1995. [ Links ]
15. Thurnham D, McCabe LD, Haldar S, Wieringa FT, Northrop-Clewes CA, McCabe GP. Adjusting ferritin concentration to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta-analysis. Am J Clin Nutr 2010;92:546-555. [ Links ]
16. Cohen J, Hass J. Hemoglobin correction factors for estimating the prevalence of iron deficiency anemia in pregnant women residing at high altitudes in Bolivia. Rev Panam Salud Publica 1999;6:392-396. [ Links ]
17. Iron deficiency anaemia. Assessment, prevention and control. A guide for programme managers. UNICEF/UNU/WHO [report no. 01.3]. 2001 [accessed 2015 July 09]. Available at: http://apps.who.int/iris/bitstream/10665/66914/1/WHO_NHD_01.3.pdf [ Links ]
18. Palma O, Shamah T, Franco A, Olaiz G, Mendez I. Metodologia. In: Encuesta Nacional de Salud y Nutrición 2006. Cuernavaca, Mexico: Instituto Nacional de Salud Pública, 2006:19-33. [ Links ]
19. Olaiz G, Rivera J, Shamah T, Rojas R, Villalpando S, Hernandez A, et al. Encuesta Nacional de Salud y Nutrición 2006. Cuernavaca, México: Instituto Nacional de Salud Pública, 2006. [ Links ]
20. Corona L, Oliveira Y, Lebrao M. Prevalence of anemia and associated factors in older adults: evidence from the SABE Study.Rev Saude Publica 2014;48(5):723-731. [ Links ]
21. Guralnik J, Eisenstaedt R, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood 2004;104:2263-2268. [ Links ]
22. Inelmen EM, D'Alessio M, Gatto MR, Baggio MB, Jimenez G, Bizzotto MG, et al. Descriptive analysis of the prevalence of anemia in a randomly selected sample of elderly people living at home: some results of an Italian multicentric study. Aging 1994;6:81-89. [ Links ]
23. Salive ME, Cornoni-Huntley J, Guralnik JM, Phillips CL, Wallace RB, Ostfeld AM, et al. Anemia and hemoglobin levels in older persons: relationship with age, gender, and health status. J Am Geriatr Soc 1992;40:489-496. [ Links ]
24. Skjelbakken T, Langbakk B, Dahl IM, Lochen ML. Haemoglobin and anemia in a gender perspective: the Tromso Study. Eur J Haematol 2005;74:381-388. [ Links ]
25. Freire WB, Brenes L, Waters WF, Paula D, Mena MB. SABE II. Situación de salud y nutrición de los adultos mayores ecuatorianos, a través de biomarcadores. MIES-Aliméntate Ecuador/USFQ. Quito, Ecuador USFQ, 2011. [ Links ]
26. Brussaard JH, Brants HA, Bouman M, Lowik MR. Iron intake and iron status among adults in the Netherlands. Eur J Clin Nutr 1997; 51 suppl 3:S51-S58. [ Links ]
27. Wang J, Shaw N. Iron status of the Taiwanese elderly: the prevalence of iron deficiency and elevated iron stores. Asia Pac J Clin Nutr 2005;14(3):278-284. [ Links ]
28. Berner LA, Clydesdale FM, Douglass JS. Fortification contributed greatly to vitamin and mineral intakes in the United States, 1989-1991. J Nutr 2001;131:2177-2183. [ Links ]
29. Hughes K. Serum ferritin and iron status in the general population of Singapore, 1993-1995. Ann Acad Med Singapore 1998;27:507-511. [ Links ]
30. Milman N, Pedersen A, Ovesen L, Schroll M. Iron status in 358 apparently healthy 80-year-old Danish men and women: relation to food composition and dietary and supplemental iron intake. Ann Hematol 2004;83:423-429. [ Links ]
31. Cepeda-Lopez A, Aeberli I, Zimmermann M. Does obesity increase risk for iron deficiency? A review of the literature and the potential mechanisms. Int J Vitam Nutr Res. 2010;80: 263-270 DOI:10.1024/0300-9831. [ Links ]
32. Yanoff LB, Menzie CM, Denkinger B, Sebring NG, McHugh T, Remaley AT, Yanovski JA. Inflammation and iron deficiency in the hypoferremia of obesity Int J Obes 2007;31:1412-1419. [ Links ]
33. Maiwall R, Maras JS, Kumar S, Sarin SK. Serum Ferritin in decompensated cirrhosis: Marker of both iron excess and inflammation. J Hepatol 2014;11(14):00819-8. [ Links ]
34. Price E, Mehra R, Holmes T, Schrier S. Anemia in older persons: Etiology and evaluation. Blood Cells Mol Dis 2011;46:159-165. [ Links ]
Received on: February 13, 2015
Accepted on: July 10, 2015
 Corresponding author:
Corresponding author:
Salvador Villalpando.
Instituto Nacional de Salud Pública. Av. Universidad 655,
col. Santa María Ahuacatitlán. 62100 Cuernavaca, Morelos, México.
E-mail: svillalp@insp.mx.
Declaration of conflict of interests. The authors declare that they have no conflict of interests.













