Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Salud Pública de México
Print version ISSN 0036-3634
Salud pública Méx vol.57 n.4 Cuernavaca Jul./Aug. 2015
Artículo original
Disease and weight loss: a prospective study of middle-aged and older adults in Costa Rica and England
Enfermedad y pérdida de peso: un estudio prospectivo de adultos y adultos mayores en Costa Rica e Inglaterra
Laura Blue, PhD,(1)*, Noreen Goldman, PhD,(1) Luis Rosero-Bixby, PhD, MPH.(2,3)
(1) Office of Population Research, Princeton University. The United States.
(2) Department of Demography, University of California, Berkeley. The United States.
(3) Centro Centroamericano de Población, Universidad de Costa Rica. Costa Rica.
Abstract
Objective. To determine whether disease predicts weight loss in population-based studies, as this may confound the relationship between weight and mortality.
Materials and methods. We used longitudinal data from the Costa Rican Longevity and Healthy Aging Study (CRELES) and the English Longitudinal Study of Ageing (ELSA). We defined two overlapping outcomes of measured weight loss between waves: >1.0 point of body mass index (BMI) and >2.0 BMI points. Logistic regression models estimated the associations with disease, adjusting for age (range 52-79), sex, smoking, and initial BMI.
Results. In ELSA, onset of diabetes, cancer, or lung disease is associated with loss >2.0 points (respectively, OR=2.25 [95%CI: 1.34-3.80]; OR=2.70 [95%CI: 1.49-4.89]; OR=1.82 [95%CI: 1.02-3.26]). In CRELES, disease-onset reports are not associated with weight loss at 5% significance, but statistical power to detect associations is poor.
Conclusion. Although it is known that some diseases cause weight loss, at the population level these associations vary considerably across samples.
Key words: weight loss; body weight changes; body mass index; diabetes mellitus; Costa Rica; England.
Resumen
Objetivo. Determinar si las enfermedades predicen pérdida de peso a partir de encuestas poblacionales, debido a que esto podría confundir la relación entre peso y mortalidad.
Material y métodos. Se utilizaron datos longitudinales de Costa Rica: Estudio de Longevidad y Envejecimiento Saludable (CRELES) y Estudio Longitudinal de Envejecimiento en Inglaterra (ELSA, por sus siglas en inglés). Se definieron dos indicadores de resultado no excluyentes de pérdida de peso entre rondas: >1.0 punto de índice de masa corporal (IMC) y >2.0 puntos de IMC. Las asociaciones de interés se estimaron con modelos de regresión logística, con controles para la edad (rango 52-79), sexo, tabaquismo actual e IMC inicial.
Resultados. En el ELSA, la incidencia de diabetes, cáncer o enfermedad pulmonar está asociada con pérdida de >2.0 puntos de IMC (respectivamente: OR=2.25 [IC95%: 1.34-3.80]; OR=2.70 [IC95%: 1.49-4.89]; OR=1.82 [IC95%: 1.02-3.26]). En el CRELES, el reporte de diagnóstico de enfermedades no muestra asociación significativa a 5% con pérdida de peso, pero el poder estadístico de la muestra para detectar asociaciones es limitado.
Conclusión. Aunque es conocido que ciertas enfermedades causan pérdida de peso, estas asociaciones a nivel poblacional varían considerablemente entre encuestas.
Palabras clave: pérdida de peso; cambios en el peso corporal; índice de masa corporal; diabetes mellitus; Costa Rica; Inglaterra.
This study tests whether disease predicts weight loss among middle-aged and older adults who participate in multiple waves of longitudinal, population-based surveys. There are two major motivations for this research. First, previous studies find an association between weight loss and subsequent mortality in such longitudinal surveys.1-3 If disease does predict weight loss, this could explain the link between weight loss and mortality even though, for many individuals, mild to moderate weight loss is recommended to manage hypertension,4 diabetes,5 and other cardiovascular risk factors.6 Second, the relationship between disease and weight loss in population-based surveys is central to interpreting many epidemiological studies that analyze impacts of body weight on mortality risk.7 In those studies, body weight measures are typically assumed to reflect some combination of nutrition, fitness, and overall metabolic health. However, if weight also reflects disease processes, it is not straightforward to treat body weight as a causal exposure. Instead, body weight is both cause and effect of health outcomes. In short, the relationship between disease and weight loss matters for interpreting body weight and body mass index (BMI) intelligently in epidemiological research.
There is no good evidence about the population-level association between disease and weight loss. Clinicians consider weight loss a symptom of many life-threatening conditions, including cancer, lung disease, and congestive heart failure,8 and disease is a demonstrated cause of weight loss in select clinical populations.9,10 Nevertheless, it is unclear whether disease drives weight loss in non-clinical samples, in which life-threatening illness is probably rare. Two retrospective studies in the USA linked unintentional weight loss to poor health,11,12 but the results have not been replicated in prospective data. In contrast, using the longitudinal Americans' Changing Lives Survey, Kahng, Dunkle, and Jackson found no effect of health on BMI change, and found, instead, that falling BMI tends to precede health decline.13 Prospective studies of weight loss and physical function or mobility yield conflicting results.14,15
In this study, we aim to clarify the relationship between disease and weight loss among longitudinal survey participants by testing for associations between self-reported clinical diagnoses and subsequent or concurrent BMI loss. We use two prospective, population-based surveys, one based in Costa Rica and one in England. These countries have very different socioeconomic and public health histories, but, in 2010, had roughly similar mean BMI (26.7 in Costa Rica v. 27.0 in the United Kingdom) and obesity prevalence (24.0 v. 25.0%).16 Both countries' surveys include three waves with measured height and weight, whereas most previous population studies have relied on self-reported values for at least one wave. Self-reports may be biased.17 Furthermore, survey respondents may be unlikely to know and report their true weight if weight is changing rapidly. Using objective weight measurements, this study allows a more robust investigation of disease and weight loss in non-clinical samples than has previously been possible.
Materials and methods
Data
Data come from two longitudinal surveys: the Costa Rican Longevity and Healthy Aging Study (CRELES) and the English Longitudinal Study of Ageing (ELSA). Data collection, including sampling scheme and measurement of height and weight, has been described elsewhere.18,19 Each survey is nationally representative, within certain age limits, of the population from which it is drawn. Each measures participants' height (by stadiometer) and weight (by scale) over at least three waves, and each collects detailed reports about health history and recent diagnoses. Because relationships between BMI and health may vary by age, especially among the very elderly,20 we limit the sample to participants below age 80. The age range for the CRELES sample is 61-79. The age range for the ELSA sample is 52-79. By studying two culturally distinct populations, we hope to assess the constancy of relationships across samples.
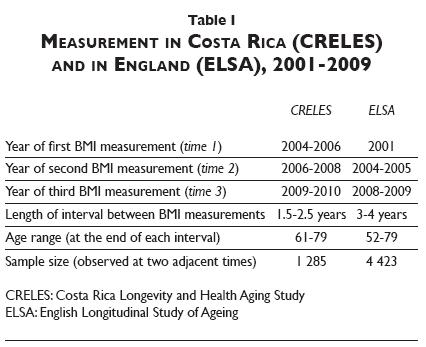
Survey waves. Table I shows measurement timing. For simplicity, we refer to waves with initial height and weight measurements as time 1; waves with the second measurements as time 2, and waves with the third as time 3, although these times do not occur in the same years in ELSA as in CRELES. In CRELES, measurements occurred roughly biennially around 2005, 2007, and 2009. In ELSA, measurements occurred first in 1998-1999 or 2001 (because ELSA's first wave was split), and then in 2004-2005 and 2008-2009. To ensure comparable BMI-change measurements, we restrict the CRELES sample to include BMI observations spaced at least 18 months and no more than 30 months apart. We restrict the ELSA sample to include observations three to four years apart (effectively removing any initial BMI measurement before 2001). Thus intervals between measurements are about the same length within each study population, but are shorter in CRELES than ELSA. This prevents direct comparison between countries of the magnitude of associations. We may still compare across them for evidence of any association—but differences could reflect variations either in the underlying relationships or in study limitations (e.g., in statistical power).
Definition of "weight loss". We calculate BMI at each wave, and define two (overlapping) categories of weight loss: a) measured BMI loss between two adjacent survey waves of >1.0 BMI point, and b) measured BMI loss between adjacent survey waves of >2.0 BMI points. We define weight loss using categorical variables because many diseases are more common among both weight losers and weight gainers (results not shown). It is plausible, therefore, that some diseases are associated with weight loss beyond a given threshold, but not with mean weight change (see Sensitivity analysis below, for discussion of additional analyses with continuous outcomes).
All height and weight values come from direct measurement rather than self-reports. We discarded outlier observations with height <1.22 m or >2.03 m, weight <31.8 kg, or a recorded height change >10 cm between waves—restrictions that eliminate roughly 0.2% of the sample from CRELES and 0.1% from ELSA. In both samples, BMI change is driven overwhelmingly by weight change, as participants' heights fluctuate only slightly between waves.
Within each population, we assume the relationship between disease and weight loss from time 1 to 2 is the same as from time 2 to 3. Analysis of each period separately supports this view (results not shown). Because simple random- and fixed-effects models do not fit the data well (an individual's weight-change observations tend to be negatively correlated), we include only one period per participant. For participants observed at all three waves, we use the weight-change observation from time 1 to 2.
Disease diagnoses. CRELES and ELSA participants report lifetime and recent disease diagnoses at time 1, and all new diagnoses since the previous interview at times 2 and 3. We focus on six major conditions: cancer, diabetes, heart attack, stroke, arthritis, and lung disease. We consider a condition present "at baseline" if a respondent reports the diagnosis (occurring in the past two years for cancer, or ever diagnosed for other conditions) in the wave starting the weight-change interval. We consider "disease onset" to occur if the respondent does not report the disease at the initial wave, but reports it at the next wave.
Disease definitions are the same in CRELES and ELSA for three of the six conditions, but three definitions are different. Diabetes status in ELSA is determined by self-report, whereas in CRELES it is determined in part by fasting glucose and glycosylated hemoglobin tests administered by CRELES; this broader definition may capture less severe cases than those identified in ELSA. In ELSA, study participants are asked if a doctor has ever diagnosed "chronic lung disease such as chronic bronchitis or emphysema"; in CRELES, the lung-disease question adds asthma and tuberculosis. Finally, in CRELES, questions about heart attack are specific to that event, whereas ELSA combines three serious manifestations of cardiovascular disease—heart attack, angina, and congestive heart failure—into a single marker that we label "heart attack".
Survey attrition and eligible sample. As noted above, the sample is the subset of CRELES and ELSA respondents with BMI measurements at two adjacent waves. Because attrition is likely correlated with disease, this study does not describe the typical magnitude or prevalence of illness-induced weight loss within a population. Instead, we examine associations between disease and weight loss over 1.5 to 4 years among a group with continued participation in voluntary, longitudinal surveys—similar to those used for many epidemiological studies. Results are not generalizable to the full population.
In each country, 3 to 5% of participants die between any two waves. Loss to follow-up among survivors ranges from just under 10% (between times 1 and 2 in CRELES) to roughly one third (between ELSA's first wave and the first follow-up).
Regression analyses. In four separate logistic regression analyses—one for each weight-loss outcome in each country—we estimate associations between diseases and weight loss, adjusting for age, sex, smoking status (current, former, never, measured at the start of the weight-change interval), and initial BMI. In each model, we include variables for each disease at baseline and each new diagnosis during the measurement interval. All analyses are performed in Stata 12 (StataCorp LP, College Station, TX). We do not use sample weights because weights in CRELES and ELSA were not designed for the present sample (participants with repeated BMI measurements), and because there is no consensus on whether to include survey weights in regression analyses.21
Odds ratios associated with continuous variables (i.e., age, BMI) are calculated for a two-standard-deviation change (approximately), as this makes the magnitude of estimates from continuous variables comparable to those from binary variables, at least when the mean of the binary variable is close to 0.5.22 Confidence intervals may be used to test whether any given odds ratio is different from 1.0. However, due to the large number of parameters tested (68, excluding sensitivity analysis), we expect to find roughly three associations significant at 5% if there are no true relationships. Each association, taken alone, should therefore be viewed as suggestive.
Sensitivity analysis. We test several alternative model specifications. First, we examine how results change with different predictor (i.e., disease) variables, including composite measures of disease and participants' self-rated overall health (details available upon request). Next, we examine how results change with a different functional form: namely, linear regression using a continuous outcome (either weight change in kilograms, or BMI change in points).
Results
Close to 20% of individuals in each sample have weight loss >1.0 BMI point and 6 to 8% have loss >2.0 points (table II). Mean BMI change is close to zero in both samples as weight gain is about as common as loss. Results are for the study samples only, not the national populations from which they were drawn, as we have no appropriate survey weights.
The proportions with self-reported disease at baseline range from less than 3% (for stroke or cancer) to 24% (for diabetes in CRELES and arthritis in ELSA). In both samples, the proportion with disease onset is less than 5% for all diseases, except arthritis in ELSA (12%). In CRELES (N=1 285), these low proportions result in poor statistical power to detect relationships between disease and weight loss—less than 50% power to reject the null for three quarters of the tests, assuming the true odds ratio is 2. This means we are unlikely to observe associations in CRELES because disease itself, especially new disease onset between waves (two years apart), is relatively rare. This fact alone is informative, suggesting that the impact of observed disease on weight loss is unlikely to be substantial. In ELSA, power is greater than 70% for nearly two-thirds of the statistical tests, and greater than 90% for one-third.
Figure 1 shows odds ratios from logistic regression of BMI loss >1.0 point on self-reported diseases, adjusted for age, sex, smoking status, and starting BMI. Figure 2 shows results from the analyses of BMI loss >2.0 points. In figure 1, we observe associations between diabetes in ELSA and BMI loss >1.0 point (OR=1.63 for diabetes at baseline [95%CI: 1.23-2.17], and OR=1.94 for onset during the weight-change interval [95%CI: 1.29-2.93]). No other conditions are associated at 5% in either country. In figure 2, however, where the outcome is weight loss >2.0 BMI points, associations with diabetes in England appear ber (OR=2.08 for disease at baseline [95%CI: 1.45-2.99], and OR=2.25 for onset [95%CI: 1.34-3.80]). We further observe associations in England between cancer onset and weight loss (OR=2.70 [95%CI: 1.49-4.89]) and between lung disease onset and weight loss (OR=1.82 [95%CI: 1.02-3.26]). In Costa Rica, no reports of new disease onset are significantly associated with weight loss, but we find a relationship between baseline arthritis and weight loss >2.0 BMI points (OR=2.00 [95%CI: 1.08-3.72]).
Results are similar when we estimate associations between weight loss and disease composite variables or self-reported health (results from sensitivity analysis are not shown). Similarly, we find no new associations with disease when we define weight change as a continuous variable, modeling associations using linear regression (results not shown). Sensitivity analysis results are available on request.
Discussion
This study is among the first to examine disease characteristics of people losing weight in population-based, prospective data. We analyze two surveys, in two countries, each collecting measured height and weight and detailed self-reports of clinical diagnoses.
Although some diseases cause weight loss,9,10 we find that associations between disease and weight loss among population-based survey participants vary considerably, both by survey and by magnitude of weight loss considered. We find more apparent relationships in ELSA than CRELES, and more apparent relationships with BMI loss >2.0 points than BMI loss >1.0 point. The larger number of significant associations between disease and major weight loss may suggest that mild loss has many causes (including, e.g., improved diet and exercise), while people with major weight loss are unusually likely to be sick. Observed variation by country could reflect differences in the burden of disease (e.g., in prevalence of different conditions, or in typical disease severity), differences in diagnosis norms or reporting, or simply different survey sampling (e.g., in age range covered, sample size and hence power, or intervals between BMI measurements). Together, the results provide equivocal support for the hypothesis that disease drives weight loss in population-based surveys. They further demonstrate that relationships may not be constant across populations. This, in turn, suggests that BMI may be difficult to interpret as a causal exposure in epidemiological research—because the effect of disease on BMI may vary across samples.
Our findings are largely exploratory, as we test for many possible associations in two different countries. This strategy is motivated by a lack of theoretical or empirical work to inform decisions about modeling disease and weight loss. However, because we find the best associations between disease and extreme weight loss, future scholars may wish to test for relationships between health and weight change at the left tails of the weight-change distribution.
This study has four limitations that prevent us from interpreting results—for people with BMI measurements in adjacent waves of a longitudinal survey—as evidence of causal effects of disease on weight loss. First, despite relatively high-quality data, measurements of weight change and disease onset remain crude because we observe people only periodically. Thus we observe net BMI change, rather than all BMI changes, between the waves. Furthermore, we do not observe the exact sequence of disease and weight-change events. When disease is diagnosed between survey waves, we cannot determine whether disease precedes or follows weight change.
Second, diagnoses are self-reported. Self-reports may be inaccurate, either because participants do not know they are sick, do not know the name of the disease that affects them, or choose not to report their illness in a survey. Reporting norms may vary across the two countries. Each disease-diagnosis category may also represent a broad range of disease severity.
Third, as noted above, we may lack statistical power to detect relationships, especially in CRELES. With few people in this sample experiencing new disease onset, in particular, we are unlikely to observe associations between disease onset and weight loss.
Fourth, we observe weight change only among survey participants who survive and have BMI measured in adjacent survey waves. In the absence of survey attrition, associations between disease and weight loss would likely be larger than observed here, since survey exit and death are very likely correlated with sickness, and death is very likely correlated with weight loss, as in other populations.1-3
Despite these limitations to generalizability, we believe our findings provide valuable information about the disease determinants of weight loss among middle-aged and older Costa Rican and English adults, who participate in two adjacent waves of longitudinal, population-based surveys. These surveys are similar to many others used in observational epidemiology, but include superior data on weight change over time. Epidemiologists should be aware that typical BMI data may reflect both the effects of body weight on health, and of health on body weight—but that processes could vary from one sample to the next, complicating causal interpretation. Our findings suggest both that one-time BMI measurements are noisy measures of long-term BMI exposure (because weight change is relatively common), and that the impact of health on BMI is complex.
Acknowledgments
We wish to thank Germán Rodríguez, Scott Lynch, and Thomas Espenshade for methodological advice and suggestions on earlier drafts. ELSA was developed at Britain's National Centre for Social Research, University College London, and the Institute for Fiscal Studies, with funding from the US National Institute on Aging and a consortium of UK government departments. Data are available through the UK Data Archive. CRELES was conducted by the University of Costa Rica's Centro Centroamericano de Población, collaborating with the Instituto de Investigaciones en Salud, and with funding from the Wellcome Trust (grant WT072406). We are deeply grateful to the participants and data collectors of both surveys. This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant 5R24HD047879) and the National Science Foundation (grant DGE-0646086).
References
1. Lee I, Paffenbarger RS. Change in body weight and mortality. J Am Med Assoc 1992;268(11): 2045-2049. [ Links ]
2. Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med 2005;165(9):1035-1040. [ Links ]
3. Myrskyla M, Chang VW. Weight change, initial BMI, and mortality among middle- and older-aged adults. Epidemiol 2009;20(6):840-848. [ Links ]
4. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension 2003;42:878-884. [ Links ]
5. Sjostrom CD, Peltonen M, Wedel H, Sjostrom L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 2000;36:20-25. [ Links ]
6. Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. J Am Med Assoc 2003;289(14):1799-1804. [ Links ]
7. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. J Am Med Assoc 2013;309(1):71-82. [ Links ]
8. Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, PA: Saunders Elsevier, 2008: 350, 619, 1353. [ Links ]
9. Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009;89:381-410. [ Links ]
10. Von Haehling S, Lainscak M, Springer J, Ander SD. Cardiac cachexia: a systematic overview. Pharmacol Therapeut 2009;121(3):227-252. [ Links ]
11. Meltzer AA, Everhart JE. Unintentional weight loss in the United States. Am J Epidemiol 1995;142(10):1039-1046. [ Links ]
12. French SA, Jeffery RW, Folsom AR, Williamson DF, Byers T. Relation of weight variability and intentionality of weight loss to disease history and health-related variables in a population-based sample of women aged 55-69 years. Am J Epidemiol 1995;142(12):1306-1314. [ Links ]
13. Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults: multilevel modeling analyses. Res Aging 2004;26(1):31-61. [ Links ]
14. Lee JS, Kritchevsky SB, Tylavsky F, Harris T, Simonsick EM, Rubin SM, et al. Weight change, weight change intention, and the incidence of mobility limitation in well-functioning community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2005;60(8):1007-1012. [ Links ]
15. St-Arnaud-McKenzie D, Payette H, Gray-Donald K. Low physical function predicts either 2-year weight loss or weight gain in healthy community-dwelling older adults: The NuAge Longitudinal Study. J Gerontol A Biol Sci Med Sci 2010;65A(12):1362-1368. [ Links ]
16. World Health Organization. WHO Global Infobase NCD Indicators [online document]. WHO, 2011 [accesed March 15, 2015]. Available at: https://apps.who.int/infobase/Indicators.aspx. [ Links ]
17. Connor-Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307-326. [ Links ]
18. Rosero-Bixby L, Fernández, X, Dow WH. CRELES: Costa Rican Longevity and Healthy Aging Study, 2005 (Costa Rica Estudio de Longevidad y Envejecimiento Saludable) ICPSR26681-v2 2010-07-21 2013 Inter-university Consortium for Political and Social Research (ICPSR) [online document]. [accesed October 2, 2014]. Available at: http://doi.org/10.3886/ICPSR26681.v2. [ Links ]
19. Marmot M, Oldfield Z, Clemens S, Blake M, Nazroo J, Steptoe A, et al. English Longitudinal Study of Ageing: Wave 0 (1998, 1999 and 2001) and Waves 1-4 (2002-2009) [computer file]. 11th Edition. Colchester, Essex: UK Data Archive [distributor]. April 2011. SN: 5050 [accesed October 2, 2014]. Available at: http://dx.doi.org/10.5255/UKDA-SN-5050-1. [ Links ]
20. Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. New Eng J Med 1998;338:1-7. [ Links ]
21. Winship C, Radbill L. Sampling weights and regression analysis. Sociol Methods Res 1994;23(2):230-257. [ Links ]
22. Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge, UK: Cambridge University Press, 2007:57. [ Links ]
Received on: May 27, 2014
Accepted on: May 20, 2015
 Corresponding author:
Corresponding author:
Laura Blue.
Mathematica Policy Research.
1100 First St NE, 12th Floor. 20002
Washington, DC. The United States.
E-mail: lblue@mathematica-mpr.com
Declaration of conflict of interests. The authors declare that they have no conflict of interests.
* The author conducted this work while a PhD candidate at Princeton University.













